Abstract
Background
Changes in the intestinal flora composition is referred to as dysbiosis, which is related to obesity development, thus supporting the potential roles of nutrients acting on intestinal flora to exert salutary effects on energetic metabolism of host. Dietary fiber has been known to affect the composition of intestinal flora. The aim of the present study was to investigate the functional effects of konjac flour (KF) on obesity control in respect to improving inflammation, metabolism, and intestinal barrier function, and the possible association of the effects with intestinal flora composition changes.
Methods
Mice (n = 30) were randomly divided into control group (n = 10), high-fat-diet (HFD) group (n = 10), and KF intervention group (n = 10), followed by feeding for 12 weeks and with adding a KF daily supplementation for the treatment group. Body weight, fat accumulation, inflammation, and energetic metabolism markers in multiple tissues and the gut microbiota of the mice were examined at the end of the experiment.
Results
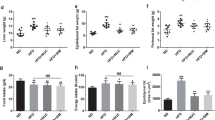
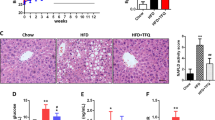
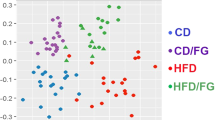
The KF supplementation significantly reduced the gains in weight, fat mass, as well as adipocyte size of HFD mice and lowered the serum TC, leptin (LEP), thiobarbituric acid-reacting substance (TBARS), IL-6, and lipopolysaccharide (LPS) levels in HFD mice. KF also upregulated the expression of intestinal mucosa protein gene Intection and tight junction ZO-1 in HFD mice, as well as upregulate the expression of energy metabolism genes PPARα and CPT-1 as well as the fat metabolism gene HLS in livers and fat tissues, and downregulate that of fat synthesis gene PPARγ (p < 0.05). The KF treatment increases the α-diversity and change the β-diversity of the intestinal microflora in HFD mice and boosted the abundances of some obesity-related beneficial microorganisms (such as Megasphaera elsdenii) in the intestinal microflora of HFD mice, while reduced those of harmful microorganisms (such as Alistipes, Alloprevotella, Bacteroides acidifaciens, and Parabacteroides goldsteinii). The abundance of Alistipes was positively correlated with weight, fat mass, serum TC, TG, LEP, IL-6, and LPS contents as well as PPARγ gene expression; while notably and negatively related to the expression of CPT-1 and HLS genes (p < 0.01). KF remarkably increased the abundance of Aerococcaceae, while reduced that of Alistipes finegoldii (p < 0.01).
Conclusions
Supplementation with KF achieves favorable effects on treating obesity, improving inflammatory response, metabolism, and intestinal barrier function, by regulating intestinal microfloral structure in HFD-fed mice.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hoyt CL, Burnette JL, Austergussman L. “Obesity is a disease”: examining the self-regulatory impact of this public-health message. Psychol Sci. 2014;25:997.
Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499:97–101.
Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med. 2012;18:363–74.
Moustafa ES, Froguel P. From obesity genetics to the future of personalized obesity therapy. Nat Rev Endocrinol. 2013;9:402.
Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341:1241214.
Dethlefsen L, Mcfall-Ngai, Amp M, Relman DA. An ecological and evolutionary perspective on human-microbe mutualism and disease. Nature. 2007;449:811–8.
Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101:15718.
Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–31.
Goodman AL, Kallstrom G, Faith JJ, Reyes A, Moore A, Dantas G, et al. Extensive personal human gut microbiota culture collections characterized and manipulated in gnotobiotic mice. Proc Natl Acad Sci USA. 2011;108:6252–7.
Cani PD, Possemiers S, Wiele TVD, Guiot Y, Everard A, Rottier O, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58:1091.
Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet–induced obesity and diabetes in mice. Diabetes. 2008;57:1470–81.
Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761.
Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–9.
Kovatcheva-Datchary P, Arora T. Nutrition, the gut microbiome and the metabolic syndrome. Best Pract Res Clin Gastroenterol. 2013;27:59–72.
Dewulf EM, Cani PD, Claus SP, Susana F, Puylaert Philippe GB, Neyrinck AM, et al. Insight into the prebiotic concept: lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Gut. 2013;62:1112–21.
Nakamura YK, Omaye ST. Metabolic diseases and pro- and prebiotics: mechanistic insights. Nutr Metab. 2012;9:60.
Everard A, Lazarevic V, Derrien M, Girard M, Muccioli GG, Neyrinck AM, et al. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes. 2011;60:2775–86.
Chen HL, Cheng HC, Liu YJ, Liu SY, Wu WT. Konjac acts as a natural laxative by increasing stool bulk and improving colonic ecology in healthy adults. Nutrition. 2006;22:1112–9.
