Abstract
Background
There is an important interplay between epigenetic factors and body weight, and previous work has identified ten sites where DNA methylation is robustly associated with body mass index (BMI) cross-sectionally. However, interpretation of the associations is complicated by the substantial changes in BMI often occurring in late-life, and the fact that methylation is often driven by genetic variation. This study therefore investigated the longitudinal association between these ten sites and BMI from midlife to late-life, and whether associations persist after controlling for genetic factors.
Methods
We used data from 535 individuals (mean age 68) in the Swedish Adoption/Twin Study of Aging (SATSA) with longitudinal measures of both DNA methylation from blood samples and BMI, spanning up to 20 years. Methylation levels were measured with the Infinium Human Methylation 450K or Infinium MethylationEpic array, with seven of the ten sites passing quality control. Latent growth curve models were applied to investigate longitudinal associations between methylation and BMI, and between–within models to study associations within twin pairs, thus adjusting for genetic factors.
Results
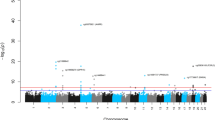
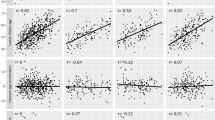
Baseline DNA methylation levels at five of the seven sites were associated with BMI level at age 65 (cg00574958 [CPT1A]; cg11024682 [SREBF1]), and/or change (cg06192883 [MYO5C]; cg06946797 [RMI2]; cg08857797 [VPS25]). For four of the five sites, the associations remained comparable within twin pairs. However, the effects of cg06192883 were substantially attenuated within pairs. No change in DNA methylation was detected for any of the seven evaluated sites.
Conclusion
Five of the seven sites investigated were associated with late-life level and/or change in BMI. The effects for four of the sites remained similar when examined within twin pairs, indicating that the associations are mainly environmentally driven. However, the substantial attenuation in the association between cg06192883 and late-life BMI within pairs points to the importance of genetic factors in this association.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Dahl AK, Reynolds CA, Fall T, Magnusson PK, Pedersen NL. Multifactorial analysis of changes in body mass index across the adult life course: a study with 65 years of follow-up. Int J Obes. 2014;38:1133–41.
Dahl AK, Fauth EB, Ernsth-Bravell M, Hassing LB, Ram N, Gerstof D. Body mass index, change in body mass index, and survival in old and very old persons. J Am Geriatr Soc. 2013;61:512–8.
Stokes A, Preston SH. Revealing the burden of obesity using weight histories. Proc Natl Acad Sci USA. 2016;113:572–7.
Min J, Chiu DT, Wang Y. Variation in the heritability of body mass index based on diverse twin studies: a systematic review. Obes Rev. 2013;14:871–82.
Winkler TW, Justice AE, Graff M, Barata L, Feitosa MF, Chu S, et al. The influence of age and sex on genetic associations with adult body size and shape: a large-scale genome-wide interaction study. PLoS Genet. 2015;11:e1005378.
Song M, Zheng Y, Qi L, Hu FB, Chan AT, Giovannucci EL. Longitudinal analysis of genetic susceptibility and BMI throughout adult life. Diabetes. 2018;67:248–55.
Benayoun BA, Pollina EA, Brunet A. Epigenetic regulation of ageing: linking environmental inputs to genomic stability. Nat Rev Mol Cell Biol. 2015;16:593–610.
Luo C, Hajkova P, Ecker JR. Dynamic DNA methylation: In the right place at the right time. Science. 2018;361:1336–40.
Demerath EW, Guan W, Grove ML, Aslibekyan S, Mendelson M, Zhou YH, et al. Epigenome-wide association study (EWAS) of BMI, BMI change and waist circumference in African American adults identifies multiple replicated loci. Hum Mol Genet. 2015;24:4464–79.
Mendelson MM, Marioni RE, Joehanes R, Liu C, Hedman AK, Aslibekyan S, et al. Association of body mass index with DNA methylation and gene expression in blood cells and relations to cardiometabolic disease: a Mendelian randomization approach. PLoS Med. 2017;14:e1002215.
Wahl S, Drong A, Lehne B, Loh M, Scott WR, Kunze S, et al. Epigenome-wide association study of body mass index, and the adverse outcomes of adiposity. Nature. 2017;541:81–6.
Sayols-Baixeras S, Subirana I, Fernandez-Sanles A, Senti M, Lluis-Ganella C, Marrugat J, et al. DNA methylation and obesity traits: an epigenome-wide association study. The REGICOR study. Epigenetics. 2017;12:909–16.
Campanella G, Gunter MJ, Polidoro S, Krogh V, Palli D, Panico S, et al. Epigenome-wide association study of adiposity and future risk of obesity-related diseases. Int J Obes. 2018;42:2022–35.
Dhana K, Braun KVE, Nano J, Voortman T, Demerath EW, Guan W, et al. An epigenome-wide association study of obesity-related traits. Am J Epidemiol. 2018;187:1662–9.
Bell CG. The epigenomic analysis of human obesity. Obesity. 2017;25:1471–81.
McRae AF, Marioni RE, Shah S, Yang J, Powell JE, Harris SE, et al. Identification of 55,000 replicated DNA methylation QTL. Sci. Rep. 2018;8:17605.
Finkel D, Pedersen N. Processing speed and longitudinal trajectories of change for cognitive abilities: the Swedish Adoption/Twin Study of Aging. Neuropsychol Dev Cognit. 2004;11:325–45.
Magnusson PK, Almqvist C, Rahman I, Ganna A, Viktorin A, Walum H, et al. The Swedish Twin Registry: establishment of a biobank and other recent developments. Twin Res Hum Genet. 2012;16:317–29.
