Abstract
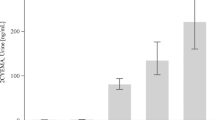
Acrylamide (AA), a probable human carcinogen, is a widely-used industrial chemical but is also present in tobacco smoke and carbohydrate-rich foods processed at high temperatures. AA is metabolized to glycidamide (GA) to cause the formation of DNA adducts. N7-(2-carbamoyl-2-hydroxyethyl) guanine (N7-GAG), the most abundant DNA adduct induced by GA, was recently detected in urine of smokers and non-smokers. In this study, we assessed the variability of AA exposure and biomarkers of AA exposure in urine samples repeatedly collected from AA-exposed workers and explored the half-life of N7-GAG. A total of 8 AA-exposed workers and 36 non-exposed workers were recruited. Pre-shift and post-shift urine samples were collected from the exposed group in parallel with personal sampling for eight consecutive days and from the control group on day 1 of the study. Urinary N7-GAG and the mercapturic acids of AA and GA, namely N-acetyl-S-(2-carbamoylethyl)-L-cysteine (AAMA) and N-(R,S)-acetyl-S-(1-carbamoyl-2-hydroxyethyl)-l-cysteine (GAMA) were analyzed using on-line solid phase extraction-liquid chromatography-electrospray ionization/tandem mass spectrometry methods. We found that N7-GAG levels in urine were significantly higher in exposed workers than in controls and that N7-GAG level correlated positively with AAMA and GAMA levels. Results from this study showed that AAMA and GAMA possibly remain the more preferred biomarkers of AA exposure and that N7-GAG levels could be elevated by occupational exposures to AA and serve as a biomarker of AA-induced genotoxicity for epidemiological studies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
IARC. IARC monographs on the evaluation of carcinogen risk to humans: some industrial chemicals. Lyon: IARC; 1994.
NSC. Chemical backgrounders index: acrylamide. 2006.
U.S. EPA (2010). Toxicological review of acrylamide (CAS No. 79-06-1) in support of summary information on the Integrated Risk Information System (IRIS). U.S. Environmental Protection Agency. Washington, DC. EPA/635/R-07/009F.
Tareke E, Rydberg P, Karlsson P, Eriksson S, Tornqvist M. Analysis of Acrylamide, a Carcinogen Formed in Heated Foodstuffs. J Agric Food Chem. 2002;50:4998–5006.
Joint FAO/WHO Expert Committee on Food Additives (2005 : Rome, Italy) Evaluation of certain food contaminants : sixty-fourth report of the Joint FAO/WHO Expert Committee on Food Additives.(WHO technical report series; 930) No. 930, 2006. p 7-17.
Smith CJ, Perfetti TA, Rumple MA, Rodgman A, Doolittle DJ. “IARC Group 2A Carcinogens” reported in cigarette mainstream smoke. Food Chem Toxicol. 2000;38:371–83.
Bull RJ, Robinson M, Laurie RD, Stoner GD, Greisiger E, Meier JR, et al. Carcinogenic effects of acrylamide in Sencar and A/J mice. Cancer Res. 1984;44:107–11.
Johnson KA, Gorzinski SJ, Bodner KM, Campbell RA, Wolf CH, Friedman MA, et al. Chronic toxicity and oncogenicity study on acrylamide incorporated in the drinking water of Fischer 344 rats. Toxicol Appl Pharmacol. 1986;85:154–68.
Friedman MA, Dulak LH, Stedham MA. A lifetime oncogenicity study in rats with acrylamide. Toxicol Sci. 1995;27:95–105.
Hogervorst JG, Schouten LJ, Konings EJ, Goldbohm RA, van den Brandt PAA. Prospective study of dietary acrylamide intake and the risk of endometrial, ovarian, and breast cancer. Cancer Epidemiol Biomark Prev. 2007;16:2304–13.
Hogervorst JGF, Baars BJ, Schouten LJ, Konings EJM, Goldbohm RA, van den Brandt PA. The carcinogenicity of dietary acrylamide intake: A comparative discussion of epidemiological and experimental animal research. Crit Rev Toxicol. 2010;40:485–512.
Sumner SC, Fennell TR, Moore TA, Chanas B, Gonzalez F, Ghanayem BI. Role of cytochrome P450 2E1 in the metabolism of acrylamide and acrylonitrile in mice. Chem Res Toxicol. 1999;12:1110–6.
