Abstract
Background/objectives
Recent studies indicate a possible role of TSH/TSHR signalling axis on adipogenesis and adipose tissue physiology. Here, we aimed to investigate the relationship between adipose tissue TSHB and adipose tissue physiology-related gene expression.
Subjects/methods
Subcutaneous and visceral adipose tissue TSHB gene expression was analysed in two independent cohorts [Cohort1 (N = 96) and Cohort2 (N = 45)] and after bariatric surgery-induced weight loss [Cohort3 (N = 22)]. Adipose tissue TSH protein expression was also analysed in a subgroup of participants from Cohort 1 (N = 16). The effects of recombinant TSH on human subcutaneous preadipocytes and adipocytes were investigated.
Results
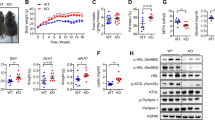
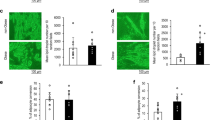
In cohort 1, both visceral and subcutaneous adipose tissue TSHB gene expression was positively correlated with the expression of mitochondrial function (PPARGC1A, ISCA2, CISD1, SIRT1, NFE2L2, NRF1) and fatty acid mobilization (CAV1, ENGL1), but not with adipogenic-related genes. Of note, adipose tissue TSH protein levels were also associated with some of these markers of mitochondrial function and fatty acid mobilization. These associations were replicated in cohort 2. Bariatric surgery-induced weight loss resulted in increased subcutaneous adipose tissue TSHB in parallel to increased PPARGC1A. In human subcutaneous adipocytes, rh-TSH administration led to increased mitochondrial respiratory capacity in parallel to increased mitochondrial function- and adipogenic-related gene expression, but no significant effects were observed during differentiation of human preadipocytes.
Conclusion
These data point to a possible role of adipose tissue TSH in the maintenance of adipocyte mitochondrial function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Pellegrinelli V, Carobbio S, Vidal-Puig A. Adipose tissue plasticity: how fat depots respond differently to pathophysiological cues. Diabetologia. 2016;59:1075–88.
Klöting N, Blüher M. Adipocyte dysfunction, inflammation and metabolic syndrome. Rev Endocr Metab Disord. 2014;15:277–87.
Heinonen S, Buzkova J, Muniandy M, Kaksonen R, Ollikainen M, Ismail K, et al. Impaired mitochondrial biogenesis in adipose tissue in acquired obesity. Diabetes. 2015;64:3135–45.
Haraguchi K, Shimura H, Lin L, Endo T, Onaya T. Differentiation of rat preadipocytes is accompanied by expression of thyrotropin receptors. Endocrinology. 1996;137:3200–5.
Bell A, Gagnon A, Grunder L, Parikh SJ, Smith TJ, Sorisky A. Functional TSH receptor in human abdominal preadipocytes and orbital fibroblasts. Am J Physiol Cell Physiol. 2000;279:C335–40.
Lu M, Lin RY. TSH stimulates adipogenesis in mouse embryonic stem cells. J Endocrinol. 2008;196:159–69.
Bell A, Gagnon A, Dods P, Papineau D, Tiberi M, Sorisky A. TSH signaling and cell survival in 3T3-L1 preadipocytes. Am J Physiol Cell Physiol. 2002;283:C1056–64.
Ma S, Jing F, Xu C, Zhou L, Song Y, Yu C, et al. Thyrotropin and obesity: increased adipose triglyceride content through glycerol-3-phosphate acyltransferase 3. Sci Rep. 2015;5:7633.
Lu S, Guan Q, Liu Y, Wang H, Xu W, Li X, et al. Role of extrathyroidal TSHR expression in adipocyte differentiation and its association with obesity. Lipids Health Dis. 2012;11:17.
Nannipieri M, Cecchetti F, Anselmino M, Camastra S, Niccolini P, Lamacchia M, et al. Expression of thyrotropin and thyroid hormone receptors in adipose tissue of patients with morbid obesity and/or type 2 diabetes: effects of weight loss. Int J Obes. 2009;33:1001–6.
Thrush AB, Gagnon A, Sorisky A. PKC activation is required for TSH-mediated lipolysis via perilipin activation. Horm Metab Res. 2012;44:825–31.
