Abstract
Background/Objectives
We previously observed that selective agonists of the sympatho-inhibitory I1 imidazoline receptors (LNP ligands) have favorable effects on several cardiovascular and metabolic disorders defining the metabolic syndrome, including body weight. The objectives of this study were to explore the effects of LNPs on adiposity and the mechanisms involved, and to evaluate their impact on metabolic homeostasis.
Methods
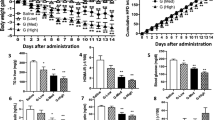
Young Zucker fa/fa rats were treated with LNP599 (10 mg/kg/day) for 12 weeks. Effects on body weight, adiposity (regional re-distribution, morphology, and function of adipose tissues), cardiovascular and metabolic homeostasis, and liver function were evaluated. Direct effects on insulin and AMP-activated protein kinase (AMPK) signaling were studied in human hepatoma HepG2 cells.
Results
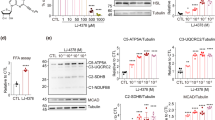
LNP599 treatment limited the age-dependent remodeling and inflammation of subcutaneous, epididymal, and visceral adipose tissues, and prevented total fat deposits and the development of obesity. Body-weight stabilization was not related to reduced food intake but rather to enhanced energy expenditure and thermogenesis. Cardiovascular and metabolic parameters were also improved and were significantly correlated with body weight but not with plasma norepinephrine. Insulin and AMPK signaling were enhanced in hepatic tissues of treated animals, whereas blood markers of hepatic disease and pro-inflammatory cytokine levels were reduced. In cultured HepG2 cells, LNP ligands phosphorylated AMPK and the downstream acetyl-CoA carboxylase and prevented oleic acid-induced intracellular lipid accumulation. They also significantly potentiated insulin-mediated AKT activation and this was independent from AMPK.
Conclusions
Selective I1 imidazoline receptor agonists protect against the development of adiposity and obesity, and the associated cardio-metabolic disorders. Activation of I1 receptors in the liver, leading to stimulation of the cellular energy sensor AMPK and insulin sensitization, and in adipose tissues, leading to improvement of morphology and function, are identified as peripheral mechanisms involved in the beneficial actions of these ligands.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Alberti KG, Zimmet P, Shaw J. Metabolic syndrome–a new world-wide definition. A consensus statement from the International Diabetes Federation. Diabet Med. 2006;23:469–80.
Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52.
Harris MF. The metabolic syndrome. Aust Fam Physician. 2013;42:524–7.
Grassi G, Dell’Oro R, Quarti-Trevano F, Scopelliti F, Seravalle G, Paleari F, et al. Neuroadrenergic and reflex abnormalities in patients with metabolic syndrome. Diabetologia. 2005;48:1359–65.
Mancia G, Bousquet P, Elghozi JL, Esler M, Grassi G, Julius S, et al. The sympathetic nervous system and the metabolic syndrome. J Hypertens. 2007;25:909–20.
Feldstein C, Julius S. The complex interaction between overweight, hypertension, and sympathetic overactivity. J Am Soc Hypertens. 2009;3:353–65.
Mahfoud F, Schlaich M, Kindermann I, Ukena C, Cremers B, Brandt MC, et al. Effect of renal sympathetic denervation on glucose metabolism in patients with resistant hypertension: a pilot study. Circulation. 2011;123:1940–6.
Schlaich MP, Hering D, Sobotka P, Krum H, Lambert GW, Lambert E, et al. Effects of renal denervation on sympathetic activation, blood pressure, and glucose metabolism in patients with resistant hypertension. Front Physiol. 2012;3:10.
Witkowski A, Prejbisz A, Florczak E, Kądziela J, Śliwiński P, Bieleń P, et al. Effects of renal sympathetic denervation on blood pressure, sleep apnea course, and glycemic control in patients with resistant hypertension and sleep apnea. Hypertension. 2011;58:559–65.
Bousquet P, Feldman J, Schwartz J. Central cardiovascular effects of alpha adrenergic drugs: differences between catecholamines and imidazolines. J Pharmacol Exp Ther. 1984;230:232–6.
