Abstract
Background/Objectives
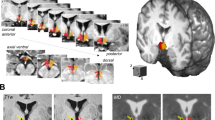
Hypothalamic neurons play a major role in the control of body mass. Obese subjects present radiologic signs of gliosis in the hypothalamus, which may reflect the damage or loss of neurons involved in whole-body energy homeostasis. It is currently unknown if hypothalamic gliosis (1) differs between obese nondiabetic (ND) and obese diabetic subjects (T2D) or (2) is modified by extensive body mass reduction via Roux-n-Y gastric bypass (RYGB).
Subjects/Methods
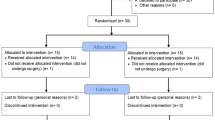
Fifty-five subjects (all female) including lean controls (CT; n = 13), ND (n = 28), and T2D (n = 14) completed at least one study visit. Subjects underwent anthropometrics and a multi-echo MRI sequence to measure mean bilateral T2 relaxation time in the mediobasal hypothalamus (MBH) and two reference regions (amygdala and putamen). The obese groups underwent RYGB and were re-evaluated 9 months later. Analyses were by linear mixed models.
Results
Analyses of T2 relaxation time at baseline showed a group by region interaction only in the MBH (P < 0.0001). T2D had longer T2 relaxation times compared to either CT or ND groups. To examine the effects of RYGB on hypothalamic gliosis a three-way (group by region by time) mixed effects model adjusted for age was executed. Group by region (P < 0.0001) and region by time (P = 0.0005) interactions were significant. There was a reduction in MBH relaxation time by RYGB, and, although the T2D group still had higher T2 relaxation time overall compared to the ND group, the T2D group had significantly lower T2 relaxation time after surgery and the ND group showed a trend. The degree of reduction in MBH T2 relaxation time by RYGB was unrelated to clinical outcomes.
Conclusion
T2 relaxation times, a marker of hypothalamic gliosis, are higher in obese women with T2D and are reduced by RYGB-induced weight loss.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Morton GJ, Meek TH, Schwartz MW. Neurobiology of food intake in health and disease. Nat Rev Neurosci. 2014;15:367–78.
Velloso LA, Schwartz MW. Altered hypothalamic function in diet-induced obesity. Int J Obes. 2011;35:1455–65.
van de Sande-Lee S, Velloso LA. Hypothalamic dysfunction in obesity. Arq Bras Endocrinol Metabol. 2012;56:341–50.
Moraes JC, Coope A, Morari J, Cintra DE, Roman EA, Pauli JR, et al. High-fat diet induces apoptosis of hypothalamic neurons. PLoS ONE. 2009;4:e5045.
Morari J, Anhe GF, Nascimento LF, de Moura RF, Razolli D, Solon C, et al. Fractalkine (CX3CL1) is involved in the early activation of hypothalamic inflammation in experimental obesity. Diabetes. 2014;63:3770–84.
Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest. 2012;122:153–62.
Valdearcos M, Robblee MM, Benjamin DI, Nomura DK, Xu AW, Koliwad SK. Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell Rep. 2014;9:2124–38.
Matsuda M, Liu Y, Mahankali S, Pu Y, Mahankali A, Wang J, et al. Altered hypothalamic function in response to glucose ingestion in obese humans. Diabetes. 1999;48:1801–6.
Vidarsdottir S, Smeets PA, Eichelsheim DL, van Osch MJ, Viergever MA, Romijn JA, et al. Glucose ingestion fails to inhibit hypothalamic neuronal activity in patients with type 2 diabetes. Diabetes. 2007;56:2547–50.
van de Sande-Lee S, Pereira FR, Cintra DE, Fernandes PT, Cardoso AR, Garlipp CR, et al. Partial reversibility of hypothalamic dysfunction and changes in brain activity after body mass reduction in obese subjects. Diabetes. 2011;60:1699–704.
Rachid B, van de Sande-Lee S, Rodovalho S, Folli F, Beltramini GC, Morari J, et al. Distinct regulation of hypothalamic and brown/beige adipose tissue activities in human obesity. Int J Obes. 2015;39:1515–22.
Kreutzer C, Peters S, Schulte DM, Fangmann D, Turk K, Wolff S, et al. Hypothalamic inflammation in human obesity is mediated by environmental and genetic factors. Diabetes. 2017;66:2407–15.
Berkseth KE, Guyenet SJ, Melhorn SJ, Lee D, Thaler JP, Schur EA, et al. Hypothalamic gliosis associated with high-fat diet feeding is reversible in mice: a combined immunohistochemical and magnetic resonance imaging study. Endocrinology. 2014;155:2858–67.
Samocha-Bonet D, Dixit VD, Kahn CR, Leibel RL, Lin X, Nieuwdorp M, et al. Metabolically healthy and unhealthy obese—the 2013 Stock Conference report. Obes Rev. 2014;15:697–708.
Souza GF, Solon C, Nascimento LF, De-Lima-Junior JC, Nogueira G, Moura R, et al. Defective regulation of POMC precedes hypothalamic inflammation in diet-induced obesity. Sci Rep. 2016;6:29290.
Schur EA, Melhorn SJ, Oh SK, Lacy JM, Berkseth KE, Guyenet SJ, et al. Radiologic evidence that hypothalamic gliosis is associated with obesity and insulin resistance in humans. Obesity. 2015;23:2142–8.
Lee D, Thaler JP, Berkseth KE, Melhorn SJ, Schwartz MW, Schur EA. Longer T(2) relaxation time is a marker of hypothalamic gliosis in mice with diet-induced obesity. Am J Physiol Endocrinol Metab. 2013;304:E1245–50.
