Abstract
A newborn screening program for Pompe disease using dried blood spots (DBSs) was initiated in Japan. Here, we summarized this screening program and described the results of the GAA gene analysis. From April 2013 to November 2016, 103,204 newborns were screened; 71 had low acid alpha-glucosidase (AαGlu) activity. GAA sequencing showed that 32 (45.1%) and 37 (52.1%) of these newborns were homozygous and heterozygous for pseudodeficiency alleles c.[1726G>A; 2965G>A], respectively. Moreover, 24 of 32 newborns with homozygous c.[1726G>A; 2965G>A] alleles had no mutations, and the other eight had one mutation each. Thirty-five of 37 newborns with heterozygous c.[1726G>A; 2965G>A] alleles had one mutation, and the other two had two mutations each. Only one newborn who had two mutations did not harbor c.[1726G>A; 2965G>A] alleles. Thus, it was difficult to distinguish newborns with c.[1726G>A; 2965G>A] alleles from newborns with pre-symptomatic Pompe disease using AαGlu assays in DBSs or fibroblasts; GAA gene sequencing was necessary. Seventy-one newborns had 50 variants, including 21 mutations or predictably pathogenic variants, and 29 polymorphisms or predictably non-pathogenic variants. Four of 21 mutations or predictably pathogenic variants and four of 29 polymorphisms or predictably non-pathogenic variants were novel. No infantile-onset Pompe disease was detected, and three newborns were diagnosed with potential late-onset Pompe disease. In the literature, 156 variants have been reported for 296 patients from 277 families in 41 articles from Japan, Korea, Taiwan, and China. Our results provide insights into GAA gene mutation profiles and the relationship between GAA and Pompe disease in Asian populations.
Similar content being viewed by others
Introduction
Glycogen storage disease type II (OMIM 232300), also known as Pompe disease, is an autosomal recessive disorder caused by a deficiency of AαGlu (EC 3.2.1.20/3), resulting in the accumulation of lysosomal glycogen in the skeletal muscles and heart [1]. The rates of accumulation and tissue damage vary depending on the residual enzyme activity. Patients with infantile-onset Pompe disease (IOPD) with an almost complete absence of AαGlu activity present with hypotonia and hypertrophic cardiomyopathy within a few months after birth. Massive amounts of glycogen accumulate in skeletal and heart muscle, and these patients eventually die due to cardiorespiratory failure. Patients with late-onset Pompe disease (LOPD) who exhibit marked reduction of AαGlu activity are predominantly characterized by skeletal muscle dysfunction, but rarely show an involvement of cardiac muscle. The onset time and phenotypes of LOPD are variable, and patients often develop symptoms in the fifth decade or later in life [2]. Enzyme replacement therapy (ERT) is often used for the treatment of IOPD and LOPD [3, 4]. ERT should be started before symptoms are apparent, prior to the development of irreversible damage, to achieve optimal outcomes [5, 6]. Early initiation of ERT in IOPD markedly improves survival, reduces the need for ventilation, results in earlier independent walking, and enhances patient quality of life [7, 8].
Newborn screening (NBS) is often the best approach for early diagnosis and treatment. Asian populations have a high frequency of pseudodeficiency alleles c.[1726G>A; 2065G>A] in the GAA gene, and these pseudodeficiency alleles reduce AαGlu activity into the abnormal range [9]. The frequency of pseudodeficiency alleles in the Japanese population is estimated to be 3.9% for homozygous alleles and 30.5% for heterozygous alleles [10]. Therefore, the presence of pseudodeficiency alleles interfered with NBS for Pompe disease in our previous pilot program [10]. Recent reports have suggested that the combination of AαGlu assays using dried blood spots (DBSs) and GAA gene mutation analysis could be used to distinguish false-positive cases from patients with Pompe disease in an NBS program for Pompe disease [11].
We have conducted a large-scale NBS program for Pompe disease at Kumamoto and Fukuoka prefectures in Japan. Overall, 103,204 newborns were screened from April 2013 to November 2016 using DBS analysis with fluorometric assays [12]. Accordingly, in this study, we describe the analysis of AαGlu activity and GAA gene sequencing in this population. We also discuss the usefulness of AαGlu assays in skin fibroblasts, which are considered to be the gold standard for the diagnosis of patients with suspected Pompe disease [13]. Moreover, we summarize previous reports of GAA mutations in Japanese, Taiwanese, Korean, and Chinese populations and discuss the mutation profiles in Asian patients.
