Abstract
Background
Electronic health records (EHRs) are potentially important components in addressing pediatric obesity in clinical settings and at the population level. This work aims to identify temporal condition patterns surrounding obesity incidence in a large pediatric population that may inform clinical care and childhood obesity policy and prevention efforts.
Methods
EHR data from healthcare visits with an initial record of obesity incidence (index visit) from 2009 through 2016 at the Children’s Hospital of Philadelphia, and visits immediately before (pre-index) and after (post-index), were compared with a matched control population of patients with a healthy weight to characterize the prevalence of common diagnoses and condition trajectories. The study population consisted of 49,694 patients with pediatric obesity and their corresponding matched controls. The SPADE algorithm was used to identify common temporal condition patterns in the case population. McNemar’s test was used to assess the statistical significance of pattern prevalence differences between the case and control populations.
Results
SPADE identified 163 condition patterns that were present in at least 1% of cases; 80 were significantly more common among cases and 45 were significantly more common among controls (p < 0.05). Asthma and allergic rhinitis were strongly associated with childhood obesity incidence, particularly during the pre-index and index visits. Seven conditions were commonly diagnosed for cases exclusively during pre-index visits, including ear, nose, and throat disorders and gastroenteritis.
Conclusions
The novel application of SPADE on a large retrospective dataset revealed temporally dependent condition associations with obesity incidence. Allergic rhinitis and asthma had a particularly high prevalence during pre-index visits. These conditions, along with those exclusively observed during pre-index visits, may represent signals of future obesity. While causation cannot be inferred from these associations, the temporal condition patterns identified here represent hypotheses that can be investigated to determine causal relationships in future obesity research.
Similar content being viewed by others
Introduction
Childhood obesity is a major public health issue in the United States. In 2016, ~35 percent of children and adolescents, ages 2–19 years, were overweight (age- and sex-specific body mass index (BMI) greater than or equal to the 85th percentile per Centers for Disease Control and Prevention (CDC) growth charts) [1] or obese (age- and sex-specific BMI greater than or equal to the 95th percentile per CDC growth charts) [1]; approximately half of these children were obese [2]. Children with obesity have elevated risks of developing numerous comorbidities including diabetes, hypertension, sleep apnea, and psychological issues in childhood and later in life [3,4,5].
Electronic health records (EHRs) have the potential to support childhood obesity diagnosis, treatment, and surveillance at the clinical [6] and population levels [7]. EHR-derived data support the surveillance of obesity and associated comorbidities such as diabetes and asthma [8]. The data are useful for generating large, diverse cohorts for population studies [9] and can be combined with community-level data on environmental factors associated with unhealthy BMI for comprehensive child obesity studies [10].
Prior research has addressed the use of EHRs for obesity diagnosis and quality improvement in clinical settings [11], for prevalence and demographic estimates of childhood obesity and associated comorbidities [8, 12, 13], and in conjunction with other sources of data to study environmental influences on childhood obesity [10]. However, there is limited research on the temporal dependency of conditions associated with childhood obesity incidence. Knowledge of such temporal condition patterns is important because it may signal impending obesity or conditions likely to follow obesity incidence which could support care providers and health policy development. This study’s objective was to identify temporally ordered condition patterns surrounding childhood obesity incidence. Specifically, we sought to identify sequences of conditions recorded in the EHR for healthcare visits immediately before, during, and after the visit in which an obese BMI was first recorded. This work presents the novel application of a sequence mining algorithm, SPADE, to a large retrospective cohort to identify common condition trajectories surrounding pediatric obesity incidence. The approach is designed to:
- (1)
Identify common temporal condition sequences surrounding pediatric obesity incidence and conditions that are more prevalent before or after incidence.
- (2)
Determine if these condition patterns occur at a statistically significant different prevalence in patients with obesity as compared with similarly matched patients with healthy BMIs.
Materials and methods
Setting
We implemented a retrospective, matched case control study using a dataset derived from the Pediatric Big Data (PBD) resource at the Children’s Hospital of Philadelphia (CHOP) (a pediatric tertiary academic medical center). The PBD resource includes clinical data collected from CHOP, the CHOP Care Network (a primary care network of over 30 sites), and CHOP Specialty Care and Surgical Centers. Both clinical and non-clinical observations (as defined by Observational Health Data Sciences and Informatics (OHDSI) condition domain standards) from a patient’s EHR are included in the PBD database [14]. The PBD resource contains health-related information, including demographic, encounter, medication, procedure, and measurement (e.g., vital signs, laboratory results) elements for a large, unselected population of children seen in the CHOP healthcare network. Non-study personnel extracted all data from the EHR and removed protected health information identifiers, with the exception of dates, prior to transfer to the study database. Date information was removed from the analysis dataset as described below. The CHOP Institutional Review Board approved this study and waived the requirement for consent.