Davis SDP, Bilke S, Triche, Jr. T and Bootwalla M. methylumi: Handle Illumina methylation data. R package version 2.18.2. 2015.
Pidsley R, CC YW, Volta M, et al. LC. A data-driven approach to preprocessing Illumina 450K methylation array data. BMC Genom. 2013;14:293.
Houseman EA, Accomando WP, Koestler DC, Christensen BC, Marsit CJ, Nelson HH, et al. DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinform. 2012;13:86.
Leek JT, Johnson WE, Parker HS, Fertig EJ, Jaffe AE and Storey JD. SVA: Surrogate Variable Analysis. R package version 3.20.0. 2016. https://bioconductor.org/packages/release/bioc/html/sva.html.
Du P, Zhang X, Huang CC, Jafari N, Kibbe WA, Hou L, et al. Comparison of beta-value and M-value methods for quantifying methylation levels by microarray analysis. BMC Bioinform. 2010;11:587.
Sjölander A, Frisell T, Öberg S. Causal interpretation of between-within models for twin research. In: Epidemiol Methods. 2012;1:216–33.
McArdle JJ. Latent variable modeling of differences and changes with longitudinal data. Annu Rev Psychol. 2009;60:577–605.
Falconer DS, Mackay TFC. Introduction to quantitative genetics, 4th ed. Longman: Harlow; 1996.
Wang Y, Karlsson R, Lampa E, Zhang Q, Hedman AK, Almgren M, et al. Epigenetic influences on aging: a longitudinal genome-wide methylation study in old Swedish twins. Epigenetics. 2018;13:975–87.
Gaunt TR, Shihab HA, Hemani G, Min JL, Woodward G, Lyttleton O, et al. Systematic identification of genetic influences on methylation across the human life course. Genome Biol. 2016;17:61.
Krementsova EB, Furuta K, Oiwa K, Trybus KM, Ali MY. Small teams of myosin Vc motors coordinate their stepping for efficient cargo transport on actin bundles. J Biol Chem. 2017;292:10998–1008.
La Rosa S, Lloyd RV, Capella C. Expression and role of myosins in pancreatic endocrine cells and related tumors. In: Loft MA editor. Trends in pancreatic cancer research. New York: Nova Publishers; 2005. pp. 23–39.
Willmer T, Johnson R, Louw J, Pheiffer C. Blood-based DNA methylation biomarkers for type 2 diabetes: potential for clinical applications. Front Endocrinol. 2018;9:744.
Braun KV, Voortman T, Dhana K, Troup J, Bramer WM, Chowdhury R, et al. The role of DNA methylation in dyslipidaemia: a systematic review. Prog Lipid Res. 2016;64:178–91.
Li S, Wong EM, Bui M, Nguyen TL, Joo JE, Stone J, et al. Inference about causation between body mass index and DNA methylation in blood from a twin family study. Int J Obes. 2019;43:243–52.
Sherling DH, Perumareddi P, Hennekens CH. Metabolic syndrome: clinical and policy implications of the new silent killer. J Cardiovasc Pharmacol Ther. 2017;22:365–7.
Kelfve S, Fors S, Lennartsson C. Getting better all the time? Selective attrition and compositional changes in longitudinal and life-course studies. Longitud Life Course Stud. 2017;8:104–20.
Li W, Christiansen L, Hjelmborg J, Baumbach J, Tan Q. On the power of epigenome-wide association studies using a disease-discordant twin design. Bioinformatics. 2018;34:4073–8.
Frisell T, Oberg S, Kuja-Halkola R, Sjolander A. Sibling comparison designs: bias from non-shared confounders and measurement error. Epidemiology. 2012;23:713–20.
Teschendorff AE, Zheng SC. Cell-type deconvolution in epigenome-wide association studies: a review and recommendations. Epigenomics. 2017;9:757–68.
Acknowledgements
SATSA was supported by National Institutes of Health (NIH; grants AG04563 and AG10175), the MacArthur Foundation Research Network on Successful Aging, the Swedish Research Council for Working Life and Social Research (FAS; Grants 97:0147:1B, 2009-0795), and the Swedish Research Council (825-2007-7460 and 825-2009-6141). This work was supported by the Swedish Research Council Grant (2016-03081) and the Swedish Research Council for Health, Working Life and Welfare (2018-01201). We acknowledge The Swedish Twin Registry for access to data. The Swedish Twin Registry is managed by Karolinska Institutet and receives funding through the Swedish Research Council under the grant no 2017-00641. Methylation profiling on the Infinium MethylationEPIC BeadChip was performed by the SNP&SEQ Technology Platform in Uppsala (www.genotyping.se). The facility is part of the National Genomics Infrastructure (NGI) Sweden and Science for Life Laboratory. The SNP&SEQ Platform is also supported by the Swedish Research Council and the Knut and Alice Wallenberg Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Karlsson, I.K., Ericsson, M., Wang, Y. et al. Replicating associations between DNA methylation and body mass index in a longitudinal sample of older twins. Int J Obes 44, 1397–1405 (2020). https://doi.org/10.1038/s41366-019-0498-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-019-0498-6
This article is cited by
-
The epigenetic etiology of cardiovascular disease in a longitudinal Swedish twin study
Clinical Epigenetics (2021)
-
Lipid Phenotypes and DNA Methylation: a Review of the Literature
Current Atherosclerosis Reports (2021)
-
DNA methylation and lipid metabolism: an EWAS of 226 metabolic measures
Clinical Epigenetics (2021)