Materials and methods
Study population and materials
The study population consisted of 103,204 newborns born in Kumamoto and Fukuoka prefectures between April 2013 and November 2016. The DBSs were prepared in each maternity clinic or obstetric department using standard procedures at 4–6 days after birth for Newborn Mass Screening in the public health system. After dropping blood spots onto filter papers (Toyo Roshi Kaisha, Ltd., Tokyo, Japan), the DBSs were dried for at least 4 h at room temperature and sent to the Newborn Screening Center at KM Biologics Co., Ltd. (Kumamoto, Japan) within 1 week after preparation. After analysis, the DBSs were transferred to Kumamoto University.
NBS program for Pompe disease
The NBS using AαGlu assays with DBSs for Pompe disease was performed in three steps (Fig. 1). In the first step, newborns with AαGlu activity under the cutoff value of 6.5 pmol/h/disk were recalled, and their DBSs were prepared again. In the second step with the Ba/Zn method, newborns with AαGlu activity under the cutoff value of 5.5 pmol/h/disk were called to the hospital within two months for the clinical examination. The newborns were examined by physical and biochemical assays to confirm symptomatic signs of IOPD, and the third AαGlu assay also was performed. Finally, GAA gene sequencing was performed in newborns with AαGlu activity under the cutoff value of 4.0 pmol/h/disk. Additionally, the AαGlu activity of the fibroblast in the newborns was measured. The period after birth until the result of the first AαGlu assay was acquired was 1–2 weeks, and the period until the result of the second AαGlu assay was acquired was within 4 weeks, the period from birth until clinical examination was performed was within 2 months, and the period from birth until GAA gene analysis and final diagnosis was achieved was up to 6 months.
AαGlu assay
The procedures for AαGlu assays using DBSs and fibroblasts were described in our previous report [13]. Briefly, a single 3.2-mm diameter disk punched from DBSs was incubated in the well of a 96-well clear microwell-plate (Corning, NY, USA) with 100 μL of 0.8 mM citrate/24 mM potassium phosphate buffer (pH 6.0) containing 0.1% Triton X-100 for 1 h at room temperature with gentle mixing. A 20 μL aliquot of the extract was then added to 40 μL of 2.0 mM 4-methylumbelliferyl α-D-glucopyranoside (4MU-αGlc) in 0.12 M citrate/0.15 M potassium phosphate buffer (pH 4.0) containing 4.5 μM acarbose (3.0 μM final concentration) in a 96-well black microwell-plate (PerkinElmer). The reaction mixture was incubated at 37 °C for 24 h, and the reaction was stopped by adding 190 μL of 0.2 M glycine/NaOH buffer (pH 10.7) containing 0.1% Triton X-100 and 0.2% SDS to measure fluorescence intensity. For the Ba/Zn method, a 3.2-mm diameter disk was placed in a 1.5 mL reaction tube and gently mixed for 10 min at room temperature in 60 μL of 0.12 M citrate/0.15 M potassium phosphate buffer (pH 4.0) containing 2.0 mM 4MU-αGlc and 3.0 μM acarbose. The reaction mixture was then incubated at 37 °C for 24 h. Next, 30 μL of 0.15 M barium hydroxide was added. The reaction tubes were mixed by vortexing and incubated at room temperature for 5 min. Thirty microliters of 0.15 M zinc sulfate was then added, and the tubes were mixed by vortexing and incubated for 10 min at room temperature. The tubes were then centrifuged for 5 min at 12,000 rpm and 5 °C. Finally, 90 μL of the supernatant was transferred to a 96-well black microwell-plate, and 160 μL of 0.4 M glycine/NaOH buffer (pH 10.7) containing 0.1% Triton X-100 was added to measure fluorescence intensity. Stock solutions of 0, 6.25, 12.5, 25, 50, and 100 μM 4-methylumbelliferone (4MU) in 20 mM sodium phosphate buffer (pH 7.0) were used to calibrate the measurement of the liberated 4MU. The enzyme activity was expressed as picomoles of 4MU released per hour per disk (pmol/h/disk). Each assay was performed in duplicate.