Inclusion criteria
This analysis used the CDC definition of childhood obesity (BMI z-score at or above the 95th percentile for age and sex) [15, 16]. Patients had at least one obesity measurement during a CHOP primary care visit and at least one visit prior to the first obesity measurement where an obese BMI was not recorded.
Data in the PBD resource are indexed by patient and visit (i.e., encounter). For purposes of this analysis, we consider a record to refer to a single patient visit and all data associated with that visit. Records that were not generated at a CHOP primary care site were excluded. Negative height, weight, and age values were removed from individual records. Height and weight measurements obtained on the same date were matched and used to calculate BMI. For records with duplicate entries of the same height or weight value recorded on the same date, only a single BMI value was used for that date. If there were different height or weight values on the same day, the most recent values recorded were used. The BMI z-scores were centrally calculated in this analysis. The same definition of obesity was used across study sites for the entire study period.
Patients must have been 2–18 years old during the index visit (the first time a patient is recorded with an obese BMI). Per CDC guidelines [17], biologically implausible height, weight, and BMI values were excluded from patient records, and we required that the BMI z-score (at or above 1.6449) during the index visit must have been biologically plausible; <1% of patients had biologically implausible BMIs at the time their BMI was first documented as obese (n = 303). Index visits coded as inpatient stays, ambulatory visits, or emergency department visits (face-to-face encounters) were included. The visit must have occurred between January 1, 2009 and December 31, 2016. Patients without a visit prior to the index visit were excluded.
The case patient data selection and cleaning processes are summarized in Fig. 1a. Data from the latest prior visit and the earliest proceeding visit for each patient were selected as a patient’s pre- and post-index visits if applicable. The pre-index and post-index visits were not necessarily face-to-face encounters, but must have occurred between January 1, 2005 and December 31, 2017. All patient visits were required to have had at least one recorded clinical finding per OHDSI condition domain standards (for example, an anemia screening is a non-clinical observation while an anemia diagnosis is a clinical observation) [14]. Condition observations are represented by ICD-9-CM and ICD-10-CM concepts [18, 19]. We note that once the pre-index, index, and post-index visits, and their corresponding separation in number of days, are identified and labeled as such, date information is not required for the subsequent analysis.
Study flow diagrams illustrating (a) data cleaning and processing of EHR data for the case population and (b) data acquisition process of visit data for potential matched controls. In a, M represents the number of observations (diagnostic codes) and N represents the number of patients. In b, K represents the number of visits and N represents the number of patients. In a, all patients had an index visit between January 1, 2009 and December 31, 2016 that was a face-to-face encounter and included height and weight measures that yielded a biologically plausible BMI. All patients had a pre-index visit. In b, all patients had BMI measurements that only fell between the 5th and 84th percentile based on height and weight measurements recorded from January 1, 2009 and December 31, 2016. Note, the Patient Encounter Status concept is used to indicate an encounter without medical findings. Use of this concept was not included in our analysis as it does not provide diagnostic information.
The final dataset was comprised of 397,337 clinical and non-clinical observations for 49,694 patients; 33.4% were recorded during a pre-index visit (n = 132,786), 40.8% were recorded during an index visit (n = 161,944), and 25.8% were recorded during a post-index visit (n = 102,607). Approximately 2/3 of patients (n = 33,839) had a non-obese BMI measurement in at least one visit prior to the index visit, and about 1/3 of patients (n = 15660) had a non-obese BMI measurement in the pre-index visit.
Study population characteristics
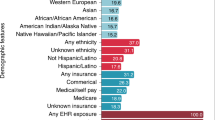
Table 1 summarizes the study population demographics. Patients were majority male (55.3%). The racial composition was 49.4% White, 34.7% Black or African–American, and 8.8% Hispanic. During the index visit, 57.1% used Private or Commercial insurance and 38.6% used Medicaid/CHIP. At the index visit, 30.5% of patients were 2–4 years old, 42.9% were 5–11 years, and 26.6% were 12–18 years.