Ghanayem BI, McDaniel LP, Churchwell MI, Twaddle NC, Snyder R, Fennell TR, et al. Role of CYP2E1 in the epoxidation of acrylamide to glycidamide and formation of DNA and hemoglobin adducts. Toxicol Sci. 2005;88:311–8.
Settels E, Bernauer U, Palavinskas R, Klaffke HS, Gundert-Remy U, Appel KE. Human CYP2E1 mediates the formation of glycidamide from acrylamide. Arch Toxicol. 2008;82:717–27.
Sumner SC, MacNeela JP, Fennell TR. Characterization and quantitation of urinary metabolites of [1,2,3-13C]acrylamide in rats and mice using 13C nuclear magnetic resonance spectroscopy. Chem Res Toxicol. 1992;5:81–9.
Sumner SC, Selvaraj L, Nauhaus SK, Fennell TR. Urinary metabolites from F344 rats and B6C3F1 mice coadministered acrylamide and acrylonitrile for 1 or 5 days. Chem Res Toxicol. 1997;10:1152–60.
Boettcher MI, Schettgen T, Kutting B, Pischetsrieder M, Angerer J. Mercapturic acids of acrylamide and glycidamide as biomarkers of the internal exposure to acrylamide in the general population. Mutat Res. 2005;580:167–76.
Huang CCJ, Li CM, Wu CF, Jao SP, Wu KY. Analysis of urinary N-acetyl-S-(propionamide)-cysteine as a biomarker for the assessment of acrylamide exposure in smokers. Environ Res. 2007;104:346–51.
Kopp EK, Sieber M, Kellert M, Dekant W. Rapid and sensitive HILIC-ESI-MS/MS quantitation of polar metabolites of acrylamide in human urine using column switching with an online trap column. J Agric Food Chem. 2008;56:9828–34.
Huang YF, Wu KY, Liou SH, Uang SN, Chen CC, Shih WC, et al. Biological monitoring for occupational acrylamide exposure from acrylamide production workers. Int Arch Occup Environ Health. 2010;84:303–13.
Gamboa da Costa, G, Churchwell, MI., Hamilton, LP, Von Tungeln, LS, Beland, FA, Marques, MM, Doerge, DR. DNA adduct formation from acrylamide via conversion to glycidamide in adult and neonatal mice. Chem Res Toxicol. 2003;16:1328–37.
Segerback D, Calleman CJ, Schroeder JL, Costa LG, Faustman EM. Formation of N-7-(2-carbamoyl-2-hydroxyethyl)guanine in DNA of the mouse and the rat following intraperitoneal administration of [14C]acrylamide. Carcinogenesis. 1995;16:1161–5.
Doerge DR, Gamboa da Costa G, McDaniel LP, Churchwell MI, Twaddle NC, Beland FA. DNA adducts derived from administration of acrylamide and glycidamide to mice and rats. Mutat Res/Genet Toxicol Environ Mutagen. 2005;580:131–41.
Koyama N, Yasui M, Kimura A, Takami S, Suzuki T, Masumura K, et al. Acrylamide genotoxicity in young versus adult gpt delta male rats. Mutagenesis. 2011;26:545–9.
Watzek N, Bohm N, Feld J, Scherbl D, Berger F, Merz KH, et al. N7-glycidamide-guanine DNA adduct formation by orally ingested acrylamide in rats: a dose-response study encompassing human diet-related exposure levels. Chem Res Toxicol. 2012;25:381–90.
Huang C-CJ, Wu C-F, Shih W-C, Luo Y-S, Chen M-F, Li C-M, et al. Potential association of urinary N7-(2-carbamoyl-2-hydroxyethyl) guanine with dietary acrylamide intake of smokers and nonsmokers. Chem Res Toxicol. 2015;28:43–50.
Farmer PB. DNA and protein adducts as markers of genotoxicity. Toxicol Lett. 2004;149:3–9.
Boysen G, Pachkowski BF, Nakamura J, Swenberg JA. The formation and biological significance of N7-guanine adducts. Mutat Res/Genet Toxicol Environ Mutagen. 2009;678:76–94.
Wu KY, Chiang SY, Shih WC, Huang CCJ, Chen MF, Swenberg JA. The application of mass spectrometry in molecular dosimetry: ethylene oxide as an example. Mass Spectrom Rev. 2011. https://doi.org/10.1002/mas.20299.