Fibroblasts were prepared from a skin biopsy and cultured under standard conditions in Dulbecco’s modified Eagle’s medium with 10% fetal calf serum and antibiotics (50 kU/L penicillin and 50 mg/L streptomycin). After growing to confluence, the fibroblasts were harvested and washed with phosphate buffered saline. The cell pellet was stored at −80 °C until use. Fibroblasts (2–4 × 106 cells) were homogenized in 150 μL water by sonication on ice, and 10 μL of the cell homogenate was added to 40 μL of the substrate solution containing 2.0 mM 4MU-αGlc in 0.12 M citrate/0.15 M potassium phosphate buffer (pH 4.0) containing 3.75 μM acarbose (3.0 μM in the final concentration) in a well of a 96-well black microwell-plate. The reaction mixture was incubated at 37 °C for 1 h, and the reaction was stopped by the addition of 200 μL of 0.2 M glycine/NaOH buffer (pH 10.7) containing 0.1% Triton X-100 to measure fluorescence intensity, with correction for the substrate blank. The stock solution of 250 μM 4MU in 20 mM sodium phosphate buffer (pH 7.0) was used to calibrate the measurement of liberated 4MU. The enzyme activity was expressed as nanomoles of 4MU released per hour per milligram of cellular protein (nmol/h/mg protein). Each assay was performed in duplicate.
Sequencing of the GAA gene by NGS
The procedures for sequencing of the GAA gene by NGS were described in our previous report [14]. Briefly, genomic DNA was extracted from total blood using a Gentra Puregene Blood Kit (Qiagen, Hilden, Germany) and stored at −80 °C until use. The 22-kbp region including the GAA gene was amplified by dividing the genomic DNA into three fragments using long-range polymerase chain reaction (Supplementary Fig. S1). The reaction was performed using a DNA polymerase (KOD FX; Toyobo, Osaka, Japan) and Veriti Thermal Cycler (Applied Biosystems, Foster City, CA, USA). PCR products were purified using an Agencourt AMP XP PCR Purification Kit (Beckman Coulter, Brea, CA, USA) and quantified with a Qubit dsDNA HS Assay Kit (Life Technologies, Carlsbad, CA, USA) using a Qubit 2.0 Fluorometer (Life Technologies). Simultaneous fragmentation of PCR products and adaptor ligation were performed using a Nextera XT Kit (Illumina, San Diego, CA, USA). Indexed DNA was purified using an Agencourt AMP XP PCR Purification Kit (Beckman Coulter). Each library was validated using High Sensitivity D1000 ScreenTape (Agilent Technologies, Santa Clara, CA, USA) with an Agilent 2200 TapeStation and quantified using a Qubit dsDNA HS Assay Kit with a Qubit 2.0 Fluorometer to allow for library normalization. Sequencing was performed with a MiSeq Reagent Kit v3 and a MiSeq sequencer (Illumina) using the “paired-end” sequencing run method. After sequencing runs, the data were aligned to target sequences on the human reference genome sequence using MiSeq Reporter software (Illumina). Sequence data analysis, mapping, and variant calling were streamlined using MiSeq Reporter v2 (Illumina). Briefly, reads were aligned to the reference sequence, from positions 80,101,882 to 80,123,207 of the genome sequence of chromosome 17 (NC_000017.11), using bwa-0.6.1. Single-nucleotide polymorphism and insertion/deletion identifications were performed using the Genome Analysis Toolkit (GATK v1.6; Broad Institute, Cambridge, MA, USA). Visualization of sequencing reads was performed with IGV_2.3.40 (Broad Institute).
Resequencing of the GAA gene by the Sanger method
Variants detected in the GAA gene by NGS were re-sequenced by the Sanger method [15]. Briefly, a region including the variant was amplified by PCR using an appropriate set of primers. PCR products were sequenced on an ABI3500xl auto sequencer (Applied Biosystems) and analyzed using Sequencher 5.0 (Gene Codes Corporation, Ann Arbor, MI, USA).