Visit characteristics
The mean and standard deviation time difference between pre-index and index visits were 303.6 and 462.8 days, respectively, and the median difference was 125 days. The mean and standard deviation time difference between index and post-index visits were 147.8 and 246.3 days, respectively, and the median difference was 49 days. More than two-thirds of clinical observations recorded during pre- and post-index visits were made within 180 days of the index visit (n = 129,095) and an additional 13.8% of observations were made between 180 and 365 days of the index visit (n = 26,446); over 80% of observations from pre- and post-index visits were made within a year of the index visit. A majority of visits for patients in the study population (90.1%) occurred in an outpatient setting; 8.7% were emergency room visits and 1.2% occurred in an inpatient setting.
Data analysis
Matched control population
To compare clinical condition trajectories between our cohort with obesity and patients with a healthy BMI, a matched control cohort of children with at least one healthy BMI measurement (measurements in the 5th–84th percentiles for age and sex) [20] between 2009 and 2016 and no recorded unhealthy BMI measurements was obtained.
The control patient data selection and cleaning processes are summarized in Fig. 1b. For each visit, patient age and the number of prior visits with a clinical observation in the CHOP system were calculated. The number of prior visits with a recorded clinical observation for each case with obesity prior to the index visit was also calculated. The number of prior visits was intended to serve as a proximate measure of healthcare utilization and clinical well-being.
There were 343,998 eligible patients with 4,936,503 visits with at least one recorded clinical condition. Controls with only one documented visit and visits where patients had an age difference greater than 180 days from the oldest or youngest cases in the study population were excluded. The final control pool consisted of 3,622,341 potential visits and 296,751 patients.
Using the R matchControls function [21], each patient with pediatric obesity was matched with a control patient by sex, number of prior visits, and index age (for the matched control this was the age at the matching visit). Controls were matched by age within 60 days of their matched case. The youngest case patients were matched first. Once a control was matched, all other visits that the patient had in the control pool were removed.
All clinical observations from controls’ matching visits, the visit before, and the visit after (if applicable) were extracted from the PBD database. All controls had a pre-index and index visit (n = 49,694) and 89% of controls had a post-index visit (n = 44,208).
Figure 2 illustrates the similarity of age distribution and prior healthcare visits among the matched case and control populations. The mean and standard deviation age difference between the matched pair index ages were 0.13 and 1.65 days, respectively, and the median age difference was 0 days. The mean and standard deviation difference in visits prior to the matched index visit were 0.34 and 4.09 visits, respectively, and the median difference was 0 visits. Among control patients, 92.1% of visits were outpatient, 1.2% were inpatient, and 5.2% were emergency room visits. Less than 2% of visits were in other categories, including Administrative or Observation visits.
a, b illustrate the distribution of matched index ages for the case and control populations and c overlays both distributions on the same axes. d, e illustrate the distribution of prior healthcare visits for the case and control populations and f overlays both distributions on the same axes. Because the distributions are almost identical for the case and control populations, c and f appear to be a single KDE. The gray color of the overlaid KDEs results from the combined, identical black and white KDEs for the case and control populations.
SPADE analysis
The sequential pattern mining algorithm, SPADE [22] was used to mine patient data for temporal patterns in clinical condition trajectories surrounding obesity incidence. SPADE first scans data to identify individual items (e.g., a singular diagnosis in a specific timing class) above a specified support level (e.g., the proportion of patients with an identified condition pattern). Using these frequent single items, SPADE then builds more complex sequences (multiple diagnoses across different timing classes) at the given support level. Thus, a complex sequence present above a given support level is comprised of individual items that also occur above the support level. Prior research has shown that SPADE demonstrates runtime efficiency and low memory usage on sparse datasets [23]. As we were working to identify temporal condition patterns in a large, sparse EHR dataset, we selected SPADE as our pattern mining algorithm for this study.
The R arules package [24] was used to apply SPADE to the clinical data for the cases at a support level of 0.01 (thereby detecting the prevalence of clinical condition sequences present in at least 1% of the cases). The control population data were analyzed to determine prevalence of the patterns found by SPADE in the cases. SPADE analysis executed quickly for our study, with a runtime on the order of single seconds (on a MacBook Pro running MacOS version 10.12.6 and with 8 GB of RAM) using the R implementation. Pairwise McNemar’s tests were used to determine if there was a statistically significant difference in the frequency of these top sequences between the case and control populations. Effect sizes for each sequence were determined by calculating the odds ratios among discordant McNemar’s pairs.