Wu KY, Huang YF, Chen MF, Shih Ts, Uang SN, Mao IF et al. Exposure assessment of airborne acrylamide for occupationally exposed workers by using an isotope-dilution gas chromatography coupled with mass spectrometry. Ann Occup Hyg. 2010;54:575–83.
Jaffe M. Uber den niederschlag, welchen pikriksaure in normalen harn erzeugt und uber eine neue reaction des kreatinins. Z Physiol Chem. 1886;10:391.
Rappaport S, Lyles R, Kupper LAN. Exposure—assessment strategy accounting for within-and between-worker sources of variability. Ann Occup Hyg. 1995;39:469–95.
Lin YS, Kupper LL, Rappaport SM. Air samples versus biomarkers for epidemiology. Occup Environ Med. 2005;62:750–60.
Maniere I, Godard T, Doerge DR, Churchwell MI, Guffroy M, Laurentie M, et al. DNA damage and DNA adduct formation in rat tissues following oral administration of acrylamide. Mutat Res/Genet Toxicol Environ Mutagen. 2005;580:119–29.
Nixon BJ, Stanger SJ, Nixon B, Roman SD. Chronic exposure to acrylamide induces DNA damage in male germ cells of mice. Toxicol Sci. 2012;129:135–45.
Watzek N, Scherbl D, Schug M, Hengstler J, Baum M, Habermeyer M, et al. Toxicokinetics of acrylamide in primary rat hepatocytes: coupling to glutathione is faster than conversion to glycidamide. Arch Toxicol. 2013;87:1545–56.
Li CM, Hu CW, Wu KY. Quantification of urinary N-acetyl-S- (propionamide)cysteine using an on-line clean-up system coupled with liquid chromatography/tandem mass spectrometry. J Mass Spectrom. 2005;40:511–15.
Urban M, Kavvadias D, Riedel K, Scherer G, Tricker AR. Urinary mercapturic acids and a hemoglobin adduct for the dosimetry of acrylamide exposure in smokers and nonsmokers. Inhal Toxicol. 2006;18:831–9.
Bjellaas T, Stolen LH, Haugen M, Paulsen JE, Alexander J, Lundanes E, et al. Urinary acrylamide metabolites as biomarkers for short-term dietary exposure to acrylamide. Food Chem Toxicol. 2007;45:1020–6.
Hartmann EC, Boettcher MI, Schettgen T, Fromme H, Drexler H, Angerer J. Hemoglobin adducts and mercapturic acid excretion of acrylamide and glycidamide in one study population. J Agric Food Chem. 2008;56:6061–8.
Fennell TR, Sumner SCJ, Snyder RW, Burgess J, Spicer R, Bridson WE, et al. Metabolism and hemoglobin adduct formation of acrylamide in humans. Toxicol Sci. 2005;85:447–59.
Fuhr U, Boettcher MI, Kinzig-Schippers M, Weyer A, Jetter A, Lazar A, et al. Toxicokinetics of acrylamide in humans after ingestion of a defined dose in a test meal to improve risk assessment for acrylamide carcinogenicity. cancer epidemiology biomarkers. Prevention. 2006;15:266–71.
Loeb LA, Preston BD. Mutagenesis by apurinic/apyrimidinic sites. Annu Rev Genet. 1986;20:201–30.
Besaratinia A, Pfeifer GP. Genotoxicity of acrylamide and glycidamide. J Natl Cancer Inst. 2004;96:1023–9.
Manjanatha MG, Aidoo A, Shelton SD, Bishop ME, McDaniel LP, Lyn-Cook LE, et al. Genotoxicity of acrylamide and its metabolite glycidamide administered in drinking water to male and female Big Blue mice. Environ Mol Mutagen. 2006;47:6–17.
Acknowledgements
This research was finically supported by a grant from the National Health Research Institute (EO-095-PP-02), a grant from the National Science Council of the Republic of China, Taiwan (MOST 95–2314-B-400-004-MY3), and a grant from Institute of Occupational Safety and Health (IOSH95-A319), Taiwan. We acknowledge the cooperation of the staff in the IOSH and our study participant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Huang, YF., Huang, CC.J., Lu, C.A. et al. Feasibility of using urinary N7-(2-carbamoyl-2-hydroxyethyl) Guanine as a biomarker for acrylamide exposed workers. J Expo Sci Environ Epidemiol 28, 589–598 (2018). https://doi.org/10.1038/s41370-018-0018-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41370-018-0018-0