Abstract
Objective
To determine the impact of progressive anemia of prematurity on cerebral regional saturation (C-rSO2) in preterm infants and identify the hemoglobin threshold below which a critical decrease (>2SD below the mean) in C-rSO2 occurs.
Study design
In a cohort of infants born ≤30 weeks EGA, weekly C-rSO2 data were prospectively collected from the second week of life through 36 weeks post-menstrual age (PMA). Clinically obtained hemoglobin values were noted at the time of recording. Recordings were excluded if they were of insufficient duration (<1 h) or if the hemoglobin was not measured within 7 days. Statistical analysis was performed using a linear mixed effects-model and ROC analysis. ROC analysis was used to determine the threshold of anemia, where C-rSO2 critically decreased >2SD below the mean normative value (<55%) in preterm infants.
Results
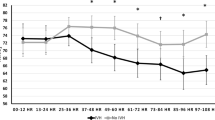
In total 253 recordings from 68 infants (mean EGA 26.9 ± 2.1 weeks, BW 1025 ± 287 g, 49% male) were included. Approximately 29 out of 68 infants (43%) were transfused during hospitalization. Mixed-model statistical analysis adjusting for EGA, BW, and PMA revealed a significant association between decreasing hemoglobin and C-rSO2 (p < 0.01) in transfusion-naive infants but not in transfused infants. In the transfusion naive group, using ROC analysis demonstrated a threshold hemoglobin of 9.5 g/dL (AUC 0.81, p < 0.01) for critical cerebral desaturation in preterm infants.
Conclusions
In transfusion-naive preterm infants, worsening anemia was associated with a progressive decrease in cerebral saturations. Analysis identified a threshold hemoglobin of 9.5 g/dL below which C-rSO2 dropped >2SD below the mean.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Whyte R, Kirpalani H. Low versus high haemoglobin concentration threshold for blood transfusion for preventing morbidity and mortality in very low birth weight infants. Cochrane Database Syst Rev. 2011;9:CD000512. https://doi.org/10.1002/14651858.CD000512.pub2.
Colombatti R, Sainati L, Trevisanuto D. Anemia and transfusion in the neonate. Semin Fetal Neonatal Med. 2016;21:2–9.
Ibrahim M, Ho SKY, Yeo CL. Restrictive versus liberal red blood cell transfusion thresholds in very low birth weight infants: a systematic review and meta-analysis. J Paediatr Child Health. 2014;50:122–30.
Christensen RD, Carroll PD, Josephson CD. Evidence-based advances in transfusion practice in neonatal intensive care units. Neonatology. 2014;106:245–53.
Juul S. Erythropoiesis and the approach to anemia in premature infants. J Matern-Fetal Neonatal Med. 2012;25:97–99.
Aher S, Malwatkar K, Kadam S. Neonatal anemia. Semin Fetal Neonatal Med. 2008;13:239–47.
Fabres J, Wehrli G, Marques Marisa B, Phillips V, Dimmitt Reed A, Westfall Andrew O, et al. Estimating blood needs for very-low-birth-weight infants. Transfusion. 2006;46:1915–20.
Kirpalani H, Whyte RK, Andersen C, Asztalos EV, Heddle N, Blajchman MA, et al. The premature infants in need of transfusion (pint) study: a randomized, controlled trial of a restrictive (LOW) versus liberal (HIGH) transfusion threshold for extremely low birth weight infants. J Pediatr. 2006;149:301–7.e303.
Bell EF, Strauss RG, Widness JA, Mahoney LT, Mock DM, Seward VJ, et al. Randomized trial of liberal versus restrictive guidelines for red blood cell transfusion in preterm infants. Pediatrics. 2005;115:1685–91.
Bifano EM. The effect of hematocrit (HCT) level on clinical outcomes in extremely low birthweight (ELBW) infants. Pediatr Res. 2001;49:311A.
Bifano EM, Bode MM, D’Eugenio DB. Prospective randomized trial of high vs. low hematocrit in extremely low birthweight (ELBW) infants: One year growth and neurodevelopmental outcome. Pediatr Res. 2002;51:325a.
Bishara N, Ohls RK. Current controversies in the management of the anemia of prematurity. Semin Perinatol. 2009;33:29–34.
Chen H-L, Tseng H-I, Lu C-C, Yang S-N, Fan H-C, Yang R-C. Effect of blood transfusions on the outcome of very low body weight preterm infants under two different transfusion criteria. Pediatr Neonatol. 2009;50:110–6.