Clinical term mapping
There were 7241 unique International Classification of Diseases concept names in the clinical observations for the case population. Many of these conditions were rarely used and, due to this granularity, SPADE would not find the support to detect meaningful condition patterns in the study population. Thus, all clinical observations were grouped into medically homogenous classes using expanded diagnostic clusters (EDCs) from the Adjusted Clinical Group System [8, 25], which places related concepts into fewer groups. Using the Python 3 programming language [26], clinical conditions from both the case and control populations were mapped to 268 unique EDC codes. Some codes had multiple mapped EDC concepts which were all included and treated as distinct conditions; 80% of clinical observations were mapped to 62 EDC codes.
Results
SPADE analysis
SPADE identified 189 sequences with a support level of 0.01 or higher among the case population. With clinician input, we removed 12 sequences with conditions that were not clinically informative, including: administrative concerns and nonspecific laboratory abnormalities, other skin disorders, preventive care, and nonspecific signs and symptoms. In addition, we removed 14 sequences with obesity since this diagnosis could only be common in the case population. During their index visit, 7119 patients in the case population (14.3%) received a formal obesity diagnosis.
After removing the 26 sequences with the aforementioned conditions, pairwise McNemar’s tests were administered on the remaining 163 sequences. The McNemar’s tests indicated that 80 sequences had statistically significant (p < 0.05) higher levels of support among cases (Table 2) and 45 had statistically significant higher levels of support among controls (Table 3). Although the sequences in Table 3 were initially identified by SPADE as condition trajectories that existed in at least 1% of the case population, they were detected at a significantly higher level of support among the controls. In addition, 23 sequences had statistically insignificant (p > 0.05) higher levels of support among cases, and 15 had statistically insignificant higher levels of support among controls.
Table 4 shows the unique EDC codes observed in significant sequences for the case population and control population respectively, as well as shared common conditions. Including obesity diagnoses, there were 40 unique EDC codes represented among statistically significant case sequences and 23 unique EDC codes represented among statistically significant control sequences. There was an overlap of 14 EDC codes between the two groups. These shared conditions can be considered common diagnoses for pediatric patients regardless of obesity status.
Conditions unique to significant sequences among the case population included autism spectrum disorder (ASD), sleep apnea, disorders of lipid metabolism, headaches, migraines, and psychological disorders of childhood. EDC codes that were represented among statistically significant sequences for both cases and controls include allergic rhinitis (although this was only common for controls in the post-index visit), otitis media, dermatitis and eczema, fever, acute upper respiratory tract infection, and developmental disorders. Seven conditions were diagnosed exclusively during pre-index visits among patients with obesity: dermatophytosis, ear, nose and throat disorders, exanthems, gastroenteritis, lacerations, nausea/vomiting, and strabismus/amblyopia. No diagnoses were significantly more common during post-index visits alone for the case population.
Asthma was strongly associated with pediatric obesity incidence. The diagnosis was present in 21 unique sequences, and were not present in any sequences with a significantly higher level of support among the control population. Asthma was observed in over 10% of both pre-index visits (n = 5332) and index (n = 6894) for patients with obesity. Aside from obesity, it was the only condition observed at a support level of 0.1 or higher among the case population.
However, asthma was not as commonly diagnosed during the post-index visit. A diagnosis of asthma without asthmaticus in the post-index visit was present in only three sequences. The sequence with the highest support (2-Asthma, 3-Asthma, indicating asthma diagnoses in the index and post-index visits) was present among 2331 cases, a number markedly lower than the diagnosis of asthma in the pre-index or index visits. A diagnosis of asthma, without status asthmaticus exclusively during the post-index visit (without a prior asthma diagnosis), was not a statistically significant sequence for the case population.
Finally, effect size calculations provided a measure of the strength of associations identified by SPADE. Effect sizes among the significant sequences for the case population ranged from 1.08 to 2.80. Sleep apnea diagnoses across visit timing classes had among the highest effect sizes (2.80, 2.37, and 2.33 for diagnoses during the pre-, index, and post-index visits, respectively). A diagnosis of ASD in the index visit had an effect size of 2.47, indicating a strong association. Asthma diagnosed during the pre-index visit had an effect size of 1.31; the effect size increased to 1.73 for diagnoses during the index visit. Allergic rhinitis diagnoses during the index visit had an effect size of 1.51. Comorbid asthma and allergic rhinitis diagnoses during the index visit had an effect size of 2.0.
Discussion
Methodological contributions
Obesity research has typically been formulated using epidemiological approaches wherein an a priori determined hypothesis (e.g., obesity incidence is more prevalent among asthmatics than non-asthmatics) is tested on a particular dataset. Furthermore, most extant obesity research has not considered temporal dependencies between obesity incidence and the occurrence of comorbidities.