Schann S, Bruban V, Pompermayer K, Feldman J, Pfeiffer B, Renard P, et al. Synthesis and biological evaluation of pyrrolinic isosteres of rilmenidine. Discovery of cis-/trans-dicyclopropylmethyl-(4,5-dimethyl-4,5-dihydro-3H-pyrrol-2-yl)-amine (LNP 509), an I1 imidazoline receptor selective ligand with hypotensive activity. J Med Chem. 2001;44:1588–93.
Bousquet P, Ehrhardt JD, Fellmann L, Gasparik V, Greney H, Hadjeri M, et al. Novel amino-pyrroline derivatives, and use thereof in the prevention and/or treatment of metabolic syndrome. WO 2012/143660 A1, 2011 [patent].
Gasparik V, Greney H, Schann S, Feldman J, Fellmann L, Ehrhardt JD, et al. Synthesis and biological evaluation of 2-aryliminopyrrolidines as selective ligands for I1 imidazoline receptors: discovery of new sympatho-inhibitory hypotensive agents with potential beneficial effects in metabolic syndrome. J Med Chem. 2015;58:878–87.
Fellmann L, Regnault V, Greney H, Gasparik V, Muscat A, Max JP, et al. A new pyrroline compound selective for i1-imidazoline receptors improves metabolic syndrome in rats. J Pharmacol Exp Ther. 2013;346:370–80.
Weiss M, Bouchoucha S, Aiad F, Ayme-Dietrich E, Dali-Youcef N, Bousquet P, et al. Imidazoline-like drugs improve insulin sensitivity through peripheral stimulation of adiponectin and AMPK pathways in a rat model of glucose intolerance. Am J Physiol Endocrinol Metab. 2015;309:E95–104.
Shehzad A, Iqbal W, Shehzad O, Lee YS. Adiponectin: regulation of its production and its role in human diseases. Hormones (Athens). 2012;11:8–20.
Whitehead JP, Richards AA, Hickman IJ, Macdonald GA, Prins JB. Adiponectin–a key adipokine in the metabolic syndrome. Diabetes Obes Metab. 2006;8:264–80.
Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439–51.
Ruderman NB, Carling D, Prentki M, Cacicedo JM. AMPK, insulin resistance, and the metabolic syndrome. J Clin Invest. 2013;123:2764–72.
Hasenour CM, Berglund ED, Wasserman DH. Emerging role of AMP-activated protein kinase in endocrine control of metabolism in the liver. Mol Cell Endocrinol. 2013;366:152–62.
Luo L, Liu M. Adipose tissue in control of metabolism. J Endocrinol. 2016;231:R77–R99.
Gómez-Hernández A, Beneit N, Díaz-Castroverde S, Escribano Ó. Differential role of adipose tissues in obesity and related metabolic and vascular complications. Int J Endocrinol. 2016;2016:1216783.
Bae YJ, Kim SH, Chung JH, Song SW, Kim KS, Kim MK, et al. Evaluation of adiposity-related biomarkers as metabolic syndrome indicators. Clin Nutr Res. 2013;2:91–9.
Aubertin G, Sayeh A, Dillenseger JP, Ayme-Dietrich E, Choquet P, Niederhoffer N. Comparison of bioimpedance spectroscopy and X-Ray micro-computed tomography for total fat volume measurement in mice. PLoS ONE. 2017;12:e0183523.
Erdös B, Snipes JA, Miller AW, Busija DW. Cerebrovascular dysfunction in Zucker obese rats is mediated by oxidative stress and protein kinase C. Diabetes. 2004;53:1352–9.
Oltman CL, Richou LL, Davidson EP, Coppey LJ, Lund DD, Yorek MA. Progression of coronary and mesenteric vascular dysfunction in Zucker obese and Zucker diabetic fatty rats. Am J Physiol Heart Circ Physiol. 2006;291:H1780–7.
Després JP, Lemieux I, Bergeron J, Pibarot P, Mathieu P, Larose E, et al. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol. 2008;28:1039–49.
Lambert E, Straznicky NE, Dawood T, Ika-Sari C, Grima M, Esler MD, et al. Change in sympathetic nerve firing pattern associated with dietary weight loss in the metabolic syndrome. Front Physiol. 2011;2:52.
Straznicky NE, Lambert GW, McGrane MT, Masuo K, Dawood T, Nestel PJ, et al. Weight loss may reverse blunted sympathetic neural responsiveness to glucose ingestion in obese subjects with metabolic syndrome. Diabetes. 2009;58:1126–32.