Hasan KM, Walimuni IS, Abid H, Frye RE, Ewing-Cobbs L, Wolinsky JS, et al. Multimodal quantitative magnetic resonance imaging of thalamic development and aging across the human lifespan: implications to neurodegeneration in multiple sclerosis. J Neurosci. 2011;31:16826–32.
Tuulari JJ, Karlsson HK, Antikainen O, Hirvonen J, Pham T, Salminen P, et al. Bariatric surgery induces white and grey matter density recovery in the morbidly obese: a voxel-based morphometric study. Hum Brain Mapp. 2016;37:3745–56.
Polimeni JR, Wald LL. Magnetic resonance Imaging technology-bridging the gap between noninvasive human imaging and optical microscopy. Curr Opin Neurobiol. 2018;50:250–60.
Akassoglou K, Merlini M, Rafalski VA, Real R, Liang L, Jin Y, et al. In vivo imaging of CNS injury and disease. J Neurosci. 2017;37:10808–16.
Patriarca L, Magerowski G, Alonso-Alonso M. Functional neuroimaging in obesity. Curr Opin Endocrinol Diabetes Obes. 2017;24:260–5.
Ogawa S, Lee TM, Nayak AS, Glynn P. Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn Reson Med. 1990;14:68–78.
Cornier MA, Salzberg AK, Endly DC, Bessesen DH, Rojas DC, Tregellas JR. The effects of overfeeding on the neuronal response to visual food cues in thin and reduced-obese individuals. PLoS ONE. 2009;4:e6310.
Fletcher PC, Napolitano A, Skeggs A, Miller SR, Delafont B, Cambridge VC, et al. Distinct modulatory effects of satiety and sibutramine on brain responses to food images in humans: a double dissociation across hypothalamus, amygdala, and ventral striatum. J Neurosci. 2010;30:14346–55.
HFA Zoon, de Bruijn SEM, Jager G, PAM Smeets, de Graaf C, Janssen IMC, et al. Altered neural inhibition responses to food cues after Roux-en-Y Gastric bypass. Biol Psychol. 2018;137:34–41.
Dallaire-Theroux C, Callahan BL, Potvin O, Saikali S, Duchesne S. Radiological-pathological correlation in Alzheimer's disease: systematic review of antemortem magnetic resonance imaging findings. J Alzheimers Dis. 2017;57:575–601.
Berkseth KE, Rubinow KB, Melhorn SJ, Webb MF, BDLM Rosalynn, Marck BT, et al. Hypothalamic gliosis by MRI and visceral fat mass negatively correlate with plasma testosterone concentrations in healthy Men. Obesity. 2018;26:1898–904.
Puig J, Blasco G, Daunis IEJ, Molina X, Xifra G, Ricart W, et al. Hypothalamic damage is associated with inflammatory markers and worse cognitive performance in obese subjects. J Clin Endocrinol Metab. 2015;100:E276–81.
Lips MA, Wijngaarden MA, van der Grond J, van Buchem MA, de Groot GH, Rombouts SA, et al. Resting-state functional connectivity of brain regions involved in cognitive control, motivation, and reward is enhanced in obese females. Am J Clin Nutr. 2014;100:524–31.
Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58:773–95.
Gastrointestinal surgery for severe obesity: National Institutes of Health Consensus Development Conference Statement. Am J Clin Nutr. 1992;55(Suppl 2):615S–9S. https://doi.org/10.1093/ajcn/55.2.615s. (Abstract).
de Carvalho CP, Marin DM, de Souza AL, Pareja JC, Chaim EA, de Barros Mazon S, et al. GLP-1 and adiponectin: effect of weight loss after dietary restriction and gastric bypass in morbidly obese patients with normal and abnormal glucose metabolism. Obes Surg. 2009;19:313–20.
Diabetes A. 2. Classification and diagnosis of diabetes. Diabetes Care. 2017;40(Suppl 1):S11–24. American
Abel TW, Rance NE. Stereologic study of the hypothalamic infundibular nucleus in young and older women. J Comp Neurol. 2000;424:679–88.
Acknowledgements
We thank ER, GF, and LS for technical assistance. This work was supported by funding provided by the Fundação de Amparo a Pesquisa do Estado de São Paulo, Conselho Nacional de Desenvolvimento Cientifico e Tecnologico, grants from the Trust in Science Initiative from Glaxo-Smithkline, UK, and the National Institutes of Health (DK089036, DK098466; EAS). The Laboratories of Cell Signaling and Experimental Endocrinology belong to the Obesity and Comorbidities Research Center and the National Institute of Science and Technology—Diabetes and Obesity.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
van de Sande-Lee, S., Melhorn, S.J., Rachid, B. et al. Radiologic evidence that hypothalamic gliosis is improved after bariatric surgery in obese women with type 2 diabetes. Int J Obes 44, 178–185 (2020). https://doi.org/10.1038/s41366-019-0399-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-019-0399-8
This article is cited by
-
Metabolic factors in the regulation of hypothalamic innate immune responses in obesity
Experimental & Molecular Medicine (2022)
-
Hypothalamic inflammation in metabolic disorders and aging
Cellular and Molecular Life Sciences (2022)
-
Effect of pioglitazone treatment on brown adipose tissue volume and activity and hypothalamic gliosis in patients with type 2 diabetes mellitus: a proof-of-concept study
Acta Diabetologica (2019)