One of the key strengths of our study is that it utilized a large, unselected population and the SPADE algorithm to find frequent temporal patterns in clinical data. This approach does not assume an a priori hypothesis regarding the association of obesity with a prespecified covariate. We view this novel data-driven approach as one that complements standard epidemiological methods with the potential to discover important hypotheses for future research and thereby expand our understanding of the complex individual and social factors that affect the obesity epidemic [27, 28]. Using this approach in a retrospective analysis of a pediatric population with obesity, we identified 80 temporal patterns present at statistically significant higher levels than in the matched control population of individuals with only healthy BMI observations. Among these patterns, there were 40 unique condition diagnoses. Seven of these conditions were commonly diagnosed only during pre-index visits and zero were commonly diagnosed only during post-index visits.
Obesity diagnosis associations
We found strong associations between asthma, allergic rhinitis, and obesity incidence. Although the influence of body weight changes and asthma outcomes requires more exploration, prior research has shown that children who are overweight or obese are more likely to develop asthma [29, 30]. The high prevalence of asthma observed during pre-index visits provides additional evidence in support of the contribution of early-life asthma to pediatric obesity onset [31] and the idea of a bidirectional asthma–obesity relationship. Children with asthma may be particularly susceptible to developing obesity and are targets for intervention efforts. In addition, the lower prevalence of asthma diagnoses during post-index visits suggests that while children with obesity are more likely to develop asthma, there may be a period of time between when children who are newly obese develop the condition.
Prior research is mixed on the relationship between allergic rhinitis and pediatric obesity. Some studies have failed to find a strong association between allergic rhinitis and obesity [32, 33], while Han et al. [34] found reduced odds of allergic rhinitis among children who were centrally obese and Lei et al. [35] found that overweight and obesity actually increased the risk of allergic rhinitis in a pediatric population. In our study, we found that allergic rhinitis was significantly more common among patients with obesity during the pre-index and index visits, but not during the post-index visit. This suggests that further investigation is needed on how body weight changes and BMI trajectory affect allergic rhinitis incidence, and also suggests that children with allergic rhinitis may be more likely to develop an unhealthy body weight. In addition, the comparably high effect size of comorbid asthma and allergic rhinitis during the index visit indicates that asthma may mediate the relationship between allergic rhinitis and pediatric obesity. Further investigation into this potential association is warranted.
Previous studies have indicated that children with intellectual disabilities and ASD have higher rates of obesity than other youth [36, 37]. In our study, developmental disorders (DD) were observed in some sequences that were more common among cases and some sequences that were more common in the control population, but ASD was a common diagnosis during the pre, post, and index visits only for the case population. In addition, DD diagnoses during pre-index visits were not present in significant sequences for either cases or controls. These findings indicate that while there is an association between ASD and obesity, there may be no temporal dependence. Furthermore, there may be differential risk factors for obesity among children with ASD compared with youth with other intellectual disabilities, and children with certain DD outside of ASD may be more at risk for developing obesity than others. Further investigation into these risk factors as well as the temporality trends in DD diagnosis and obesity incidence observed in this study is necessary.
Obesity diagnosis using EHR data
Although all patients in the case population had an obese BMI measurement during their index visit, only a fraction (approximately one in seven) received a formal obesity diagnosis. Pediatric obesity remains underdiagnosed in clinical practice, and children who are overweight or obese lack comprehensive access to nutrition and physical activity counseling [38, 39]. Unhealthy BMI identification and documentation improves clinical weight management [40, 41]. While low physician diagnosis of child overweight and obesity is well documented, past studies that investigated EHR use to address childhood obesity in a clinical context have relied on retrospective chart review [40, 42, 43], clinician surveys [44], or mixed methods of surveys and patient record review [45], and utilized prevalence estimates of overweight and obesity in a pediatric population to calculate diagnosis. In contrast, our study employs EHR data to characterize clinical weight management at the time a child’s BMI first was classified as obese, and provides critical support for integrating recommendations from clinical practice guidelines regarding childhood obesity directly into EHR systems (such as BMI alerts) and scaling up weight management and education in pediatric clinical care settings.