Whyte RK, Kirpalani H, Asztalos EV, Andersen C, Blajchman M, Heddle N, et al. Neurodevelopmental outcome of extremely low birth weight infants randomly assigned to restrictive or liberal hemoglobin thresholds for blood transfusion. Pediatrics. 2009;123:207–13.
Nopoulos PC, Conrad AL, Bell EF, Strauss RG, Widness JA, Magnotta VA, et al. Long-term outcome of brain structure in premature infants: effects of liberal vs restricted red blood cell transfusions. Arch Pediatr Adolesc Med. 2011;165:443–50.
Josephson CD, Glynn SA, Kleinman SH, Blajchman MA. A multidisciplinary “think tank”: the top 10 clinical trial opportunities in transfusion medicine from the National Heart, Lung, and Blood Institute-sponsored 2009 state-of-the-science symposium. Transfusion. 2011;51:828–41.
Fredrickson LK, Bell EF, Cress GA, Johnson KJ, Zimmerman MB, Mahoney LT, et al. Acute physiological effects of packed red blood cell transfusion in preterm infants with different degrees of anaemia. Arch Dis Child Fetal Neonatal Ed. 2011;96:F249–53.
Hudson I, Cooke A, Holland B, Houston A, Jones JG, Turner T, et al. Red cell volume and cardiac output in anaemic preterm infants. Arch Dis Child. 1990;65:672–5.
Bailey SM, Hendricks-Munoz KD, Wells JT, Mally P. Packed red blood cell transfusion increases regional cerebral and splanchnic tissue oxygen saturation in anemic symptomatic preterm infants. Am J Perinatol. 2010;27:445–53.
El-Dib M, Aly S, Govindan R, Mohamed M, du Plessis A, Aly H. Brain maturity and variation of oxygen extraction in premature infants. Am J Perinatol. 2016;33:814–20.
Wardle SP, Yoxall CW, Weindling AM. Determinants of cerebral fractional oxygen extraction using near infrared spectroscopy in preterm neonates. J Cereb blood Flow Metab. 2000;20:272–9.
Andersen CC, Collins CL. Poor circulation, early brain injury, and the potential role of red cell transfusion in premature newborns. Pediatrics. 2006;117:1464–6.
Back SA, Riddle A, McClure MM. Maturation-dependent vulnerability of perinatal white matter in premature birth. Stroke. 2007;38:724–30.
Glass HC, Costarino AT, Stayer SA, Brett C, Cladis F, Davis PJ. Outcomes for extremely premature infants. Anesth Analg. 2015;120:1337–51.
Litt J, Taylor HG, Klein N, Hack M. Learning disabilities in children with very low birthweight: prevalence, neuropsychological correlates, and educational interventions. J Learn Disabil. 2005;38:130–41.
Taylor HG, Minich NM, Klein N, Hack M. Longitudinal outcomes of very low birth weight: neuropsychological findings. J Int Neuropsychol Soc: JINS. 2004;10:149–63.
Back SA. Perinatal white matter injury: the changing spectrum of pathology and emerging insights into pathogenetic mechanisms. Ment Retard Dev Disabil Res Rev. 2006;12:129–40.
Ment LR, Schwartz M, Makuch RW, Stewart WB. Association of chronic sublethal hypoxia with ventriculomegaly in the developing rat brain. Dev Brain Res. 1998;111:197–203.
Raman L, Georgieff MK, Rao R. The role of chronic hypoxia in the development of neurocognitive abnormalities in preterm infants with bronchopulmonary dysplasia. Dev Sci. 2006;9:359–67.
Raman L, Tkac I, Ennis K, Georgieff MK, Gruetter R, Rao R. In vivo effect of chronic hypoxia on the neurochemical profile of the developing rat hippocampus. Dev Brain Res. 2005;156:202–9.
Tao JD, Barnette AR, Griffith JL, Neil JJ, Inder TE. Histopathologic correlation with diffusion tensor imaging after chronic hypoxia in the immature ferret. Pediatr Res. 2012;71:192–8.
Dix LML, van Bel F, Lemmers PMA. Monitoring cerebral oxygenation in neonates: an update. Front Pediatr. 2017;5:46.
van Bel F, Lemmers P, Naulaers G. Monitoring neonatal regional cerebral oxygen saturation in clinical practice: value and pitfalls. Neonatology. 2008;94:237–44.