Gagnon A, Antunes TT, Ly T, Pongsuwan P, Gavin C, Lochnan HA, et al. Thyroid-stimulating hormone stimulates lipolysis in adipocytes in culture and raises serum free fatty acid levels in vivo. Metabolism. 2010;59:547–53.
Moreno-Navarrete JM, Moreno M, Ortega F, Xifra G, Hong S, Asara JM, et al. TSHB mRNA is linked to cholesterol metabolism in adipose tissue. FASEB J. 2017;31:4482–91.
American Diabetes Association 2 Classification and diagnosis of diabetes. Diabetes Care. 2015;38:S8–16.
Ortega FJ, Mercader JM, Moreno-Navarrete JM, Nonell L, Puigdecanet E, Rodriquez-Hermosa JI, et al. Surgery-induced weight loss is associated with the downregulation of genes targeted by microRNAs in adipose tissue. J Clin Endocrinol Metab. 2015;100:E1467–76.
Moreno-Navarrete JM, Ortega F, Rodríguez A, Latorre J, Becerril S, Sabater-Masdeu M, et al. HMOX1 as a marker of iron excess-induced adipose tissue dysfunction, affecting glucose uptake and respiratory capacity in human adipocytes. Diabetologia. 2017;60:915–26.
Moreno-Navarrete JM, Ortega FJ, Rodríguez-Hermosa JI, Sabater M, Pardo G, Ricart W, et al. OCT1 expression in adipocytes could contribute to increased metformin action in obese subjects. Diabetes. 2011;60:168–76.
Moreno-Navarrete JM, Ortega F, Sabater M, Ricart W, Fernández-Real JM. Proadipogenic effects of lactoferrin in human subcutaneous and visceral preadipocytes. J Nutr Biochem. 2011;22:1143–9.
Park JH, Kang HJ, Lee YK, Kang H, Kim J, Chung JH, et al. Inactivation of EWS reduces PGC-1α protein stability and mitochondrial homeostasis. Proc Natl Acad Sci USA. 2015;112:6074–9.
Stanford KI, Middelbeek RJ, Townsend KL, Lee MY, Takahashi H, So K, et al. A novel role for subcutaneous adipose tissue in exercise-induced improvements in glucose homeostasis. Diabetes. 2015;64:2002–14.
Jahansouz C, Serrot FJ, Frohnert BI, Foncea RE, Dorman RB, Slusarek B, et al. Roux-en-Y gastric bypass acutely decreases protein carbonylation and increases expression of mitochondrial biogenesis genes in subcutaneous adipose tissue. Obes Surg. 2015;25:2376–85.
Sheftel AD, Wilbrecht C, Stehling O, Niggemeyer B, Elsässer HP, Mühlenhoff U, et al. The human mitochondrial ISCA1, ISCA2, and IBA57 proteins are required for [4Fe–4S] protein maturation. Mol Biol Cell. 2012;23:1157–66.
Wiley SE, Murphy AN, Ross SA, van der Geer P, Dixon JE. MitoNEET is an iron-containing outer mitochondrial membrane protein that regulates oxidative capacity. Proc Natl Acad Sci USA. 2007;104:5318–23.
Kusminski CM, Holland WL, Sun K, Park J, Spurgin SB, Lin Y, et al. MitoNEET-driven alterations in adipocyte mitochondrial activity reveal a crucial adaptive process that preserves insulin sensitivity in obesity. Nat Med. 2012;18:1539–49.
Ferecatu I, Gonçalves S, Golinelli-Cohen MP, Clémancey M, Martelli A, Riquier S, et al. The diabetes drug target MitoNEET governs a novel trafficking pathway to rebuild an Fe–S cluster into cytosolic aconitase/iron regulatory protein 1. J Biol Chem. 2014;289:28070–86.
Zhao Y, Ling F, Griffin TM, He T, Towner R, Ruan H, et al. Up-regulation of the Sirtuin 1 (Sirt1) and peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α) genes in white adipose tissue of Id1 protein-deficient mice: implications in the protection against diet and age-induced glucose intolerance. J Biol Chem. 2014;289:29112–22.
Jukarainen S, Heinonen S, Rämö JT, Rinnankoski-Tuikka R, Rappou E, Tummers M, et al. Obesity is associated with low NAD(+)/SIRT pathway expression in adipose tissue of BMI-discordant monozygotic twins. J Clin Endocrinol Metab. 2016;101:275–83.