Limitations of the study
Our findings are descriptive and the discovered temporal patterns and comorbidities should be viewed in this light. No causality can be attributed to the associations uncovered in this study. In addition, a greater proportion of our controls had a post-index visit than the cases which may have affected the associations in sequential patterns with conditions recorded during the post-index visit. Potential explanations include pure chance in the matching process or that vulnerable children are more likely to become obese and may face greater barriers to obtaining healthcare. These disparities may manifest in fewer primary care visits, which may explain the lower proportion of children with obesity who had a post-index visit. Another limitation is that approximately one-third of study cases had no BMI measurements in the EHR prior to the index visit. Assuming some of these individuals were obese prior to the index visit could imply a reduction in support for sequences with conditions in pre-index and index visit and shift to sequences with those conditions in the index and post-index visit. A final limitation to the study lies in the data itself. Relying on diagnostic codes within EHRs may lead to an underdiagnosis of certain conditions (which contributed to the use of BMI z-score measurements instead of a formal medical diagnosis of “obesity” in this study) [46, 47]. However, resolution of this concern is likely condition dependent and could involve complex methodology that was outside the scope of this study. However, assuming underdiagnosis rates are similar between cases and controls, which we expect given that healthcare utilization was a criterion for matching, we anticipate that the effect of underdiagnosis would be a decrease in sensitivity. That is, our methodology may fail to discover some significant patterns in the presence of underdiagnosis, but the patterns that are discovered will retain the specified support level and should retain the same level differences between the cases and controls.
Future work
This study revealed key areas of future investigation. Associations between pediatric obesity incidence and comorbidities including asthma and allergic rhinitis should be further investigated to uncover potential causal relationships, as should unique and differential causal risk factors for obesity among children with ASD and other DD. In addition, unique causal risk factors for obesity among patients with conditions only associated with obesity in the pre-index visit should be investigated. Future work can also examine the effect of mediating factors such as demographic and socioeconomic indicators on the uncovered associations. Finally, the low rates of formal documentation of obesity in patients’ EHRs identified in this study suggest the need for improved clinician education on the importance of obesity diagnosis and implementation of pediatric weight management guidelines. Future research should focus on understanding optimal methods for integrating pediatric weight management clinical decision support tools into EHR systems and promoting clinical adherence to pediatric weight management guidelines.
Code availability
The code used for data acquisition, processing, and analysis in this study may be found at: https://github.com/chop-dbhi/masino-lab-obesity-incidence.
References
Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. CDC growth charts for the United States: methods and development. Vital Health Stat. 2002;11:1–190.
Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC. Prevalence of obesity and severe obesity in US children, 1999–2016. Pediatrics. 2018;141:e20173459.
Yanovski JA. Pediatric obesity. An introduction. Appetite. 2015;93:3–12.
Condition Domain: Observational Health Data Sciences and Informatics. 2016. http://www.ohdsi.org/web/wiki/doku.php?10.1038/s41366-020-0614-7id=documentation:vocabulary:condition.
Freedman DS, Mei Z, Srinivasan SR, Berenson GS, Dietz WH. Cardiovascular risk factors and excess adiposity among overweight children and adolescents: the Bogalusa Heart Study. J Pediatr. 2007;150:12–7 e2.
Gance-Cleveland B, Gilbert K, Gilbert L, Dandreaux D, Russell N. Decision support to promote healthy weights in children. J Nurse Pract. 2014;10:803–12.
Richardson M, Paulukonis S, Roberts E, English P. Electronic health records as a resource for public health surveillance. California Environmental Health Tracking Program (CEHTP); 2016. http://www.phi.org/resources/?resource=electronic-health-records-as-a-resource-for-public-health-surveillance.
Bailey LC, Milov DE, Kelleher K, Kahn MG, Del Beccaro M, Yu F, et al. Multi-institutional sharing of electronic health record data to assess childhood obesity. PLoS ONE. 2013;8:e66192.
Hawkins SS, Gillman MW, Rifas-Shiman SL, Kleinman KP, Mariotti M, Taveras EM. The linked century study: linking three decades of clinical and public health data to examine disparities in childhood obesity. BMC Pediatr. 2016;16:32.
Roth C, Foraker RE, Payne PRO, Embi PJ. Community-level determinants of obesity: harnessing the power of electronic health records for retrospective data analysis. BMC Med Inform Decis Mak. 2014;14:36.
Yabut L, Rosenblum R. An integrative review of the use of EHR in childhood obesity identification and management. Online J Nurs Inform. 2017;21.
Flood TL, Zhao YQ, Tomayko EJ, Tandias A, Carrel AL, Hanrahan LP. Electronic health records and community health surveillance of childhood obesity. Am J Prev Med. 2015;48:234–40.
Cochran J, Baus A. Developing interventions for overweight and obese children using electronic health records data. Online J Nurs Inform. 2015;19. http://www.himss.org/ResourceLibrary/GenResourceDetail.aspx?ItemNumber=39758.