Andersen CC, Hodyl NA, Kirpalani HM, Stark MJ. A theoretical and practical approach to defining “Adequate Oxygenation” in the preterm newborn. Pediatrics. 2017;139;pii: e20161117. https://doi.org/10.1542/peds.2016-1117.
Mintzer JP, Parvez B, Chelala M, Alpan G, LaGamma EF. Monitoring regional tissue oxygen extraction in neonates < 1250 g helps identify transfusion thresholds independent of hematocrit. J Neonatal–Perinat Med. 2014;7:89–100.
Alderliesten T, Dix L, Baerts W, Caicedo A, van Huffel S, Naulaers G, et al. Reference values of regional cerebral oxygen saturation during the first 3 days of life in preterm neonates. Pediatr Res. 2016;79:55–64.
Parry G, Tucker J, Tarnow-Mordi W. CRIB II: an update of the clinical risk index for babies score. Lancet. 2003;361:1789–91.
Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92:529–34.
Omar RZ, Wright EM, Turner RM, Thompson SG. Analysing repeated measurements data: a practical comparison of methods. Stat Med. 1999;18:1587–603.
Vrieze SI. Model selection and psychological theory: a discussion of the differences between the Akaike information criterion (AIC) and the Bayesian information criterion (BIC). Psychol Methods. 2012;17:228–43.
Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39:561–577.
Schisterman EF, Perkins NJ, Liu A, Bondell H. Optimal cut-point and its corresponding Youden Index to discriminate individuals using pooled blood samples. Epidemiology. 2005;16:73–81.
Pichler G, Binder C, Avian A, Beckenbach E, Schmölzer GM, Urlesberger B. Reference ranges for regional cerebral tissue oxygen saturation and fractional oxygen extraction in neonates during immediate transition after birth. J Pediatr. 2013;163:1558–63.
McNeill S, Gatenby JC, McElroy S, Engelhardt B. Normal cerebral, renal and abdominal regional oxygen saturations using near-infrared spectroscopy in preterm infants. J Perinatol. 2011;31:51–57.
van Hoften JC, Verhagen EA, Keating P, ter Horst HJ, Bos AF. Cerebral tissue oxygen saturation and extraction in preterm infants before and after blood transfusion. Arch Dis Child Fetal Neonatal Ed. 2010;95:F352–358.
Sood BG, McLaughlin K, Cortez J. Near-infrared spectroscopy: applications in neonates. Semin Fetal Neonatal Med. 2015;20:164–72.
Vesoulis ZA, Lust CE, Liao SM, Trivedi SB, Mathur AM. Early hyperoxia burden detected by cerebral near-infrared spectroscopy is superior to pulse oximetry for prediction of severe retinopathy of prematurity. J Perinatol. 2016;36:966–71.
Balegar KK, Stark MJ, Briggs N, Andersen CC. Early cerebral oxygen extraction and the risk of death or sonographic brain injury in very preterm infants. J Pediatr. 2014;164:475–80.e471.
Acknowledgements
We like to thank our study coordinator, Anthony Barton, and our research assistant, Laura Atwood, for their tireless efforts. We also thank all the patients and families who participated in this study.
Funding
This work was supported by the following grants: Washington University Institute of Clinical and Translational Sciences KL2 Training Program (NIH/NCATS KL2 TR000450); The Gerber Foundation; The Barnes-Jewish Hospital Foundation and the Washington University Institute of Clinical and Translational Sciences Clinical and Translational Funding Program (NIH/NCATS UL1 TR000448); NIH award R01HL124078.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Whitehead, H.V., Vesoulis, Z.A., Maheshwari, A. et al. Anemia of prematurity and cerebral near-infrared spectroscopy: should transfusion thresholds in preterm infants be revised?. J Perinatol 38, 1022–1029 (2018). https://doi.org/10.1038/s41372-018-0120-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-018-0120-0
This article is cited by
-
Abdominal Near Infrared Spectroscopy can be reliably used to measure splanchnic oxygenation changes in preterm infants
Journal of Perinatology (2023)
-
Hematological changes in neonatal mice with phlebotomy-induced anemia
Pediatric Research (2022)
-
Cardiorespiratory monitoring of red blood cell transfusions in preterm infants
European Journal of Pediatrics (2022)
-
Early brain and abdominal oxygenation in extremely low birth weight infants
Pediatric Research (2022)
-
Anemia of prematurity: how low is too low?
Journal of Perinatology (2021)