Li B, Xia J, Wang Y, Xie B. Grain-size effect on the structure and antiobesity activity of konjac flour. J Agric Food Chem. 2005;53:7404–7.
Sun HQ, Tan CQ, Wei HK, Zou Y, Long G, Ao JT, et al. Effects of different amounts of konjac flour inclusion in gestation diets on physio-chemical properties of diets, postprandial satiety in pregnant sows, lactation feed intake of sows and piglet performance. Anim Reprod Sci. 2015;152:55–64.
Young W, Roy NC, Lee J, Lawley B, Otter D, Henderson G, et al. Bowel microbiota moderate host physiological responses to dietary konjac in weanling rats. J Nutr. 2013;143:1052–60.
Tan CQ, Wei HK, Sun HQ, Long G, Ao JT, Jiang SW, et al. Effects of supplementing sow diets during two gestations with konjac flour and Saccharomyces boulardii on constipation in peripartal period, lactation feed intake and piglet performance. Anim Feed Sci Technol. 2015;210:254–62.
Wu CF, Dong YY, Li JJ, Tang XH, Huang ZX. Study on the preparation of Konjac Oligo-glucomannan by β-mannanase. Biotechnol Bull. 2010;2010:118–22.
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–6.
Sato M, Uzu K, Yoshida T, Hamad EM, Kawakami H, Matsuyama H, et al. Effects of milk fermented by Lactobacillus gasseri SBT2055 on adipocyte size in rats. Br J Nutr. 2008;99:1013.
Neyrinck AM, Hée VFV, Piront N, Backer FD, Toussaint O, Cani PD, et al. Wheat-derived arabinoxylan oligosaccharides with prebiotic effect increase satietogenic gut peptides and reduce metabolic endotoxemia in diet-induced obese mice. Nutr Diabetes. 2012;2:e28.
Keithley JK, Barbara S, Mikolaitis SL, Mark DM, Zeller JM, Lou F, et al. Safety and efficacy of glucomannan for weight loss in overweight and moderately obese adults. J Obes. 2013;2013:610908.
SáInz N, Barrenetxe J, Morenoaliaga MJ, MartíNez JA. Leptin resistance and diet-induced obesity: central and peripheral actions of leptin. Metabolism. 2015;64:35–46.
Correia ML, Haynes WG. Leptin, obesity and cardiovascular disease. Curr Opin Nephrol Hypertens. 2004;13:215–23.
Halliwell B, Chirico S. Lipid peroxidation: its mechanism, measurement, and significance. Am J Clin Nutr. 1993;57:715S.
Kang Y, Zhang X, Cai Y, Su J, Kong X. Gut microbiota and metabolic disease: from pathogenesis to new therapeutic strategies. Allergol Immunopathol. 2017;28:1.
Musso G, Gambino R, Cassader M. Interactions between gut microbiota and host metabolism predisposing to obesity and diabetes. Annu Rev Med. 2011;62:361–80.
Ohland CL, Macnaughton WK. Probiotic bacteria and intestinal epithelial barrier function. Am J Physiol Gastrointest Liver Physiol. 2010;298:807–19.
Zhang Y, Ma K, Song S, Elam MB, Cook GA, Park EA. Peroxisomal proliferator-activated receptor-gamma coactivator-1 alpha (PGC-1 alpha) enhances the thyroid hormone induction of carnitine palmitoyltransferase I (CPT-I alpha). J Biol Chem. 2004;279:53963–71.
Finck BN, Kelly DP. Peroxisome proliferator–activated receptor γ coactivator-1 (PGC-1) regulatory cascade in cardiac physiology and disease. Circulation. 2007;115:2540.
Preiss-Landl K, Zimmermann R, Hämmerle G, Zechner R. Lipoprotein lipase: the regulation of tissue specific expression and its role in lipid and energy metabolism. Curr Opin Lipidol. 2002;13:471.
Lafontan M, Langin D. Lipolysis and lipid mobilization in human adipose tissue. Prog Lipid Res. 2009;48:275–97.
Wang J, Jiang M, Xia JH, Li X, Yi GH, Yuan CG. The role of PPARδ in lipid metabolism. Chem Life. 2008;28:175–7.
Clarke SF, Murphy EF, O’Sullivan O, Ross RP, O’Toole PW, Shanahan F, et al. Targeting the microbiota to address diet-induced obesity: a time dependent challenge. PLoS ONE. 2013;8:e65790.
Dinh DM, Volpe GE, Duffalo C, Bhalchandra S, Tai AK, Kane AV, et al. Intestinal microbiota, microbial translocation, and systemic inflammation in chronic HIV infection. J Infect Dis. 2015;211:19–27.