Rutanen J, Yaluri N, Modi S, Pihlajamäki J, Vänttinen M, Itkonen P, et al. SIRT1 mRNA expression may be associated with energy expenditure and insulin sensitivity. Diabetes. 2010;59:829–35.
Scarpulla RC. Nuclear control of respiratory gene expression in mammalian cells. J Cell Biochem. 2006;97:673–83.
Merry TL, Ristow M. Nuclear factor erythroid-derived 2-like 2 (NFE2L2, Nrf2) mediates exercise-induced mitochondrial biogenesis and the anti-oxidant response in mice. J Physiol. 2016;594:5195–207.
Cohen AW, Razani B, Schubert W, Williams TM, Wang XB, Iyengar P, et al. Role of caveolin-1 in the modulation of lipolysis and lipid droplet formation. Diabetes. 2004;53:1261–70.
Michailidou Z, Morton NM, Moreno-Navarrete JM, West CC, Stewart KJ, Fernández-Real JM, et al. Adipocyte pseudohypoxia suppresses lipolysis and facilitates benign adipose tissue expansion. Diabetes. 2015;64:733–45.
Hansen M, Lund MT, Gregers E, Kraunsøe R, Van Hall G, Helge JW, et al. Adipose tissue mitochondrial respiration and lipolysis before and after a weight loss by diet and RYGB. Obesity. 2015;23:2022–9.
Mardinoglu A, Heiker JT, Gärtner D, Björnson E, Schön MR, Flehmig G, et al. Extensive weight loss reveals distinct gene expression changes in human subcutaneous and visceral adipose tissue. Sci Rep. 2015;5:14841.
González-Plaza JJ, Gutiérrez-Repiso C, García-Serrano S, Rodriguez-Pacheco F, Garrido-Sánchez L, Santiago-Fernández C, et al. Effect of Roux-en-Y gastric bypass-induced weight loss on the transcriptomic profiling of subcutaneous adipose tissue. Surg Obes Relat Dis. 2016;12:257–63.
Xie X, Sinha S, Yi Z, Langlais PR, Madan M, Bowen BP, et al. Role of adipocyte mitochondria in inflammation, lipemia and insulin sensitivity in humans: effects of pioglitazone treatment. Int J Obes (Lond) 2017; https://doi.org/10.1038/ijo.2017.192.
Bogacka I, Xie H, Bray GA, Smith SR. Pioglitazone induces mitochondrial biogenesis in human subcutaneous adipose tissue in vivo. Diabetes. 2005;54:1392–9.
Boudina S, Graham TE. Mitochondrial function/dysfunction in white adipose tissue. Exp Physiol. 2014;99:1168–78.
Haraguchi K, Shimura H, Lin L, Saito T, Endo T, Onaya T. Functional expression of thyrotropin receptor in differentiated 3T3-L1 cells: a possible model cell line of extrathyroidal expression of thyrotropin receptor. Biochem Biophys Res Commun. 1996;223:193–8.
Acknowledgements
This work was partially supported by research grant PI15/01934 and PI16/01173 from the Instituto de Salud Carlos III from Spain and was also supported by Fondo Europeo de Desarrollo Regional (FEDER). Xunta de Galicia (ML: 2015-CP079), Ministry of Economy and Competitiveness (ML: SAF2015-71026-R) and AtresMedia. CIBEROBN Fisiopatología de la Obesidad y Nutrición is an initiative from the Instituto de Salud Carlos III from Spain. We acknowledge the technical assistance of E. Loshuertos and O. Rovira (both from Endocrinology, IdIBGi, Spain). We want to particularly acknowledge the patients, the FATBANK platform promoted by the CIBEROBN and the IDIBGI Biobank (Biobanc IDIBGI, B.0000872), integrated in the Spanish National Biobanks Network, for their collaboration and coordination.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Comas, F., Lluch, A., Sabater, M. et al. Adipose tissue TSH as a new modulator of human adipocyte mitochondrial function. Int J Obes 43, 1611–1619 (2019). https://doi.org/10.1038/s41366-018-0203-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-018-0203-1
This article is cited by
-
Pituitary crosstalk with bone, adipose tissue and brain
Nature Reviews Endocrinology (2023)
-
Atypical pituitary hormone-target tissue axis
Frontiers of Medicine (2023)
-
Insulin-inducible THRSP maintains mitochondrial function and regulates sphingolipid metabolism in human adipocytes
Molecular Medicine (2022)