Abstract
Background
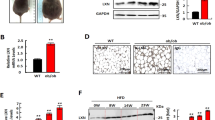
CDH13, an atypical member of the cadherin superfamily, has been identified in adipocyte secretomes of lean mouse models. CDH13 abundance differs in mouse models according to their susceptibility to develop metabolic disorders, but the role of CDH13 in adipose tissue is unknown.
Methods
Secreted CDH13 protein levels and mRNA levels in visceral adipose tissue were determined in lean and obese mouse models. In vitro studies were performed in 3T3-L1 adipocytes to determine the role of CDH13 in adipocyte differentiation. The pathophysiological impact of visceral adipose tissue CDH13 mRNA and circulating CDH13 levels were determined in humans (normal-weight men n = 37, obese men n = 109 including n = 51 type 2 diabetes patients) and in obese patients (n = 14) pre- and post-metabolic surgery.
Results
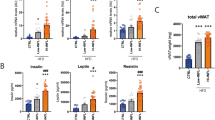
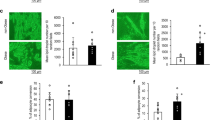
This study shows that in visceral adipose tissue CDH13 protein secretion and mRNA levels were decreased in obese mouse models. Mechanistically, CDH13 affects lipid metabolism during adipogenesis but not in mature adipocytes. CDH13 knockdown during adipogenesis reduced fatty acid uptake and lipid content in developing adipocytes. Furthermore, CDH13 depletion during adipogenesis lowered the induction of PPARγ and C/EBPα expression. These observations are of pathophysiological impact since visceral adipose tissue CDH13 mRNA and circulating CDH13 levels were decreased in obese men compared to normal-weight controls. Weight loss induced by bariatric surgery restored circulating CDH13 to levels found in normal-weight controls.
Conclusions
CDH13 levels in adipose tissue and the circulation are affected by obesity in mouse models and humans and are restored by weight loss in humans. CDH13 interferes with the differentiation potential of adipocytes and therefore is a marker for plasticity of fat tissue that might reflect the health status of adipose tissue.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Unger RH, Clark GO, Scherer PE, Orci L. Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim Biophys Acta. 2010;1801:209–14.
Tang QQ, Lane MD. Adipogenesis: from stem cell to adipocyte. Annu Rev Biochem. 2012;81:715–36.
Gupta RK, et al. Zfp423 expression identifies committed preadipocytes and localizes to adipose endothelial and perivascular cells. Cell Metab. 2012;15:230–9.
Zhuang H, et al. Molecular mechanisms of PPAR-gamma governing MSC osteogenic and adipogenic differentiation. Curr Stem Cell Res Ther. 2016;11:255–64.
Hartwig S, et al. Identification of novel adipokines differential regulated in C57BL/Ks and C57BL/6. Arch Physiol Biochem. 2014;120:208–15.
Chung CM, et al. A genome-wide association study reveals a quantitative trait locus of adiponectin on CDH13 that predicts cardiometabolic outcomes. Diabetes. 2011;60:2417–23.
Fava C, et al. A variant upstream of the CDH13 adiponectin receptor gene and metabolic syndrome in Swedes. Am J Cardiol. 2011;108:1432–7.
Gao H, et al. Genetic variation in CDH13 is associated with lower plasma adiponectin levels but greater adiponectin sensitivity in East Asian populations. Diabetes. 2013;62:4277–83.
Morisaki H, et al. CDH13 gene coding T-cadherin influences variations in plasma adiponectin levels in the Japanese population. Hum Mutat. 2012;33:402–10.
Nicolas A, et al. T-cadherin gene variants are associated with type 2 diabetes and the Fatty Liver Index in the French population. Diabetes Metab. 2017;43:33–9.
Killen AC, et al. Protective role of Cadherin 13 in interneuron development. Brain Struct Funct. 2017;222:3567–85.
Fukuda S, et al. The unique prodomain of T-cadherin plays a key role in adiponectin binding with the essential extracellular cadherin repeats 1 and 2. J Biol Chem. 2017;292:7840–9.