Skrapari I, Tentolouris N, Katsilambros N. Baroreflex function: determinants in healthy subjects and disturbances in diabetes, obesity and metabolic syndrome. Curr Diabetes Rev. 2006;2:329–38.
Huber DA, Schreihofer AM. Attenuated baroreflex control of sympathetic nerve activity in obese Zucker rats by central mechanisms. J Physiol. 2010;588(Pt 9):1515–25.
Straznicky NE, Lambert GW, Masuo K, Dawood T, Eikelis N, Nestel PJ, et al. Blunted sympathetic neural response to oral glucose in obese subjects with the insulin-resistant metabolic syndrome. Am J Clin Nutr. 2009;89:27–36.
Lidell ME, Betz MJ, Enerbäck S. Brown adipose tissue and its therapeutic potential. J Intern Med. 2014;276:364–77.
Thoonen R, Hindle AG, Scherrer-Crosbie M. Brown adipose tissue: the heat is on the heart. Am J Physiol Heart Circ Physiol. 2016;310:H1592–605.
Peterson CM, Orooji M, Johnson DN, Naraghi-Pour M, Ravussin E. Brown adipose tissue does not seem to mediate metabolic adaptation to overfeeding in men. Obesity (Silver Spring). 2017;25:502–5.
Pita J, Panadero A, Soriano-Guillén L, Rodríguez E, Rovira A. The insulin sensitizing effects of PPAR-γ agonist are associated to changes in adiponectin index and adiponectin receptors in Zucker fatty rats. Regul Pept. 2012;174:18–25.
Masaki T, Chiba S, Yasuda T, Tsubone T, Kakuma T, Shimomura I, et al. Peripheral, but not central, administration of adiponectin reduces visceral adiposity and upregulates the expression of uncoupling protein in agouti yellow (Ay/a) obese mice. Diabetes. 2003;52:2266–73.
Ohno H, Shinoda K, Spiegelman BM, Kajimura S. PPARγ agonists induce a white-to-brown fat conversion through stabilization of PRDM16 protein. Cell Metab. 2012;15:395–404.
Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–7.
Edwards L, Fishman D, Horowitz P, Bourbon N, Kester M, Ernsberger P. The I1-imidazoline receptor in PC12 pheochromocytoma cells activates protein kinases C, extracellular signal-regulated kinase (ERK) and c-jun N-terminal kinase (JNK). J Neurochem. 2001;79:931–40.
Greney H, Ronde P, Magnier C, Maranca F, Rascente C, Quaglia W, et al. Coupling of I(1) imidazoline receptors to the cAMP pathway: studies with a highly selective ligand, benazoline. Mol Pharmacol. 2000;57:1142–51.
Regunathan S, Evinger MJ, Meeley MP, Reis DJ. Effects of clonidine and other imidazole-receptor binding agents on second messenger systems and calcium influx in bovine adrenal chromaffin cells. Biochem Pharmacol. 1991;42:2011–8.
Ernsberger P, Ishizuka T, Liu S, Farrell CJ, Bedol D, Koletsky RJ, et al. Mechanisms of antihyperglycemic effects of moxonidine in the obese spontaneously hypertensive Koletsky rat (SHROB). J Pharmacol Exp Ther. 1999;288:139–47.
O’Neill HM, Holloway GP, Steinberg GR. AMPK regulation of fatty acid metabolism and mitochondrial biogenesis: implications for obesity. Mol Cell Endocrinol. 2013;366:135–51.
Acknowledgements
This work was financially supported by the SATT Conectus Alsace, the University of Strasbourg, and the Région Alsace (fellowship to MW). We acknowledge Stéphanie Dal and Elodie Seyfritz from the Centre Européen d’Etude du Diabète for their advice and help at imunofluorescence analysis, and Lucie Tischmacher for her technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Aubertin, G., Weiss, M., Traversi, F. et al. Effects of imidazoline-like drugs on liver and adipose tissues, and their role in preventing obesity and associated cardio-metabolic disorders. Int J Obes 43, 2163–2175 (2019). https://doi.org/10.1038/s41366-019-0342-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-019-0342-z
This article is cited by
-
Protective effects of the imidazoline-like drug lnp599 in a marmoset model of obesity-induced metabolic disorders
International Journal of Obesity (2021)