Mutation analysis of the variants
The mRNA reference sequence (RefSeq) NM_000152.4 was used for this study, whereby the “A” nucleotide of the ATG codon at nucleotide position 398 of the RefSeq constituted+1 numbering of the cDNA sequence. The ATG codon also represented+1 for the amino acid numbering as set forth by the AαGlu preprotein sequence NP_000143.2. Mutation nomenclature followed the guidelines established by the Human Genome Variation Society (http://varnomen.hgvs.org/). The public databases, Pompe Center (http://cluster15.erasmusmc.nl/klgn/pompe/mutations.html, updated May 2016), and ClinVar [16] (http://www.ncbi.nlm.nih.gov/clinvar) were used for the classification of each variant. The software PolyPhen-2 [17] (http://genetics.bwh.harvard.edu/pph2) was used for missense mutations to predict the potential impact of an amino acid alteration on the function of AαGlu. Online bioinformatics tools, e.g., Human Splicing Finder [18] (http://www.umd.be/HSF3/) and Mutation Taster [19] (http://www.mutationtaster.org), were used to estimate the effects of mutations on splicing signals. The database of the 1,000 Genome Project (http://browser.1000genomes.org) and the Web application Mutation@A Glance [20] (http://harrier.nagahama-i-bio.ac.jp/mutation/) were used to estimate the variants as polymorphisms.
Literature search
An electronic literature search of the PubMed database was performed for current and past findings of the GAA mutation in Japanese, Taiwanese, Korean, and Chinese patients for the years 1990 through 2018 using the keywords “acid alpha-glucosidase deficiency”, “acid maltase deficiency”, “Pompe disease”, “GAA”, “mutation”, “variant”, etc. When there were overlaps between multiple studies, the more relevant articles were used.
Results
NBS for Pompe disease
The flow chart and results for the NBS program are shown in Fig. 1. In total, 103,204 newborns were screened, 225 newborns were recalled for a second AαGlu assay, and 111 newborns with low AαGlu activities under the cutoff at the second AαGlu assay were examined at the outpatient clinic. These patients were evaluated to detect IOPD using physical and biochemical examinations (creatine kinase, alanine transaminase, aspartate aminotransferase, and lactate dehydrogenase) and echocardiogram assessments at Kumamoto University Hospital or Fukuoka University Hospital, and the third AαGlu assay was performed. In this study, no patients were found to have IOPD. For the 71 newborns with low AαGlu activity under the cutoff at the third AαGlu assay, GAA gene sequencing was performed using NGS. Moreover, the AαGlu activities in fibroblasts were measured in 32 of the 71 newborns.
Variants detected in the newborns
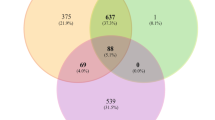
The GAA gene is highly polymorphic, and many novel mutations are still being discovered. The Pompe Center contains a list of over 550 sequence variations in the version from May 2016. In this study, 50 variants were detected in the 71 newborns (Table 1). Thirty-seven of these 50 variants were registered at the Pompe Center. The other 13 variants, i.e., c.317G>A (p.R106H), c.705G>A (p.T235T), c.1082C>A (p.P361Q), c.1244C>T (p.T415M), c.2003A>G (p.Y668C), c.2055C>G (p.Y685*), c.-260G>C, c.547-67C>G, c.547-39T>G, c.692 + 38C>T, c.858 + 8G>A, c.1552-52C>A, and c.1638 + 43G>T, were not registered. Five of the 13 variants, i.e., c.317G>A (p.R106H), c.705G>A (p.T235T), c.-260G>C, c.547-39T>G, and c.858 + 8G>A were registered at the ClinVar. Eight of the 13 variants, i.e., c.1082C>A (p.P361Q), c.1244C>T (p.T415M), c.2003A>G (p.Y668C), c.2055C>G (p.Y685*), c.547-67C>G, c.692 + 38C>T, c.1552-52C>A, and c.1638 + 43G>T, were novel. Additionally, six missense variants, i.e., c.317G>A, c.1082C>A, c.1124G>A, c.1244C>T, c.1562A>T, and c.2003A>G, were predicted by PolyPhen-2 as “probably damaging”. The silent variant c.705G>A was predicted by the Human Splicing Finder [18] as a “potential alteration of splicing”.