Condition domain: observational health data sciences and informatics. 2016. http://www.ohdsi.org/web/wiki/doku.php?id=documentation:vocabulary:condition.
Defining childhood obesity. Atlanta, GA: Centers for Disease Control and Prevention; 2016. https://www.cdc.gov/obesity/childhood/defining.html.
National health and nutrition examination survey. Atlanta, GA: Centers for Disease Control and Prevention; 2018. https://www.cdc.gov/nchs/nhanes/index.htm.
A SAS Program for the 2000 CDC Growth Charts (ages 0 to <20 years). Atlanta, GA: Centers for Disease Control and Prevention; 2016. https://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm.
International Classification of Diseases, Ninth revision, Clinical Modification (ICD-9-CM). Atlanta, GA: Centers for Disease Control and Prevention; 2013. https://www.cdc.gov/nchs/icd/icd9cm.htm.
International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM). Atlanta, GA: Centers for Disease Control and Prevention; 2018. https://www.cdc.gov/nchs/icd/icd10cm.htm.
About Child & Teen BMI. Atlanta, GA: Centers for Disease Control and Prevention; 2018. https://www.cdc.gov/healthyweight/assessing/bmi/childrens_bmi/about_childrens_bmi.html.
Meyer DE, Hornik K, Weingessel A, Leisch F, Chang C, et al. e1071: Misc Functions of the Department of Statistics, Probability Theory Group (Formerly: E1071),TU Wien; 2018. https://CRAN.R-project.org/package=e1071.
Zaki MJ. SPADE: an efficient algorithm for mining frequent sequences. Mach Learn. 2001;42:31–60.
Reshamwala A, Mishra N. Analysis of sequential pattern mining algorithms. Int J Sci Eng Res. 2014;5:1034–8.
Hahsler MBC, Gruen B, Hornik K. arules: Mining Association Rules and Frequent Itemsets; 2018. https://CRAN.R-project.org/package=arules.
Weiner JPAC. The Johns Hopkins ACG System Technical Reference Guide Version 9.0: Johns Hopkins Bloomberg School of Public Health; 2009. https://www.healthpartners.com/ucm/groups/public/@hp/@public/documents/documents/dev_057914.pdf.
Python 3.6.0. Wilmington, DE: Python Software Foundation; 2016. https://www.python.org/downloads/release/python-360/.
Morris MA, Wilkins E, Timmins KA, Bryant M, Birkin M, Griffiths C. Can big data solve a big problem? Reporting the obesity data landscape in line with the foresight obesity system map. Int J Obes. 2018;42:1963–76.
Timmins KA, Green MA, Radley D, Morris MA, Pearce J. How has big data contributed to obesity research? A review of the literature. Int J Obes. 2018;42:1951–62.
Azizpour Y, Delpisheh A, Montazeri Z, Sayehmiri K, Darabi B. Effect of childhood BMI on asthma: a systematic review and meta-analysis of case-control studies. BMC Pediatr. 2018;18:143.
Papoutsakis C, Priftis KN, Drakouli M, Prifti S, Konstantaki E, Chondronikola M, et al. Childhood overweight/obesity and asthma: is there a link? A systematic review of recent epidemiologic evidence. J Acad Nutr Diet. 2013;113:77–105.
Contreras ZA, Chen Z, Roumeliotaki T, Annesi-Maesano I, Baiz N, von Berg A, et al. Does early onset asthma increase childhood obesity risk? A pooled analysis of 16 European cohorts. Eur Respir J. 2018;52:1800504.
Sidell D, Shapiro NL, Bhattacharyya N. Obesity and the risk of chronic rhinosinusitis, allergic rhinitis, and acute otitis media in school-age children. Laryngoscope. 2013;123:2360–3.
Weinmayr G, Forastiere F, Buchele G, Jaensch A, Strachan DP, Nagel G. Overweight/obesity and respiratory and allergic disease in children: International Study of Asthma and Allergies in Childhood (ISAAC) phase two. PLoS ONE. 2014;9:e113996.
Han YY, Forno E, Gogna M, Celedon JC. Obesity and rhinitis in a nationwide study of children and adults in the united states. J Allergy Clin Immunol. 2016;137:1460–5.
Lei Y, Yang H, Zhen L. Obesity is a risk factor for allergic rhinitis in children of Wuhan (China). Asia Pac Allergy. 2016;6:101–4.
Maiano C, Hue O, Morin AJ, Moullec G. Prevalence of overweight and obesity among children and adolescents with intellectual disabilities: a systematic review and meta-analysis. Obes Rev. 2016;17:599–611.