Dasen B, et al. T-cadherin in prostate cancer: relationship with cancer progression, differentiation and drug resistance. J Pathol Clin Res. 2017;3:44–57.
Hulpiau P, van Roy F. New insights into the evolution of metazoan cadherins. Mol Biol Evol. 2011;28:647–57.
Knebel B, et al. Peroxisomes compensate hepatic lipid overflow in mice with fatty liver. Biochim Biophys Acta. 2015;1851:965–76.
Ruige JB, et al. Sex steroid-induced changes in circulating monocyte chemoattractant protein-1 levels may contribute to metabolic dysfunction in obese men. J Clin Endocrinol Metab. 2012;97:E1187–91.
Bekaert M, et al. Determinants of testosterone levels in human male obesity. Endocrine. 2015;50:202–11.
Goddeke S, Kotzka J, Lehr S. Investigating the adipose tissue secretome: a protocol to generate high-quality samples appropriate for comprehensive proteomic profiling. Methods Mol Biol. 2015;1295:43–53.
Kotzka J, et al. Phosphorylation of sterol regulatory element-binding protein (SREBP)-1a links growth hormone action to lipid metabolism in hepatocytes. Atherosclerosis. 2010;213:156–65.
Dunach M, Del Valle-Perez B, Garcia de Herreros A. p120-catenin in canonical Wnt signaling. Crit Rev Biochem Mol Biol. 2017;52:327–39.
Spalding KL, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–7.
Strissel KJ, et al. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 2007;56:2910–8.
Matsushita K, Dzau VJ. Mesenchymal stem cells in obesity: insights for translational applications. Lab Invest. 2017;97:1158–66.
Muir LA, et al. Adipocyte hypertrophy-hyperplasia balance contributes to weight loss after bariatric surgery. Adipocyte. 2017;6:134–40.
Siersbaek R, Nielsen R, Mandrup S. Transcriptional networks and chromatin remodeling controlling adipogenesis. Trends Endocrinol Metab. 2012;23:56–64.
Joshi MB, et al. A requirement for thioredoxin in redox-sensitive modulation of T-cadherin expression in endothelial cells. Biochem J. 2008;416:271–80.
Kitamoto A, et al. CDH13 polymorphisms are associated with adiponectin levels and metabolic syndrome traits independently of visceral fat mass. J Atheroscler Thromb. 2016;23:309–19.
Dasgupta A, Hughey R, Lancin P, Larue L, Moghe PV. E-cadherin synergistically induces hepatospecific phenotype and maturation of embryonic stem cells in conjunction with hepatotrophic factors. Biotechnol Bioeng. 2005;92:257–66.
Conant K, et al. Matrix metalloproteinase activity stimulates N-cadherin shedding and the soluble N-cadherin ectodomain promotes classical microglial activation. J Neuroinflamm. 2017;14:56.
Bosserhoff AK, et al. Loss of T-cadherin (CDH-13) regulates AKT signaling and desensitizes cells to apoptosis in melanoma. Mol Carcinog. 2014;53:635–47.
Marie PJ, et al. Cadherin-mediated cell-cell adhesion and signaling in the skeleton. Calcif Tissue Int. 2014;94:46–54.
Fujishima Y, et al. Adiponectin association with T-cadherin protects against neointima proliferation and atherosclerosis. FASEB J. 2017;31:1571–83.
Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4:45–60.
Funding
The work was supported by the German Diabetes Center (DDZ), which is funded by the German Federal Ministry of Health and the Ministry of Innovation, Science, Research and Technology of the state North Rhine-Westphalia. This study was supported in part by a grant from the German Federal Ministry of Education and Research (BMBF) to the German Center for Diabetes Research (DZD e. V.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Göddeke, S., Knebel, B., Fahlbusch, P. et al. CDH13 abundance interferes with adipocyte differentiation and is a novel biomarker for adipose tissue health. Int J Obes 42, 1039–1050 (2018). https://doi.org/10.1038/s41366-018-0022-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-018-0022-4