Although Qiu et al. reported that the c.2132C>G variant was responsible for LOPD [21], and the polyphen-2 predicted the c.2132C>G variant as probably damaging (Table 1), we considered c.2132C>G as a non-pathogenic variant because this variant is registered as “non-pathogenic” at the Pompe Center, and the mother of subject ID 106, with homozygous variants of c.2132C>G, has never presented signs of LOPD. Therefore, we considered these seven variants (c.317G>A, c.705G>A, c.1082C>A, c.1124G>A, c.1244C>T, c.1562A>T, and c.2003A>G) as predicted pathogenic mutations. As a result, we classified 21 of the 50 variants as mutations or predicted pathogenic variants, and the other 29 variants were classified as polymorphisms or predicted non-pathogenic variants (Table 1). The most common mutation was c.752C>T+c.761C>T, which accounted for 20 alleles (14.1%, 20/142). The pseudodeficiency alleles c.1726G>A (p.G576S) and c.2065G>A (p.E689K) were detected in 71.8% (102/142) and 72.5% (103/142) of all newborns with low AαGlu activity, respectively. Additionally, 32 newborns had the homozygous c.1726G>A (p.G576S) mutation, 33 newborns had the homozygous c.2065G>A (p.E689K) mutation, and 32 newborns (45.1%) had a combination of homozygous c.1726G>A (p.G576S) and c.2065G>A (p.E689K) mutations. Eight of 32 newborns had one mutation, and the other 24 newborns had no mutations. Additionally, 38 newborns had the heterozygous c.1726G>A (p.G576S) mutation, 37 newborns had the heterozygous c.2065G>A (p.E689K) mutation, and 37 newborns (52.1%) had a combination of heterozygous c.1726G>A (p.G576S) and c.2065G>A (p.E689K) mutations. Thirty-five of 37 newborns had one mutation, and the other two newborns had two mutations. One newborn had two mutations as a combination of heterozygous c.1726G>A (p.G576S) and homozygous c.2065G>A (p.E689K) mutations. Only one newborn with two mutations had no pseudodeficiency alleles.
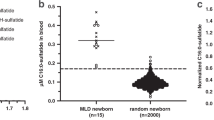
Although we did not detect IOPD in any of the patients in this study, we did identify three newborns with potential LOPD (subject IDs 93, 87, and 84). Although these three patients developed no symptoms related to Pompe disease and received no treatment, we considered them as patients with potential LOPD based on the impact of variants predicted by PolyPhen-2. The AαGlu activities and gene mutations in the three patients with potential LOPD are shown in Fig. 2 and Table 2. The c.317G>A (p.R106H), c.1244C>T (p.T415M), and c.2003A>G (p.Y668C) mutations were considered novel mutations. The subject ID 8 was not considered a patient with potential LOPD because the AαGlu activity in fibroblasts was relatively normal, although the variants, i.e., c.705G>A (p.T235T) and c.2560C>T (p.R854*), were considered as mutations by the Splicing Finder or the Pompe Center.
Literature search and mutation profiles of the GAA gene in Asian patients
In this study, we collected information on GAA gene variants from 296 patients and 277 families with Pompe disease from 41 studies conducted in Asia. The numbers of families and articles from Japan, Korea, Taiwan, and China were 64 and 12, 13 and 4, 108 and 11, and 92 and 14, respectively. Additionally, 39, 15, 49, and 89 variants, including those in pseudodeficiency alleles, were reported from Japan, Korea, Taiwan, and China, respectively. Some variants were overlapped; thus, 156 variants were reported in Asia (Table 3). Thirty-nine variants were reported in 70 Japanese patients from 64 families (Supplementary Table 1). Five of 39 variants were not registered at the Pompe Center or ClinVar. Three of five were missense mutations and predicted as “probably damaging” by PolyPhen-2. Of the remaining two, c.547-1G>C was predicted as a “potential alteration of splicing” by the Human Splicing Finder, and c.756insT was predicted to be a frameshift mutation resulting in a stop codon. The first, second, and third most common variants were c.546G>T (allele frequency: 19.53%, 25/128), c.1798C>T (p.R600C) (14.84%, 19/128), and c.1857C>G (p.S619R) (11.72%, 15/128; Table 4). Among the 64 families, 32 were compound heterozygotes, and 22 were homozygotes, including five consanguineous families. The other ten families had one pathogenic variant and one unknown variant. Eleven and 53 of the 64 families had histories of IOPD and LOPD, respectively.