Hill AP, Zuckerman KE, Fombonne E. Obesity and autism. Pediatrics. 2015;136:1051–61.
Shaikh U, Berrong J, Nettiksimmons J, Byrd RS. Impact of electronic health record clinical decision support on the management of pediatric obesity. Am J Med Qual. 2015;30:72–80.
Baer HJ, Cho I, Walmer RA, Bain PA, Bates DW. Using electronic health records to address overweight and obesity: a systematic review. Am J Prev Med. 2013;45:494–500.
Adhikari PD, Parker LA, Binns HJ, Ariza AJ. Influence of electronic health records and in-office weight management support resources on childhood obesity care. Clin Pediatr. 2012;51:788–92.
Young EL. Increasing diagnosis and treatment of overweight and obese pediatric patients. Clin Pediatr. 2015;54:1359–65.
Savinon C, Taylor JS, Canty-Mitchell J, Blood-Siegfried J. Childhood obesity: can electronic medical records customized with clinical practice guidelines improve screening and diagnosis? J Am Acad Nurse Pract. 2012;24:463–71.
Bode DV, Roberts TA, Johnson C. Increased adolescent overweight and obesity documentation through a simple electronic medical record intervention. Mil Med. 2016;181:1391.
Bronder KL, Dooyema CA, Onufrak SJ, Foltz JL. Electronic health records to support obesity-related patient care: results from a survey of United States physicians. Prev Med. 2015;77:41–7.
Naureckas SM, Zweigoron R, Haverkamp KS, Kaleba EO, Pohl SJ, Ariza AJ. Developing an electronic clinical decision support system to promote guideline adherence for healthy weight management and cardiovascular risk reduction in children: a progress update. Transl Behav Med. 2011;1:103–7.
Patel AI, Madsen KA, Maselli JH, Cabana MD, Stafford RS, Hersh AL. Underdiagnosis of pediatric obesity during outpatient preventive care visits. Acad Pediatr. 2010;10:405–9.
Banerjee D, Chung S, Wong EC, Wang EJ, Stafford RS, Palaniappan LP. Underdiagnosis of hypertension using electronic health records. Am J Hypertens. 2012;25:97–102.
Acknowledgements
This study is part of the Pediatric Big Health Data initiative funded by the State of Pennsylvania and led by the Children’s Hospital of Philadelphia, University of Pennsylvania, and Drexel University. We would like to thank the investigators of the Pediatric Big Health Data initiative for their contributions. These individuals include: Christopher B. Forrest, MD, PhD; L. Charles Bailey, MD, PhD; Shweta P. Chavan, MSEE; Rahul A. Darwar, MPH; Daniel Forsyth; Chén C. Kenyon, MD, MSHP; Ritu Khare, PhD; Mitchell G. Maltenfort, PhD; Xueqin Pang, PhD; Hanieh Razzaghi, MPH; Justine Shults, PhD; Levon H. Utidjian, MD, MBI from the Children’s Hospital of Philadelphia; Ana Diez Roux, MD, PhD, MPH; Amy H. Auchincloss, PhD, MPH; Kimberly Daniels, MS; Anneclaire J. De Roos, PhD, MPH; J. Felipe Garcia-Espana, MS, PhD; Irene Headen, PhD, MS; Félice Lê-Scherban, PhD, MPH; Steven Melly, MS, MA; Yvonne L. Michael, ScD, SM; Kari Moore, MS; Abigail E. Mudd, MPH; Leah Schinasi, PhD, MSPH from Drexel University and, Yong Chen, PhD; John H. Holmes, PhD; Rebecca A. Hubbard, PhD; A. Russell Localio, JD, MPH, PhD from the University of Pennsylvania. This work was also supported by funding from The Children’s Hospital of Philadelphia (CHOP)-Drexel Research Fellowship Program: Informatics and Analytics Collaborative Research.
Funding
This work was supported by a grant from the Commonwealth Universal Research Enhancement (C.U.R.E.) program funded by the Pennsylvania Department of Health—2015 Formula award—SAP #4100072543. This work was also supported by funding from The Children’s Hospital of Philadelphia (CHOP)-Drexel Research Fellowship Program: Informatics and Analytics Collaborative Research. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Campbell, E.A., Qian, T., Miller, J.M. et al. Identification of temporal condition patterns associated with pediatric obesity incidence using sequence mining and big data. Int J Obes 44, 1753–1765 (2020). https://doi.org/10.1038/s41366-020-0614-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-020-0614-7