Mchardy IH, Li X, Tong M, Ruegger P, Jacobs J, Borneman J, et al. HIV Infection is associated with compositional and functional shifts in the rectal mucosal microbiota. Microbiome. 2013;1:1–12.
Xu J, Lian F, Zhao L, Zhao Y, Chen X, Zhang X, et al. Structural modulation of gut microbiota during alleviation of type 2 diabetes with a Chinese herbal formula. ISME J. 2015;9:552–62.
Saulnier DM, Riehle K, Mistretta TA, Diaz MA, Mandal D, Raza S, et al. Gastrointestinal microbiome signatures of pediatric patients with irritable bowel syndrome. Gastroenterology. 2011;141:1782.
Rautio M, Eerola E, Väisänen-Tunkelrott ML, Molitoris D, Lawson P, Collins MD, et al. Reclassification of Bacteroides putredinis (Weinberg et al., 1937) in a new genus Alistipes gen. nov., as Alistipes putredinis comb. nov., and description of Alistipes finegoldii sp. nov., from human sources. Syst Appl Microbiol. 2003;26:182–8.
Louis S, Tappu RM, Damms-Machado A, Huson DH, Bischoff SC. Characterization of the gut microbial community of obese patients following a weight-loss intervention using whole metagenome shotgun sequencing. PLoS ONE. 2016;11:e0149564.
Petriz BA, Castro AP, Almeida JA, Gomes CP, Fernandes GR, Kruger RH, et al. Exercise induction of gut microbiota modifications in obese, non-obese and hypertensive rats. BMC Genomics. 2014;15:511.
Yanagibashi T, Hosono A, Oyama A, Tsuda M, Suzuki A, Hachimura S, et al. IgA production in the large intestine is modulated by a different mechanism than in the small intestine: Bacteroides acidifaciens promotes IgA production in the large intestine by inducing germinal center formation and increasing the number of IgA + B cells. Immunobiology. 2013;218:645–51.
Peterson DA, Mcnulty NP, Guruge JL, Gordon JI. IgA response to symbiotic bacteria as a mediator of gut homeostasis. Cell Host Microbe. 2007;2:328.
Rodriguezsegade S, Camiña MF, Carnero A, Lorenzo MJ, Alban A, Quinteiro C, et al. High serum IgA concentrations in patients with diabetes mellitus: agewise distribution and relation to chronic complications. Clin Chem. 1996;42:1064–7.
Hotamisligil GS, Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2007;444:860–7.
Liu Z, Chen Z, Guo H, He D, Zhao H, Wang Z, et al. The modulatory effect of infusions of green tea, oolong tea, and black tea on gut microbiota in high-fat-induced obese mice. Food Funct. 2016;7:4869–79.
Patrone V, Vajana E, Minuti A, Callegari ML, Federico A, Loguercio C. et al. Postoperative changes in fecal bacterial communities and fermentation products in obese patients undergoing bilio-intestinal bypass. Front Microbiol. 2016;7:200
Federico A, Dallio M, Tolone S, Gravina AG, Patrone V, Romano M, et al. Gastrointestinal hormones, intestinal microbiota and metabolic homeostasis in obese patients: effect of bariatric surgery. In Vivo. 2016;30:321.
Shetty SA, Marathe NP, Lanjekar V, Ranade D, Shouche YS. Comparative genome analysis of Megasphaera sp. reveals niche specialization and its potential role in the human gut. PLoS ONE. 2013;8:e79353.
Marounek M, Fliegrova K, Bartos S. Metabolism and some characteristics of ruminal strains of Megasphaera elsdenii. Appl Environ Microbiol. 1989;55:1570–3.
Acknowledgements
We thank Engineering Research Center of Sustainable Development and Utilization of Biomass Energy Ministry of Education for supplying KF.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Kang, Y., Li, Y., Du, Y. et al. Konjaku flour reduces obesity in mice by modulating the composition of the gut microbiota. Int J Obes 43, 1631–1643 (2019). https://doi.org/10.1038/s41366-018-0187-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-018-0187-x
This article is cited by
-
A study on the association between gut microbiota, inflammation, and type 2 diabetes
Applied Microbiology and Biotechnology (2024)
-
Konjac glucomannan attenuate high-fat diet-fed obesity through enhancing β-adrenergic-mediated thermogenesis in inguinal white adipose tissue in mice
Glycoconjugate Journal (2023)
-
Peony seed oil decreases plasma cholesterol and favorably modulates gut microbiota in hypercholesterolemic hamsters
European Journal of Nutrition (2022)
-
Characterization of the gut microbiota in hemodialysis patients with sarcopenia
International Urology and Nephrology (2022)
-
Similarities and differences of oligo/poly-saccharides’ impact on human fecal microbiota identified by in vitro fermentation
Applied Microbiology and Biotechnology (2021)