Fifteen variants were reported in 15 Korean patients from 13 families (Supplementary Table 2). Three of 15 variants were not registered at the Pompe Center or ClinVar. One of these three was a missense mutation and predicted as “benign” by PolyPhen-2, and the remaining two were frameshift mutations resulting in stop codons. The first and second common variants were c.1316T>A (p.M439K) (23.08%, 6/26) and c.1857C>G (p.S619R) (11.54%, 3/26; Table 4). Among the 13 families, 12 were compound heterozygotes, and one family had one pathogenic variant and one unknown variant. Ten and three of the 15 families had histories of IOPD and LOPD, respectively.
Forty-nine variants were reported in 110 Taiwanese patients from 108 families (Supplementary Table 3). All variants were registered at the Pompe Center or ClinVar. The first, second, and third most common mutations were c.1935C>A (p.D645E) (47.69%, 103/216), c.2238G>C (p.W746C) (7.87%, 17/216), and c.1411_1414del (p.E471Pfs*5) (5.56%, 12/216), respectively, excluding the pseudodeficiency allele c.1726G>A (19.91%, 43/216; Table 4). Among the 108 families, 72 were compound heterozygotes, and 29 were homozygotes, including one consanguineous family. The remaining seven families had one pathogenic variant and one unknown variant. Out of 108 families, 68 and 33 had histories of IOPD and LOPD, respectively.
Eighty-nine variants were reported in 101 Chinese patients from 92 families (Supplementary Table 4). Twenty-two of 89 variants were not registered at the Pompe Center or ClinVar. Thirteen of 22 were missense mutations and predicted as “probably damaging” for 10 of 13 and “benign” for three of 13 by PolyPhen-2. Eight of 22 were nonsense or frameshift mutations resulting in stop codons. The deletion mutant c.1320_1322del (p.M440del) was predicted to be “disease-causing” by the Mutation Taster. The first, second, and third most common mutations were c.1935C>A (p.D645E) (10.87%, 20/184), c.2238G>C (p.W746C) (9.24%, 17/184), and c.2662G>T (p.E888*) (8.15%, 15/184; Table 4). Among the 92 families, 80 were compound heterozygotes, and four were homozygotes. Eight families had one pathogenic variant and one unknown variant. Fifty-two and 40 of 92 families had histories of IOPD and LOPD, respectively.
Relationship of variants detected in the newborns and patients
Eleven of 50 variants, i.e., c.546G>T, c.752C>T+c.761C>T, c.796C>T, c.1124G>A, c.1562A>T, c.1669A>T, c.1857C>G, c.2238G>C, c.2560C>T, c.2132C>G, and c.2446G>A, were reported in patients with Pompe disease. The c.546G>T mutation, being the most common mutation in Japanese patients (19.53%, 25/128), was present in 2.82% (4/142) of the newborns in our study. The c.1798C>T mutation, the second most common mutation in Japanese patients (14.84%, 19/128), was not detected in our NBS. The c.1857C>G mutation, the third most common mutation in Japanese patients (11.72%, 15/128), was present in 0.70% (1/142) of the newborns in our study. The combination of c.752C>T+c.761C>T mutations, the most common mutation in newborns (14.08%, 20/142), was present in 1.56% (2/128) of Japanese patients. The c.2238G>C mutation, the second most common mutation in Taiwanese (7.87%, 17/216) and Chinese patients (9.24%, 17/184), was present in 0.70% (1/142) of the newborns in our study (Table 4).
Discussion
In this NBS program for Pompe disease, 225 out of 103,204 newborns were recalled for a second AαGlu assay, and 111 of these newborns were examined at the outpatient clinic. No patient with IOPD was detected, 40 of the 111 newborns were diagnosed as normal, and the other 71 newborns (0.07%, 1/1,453) who had low AαGlu activity were enrolled for further studies. Moreover, the AαGlu activity of fibroblasts in 32 patients was measured with the GAA gene sequence. The AαGlu assays in fibroblasts have been considered to be the gold standard for the diagnosis of patients with suspected Pompe disease [13], though this method is invasive and time-consuming. Our study demonstrated that the AαGlu activity in fibroblasts was variable even between newborns with pseudodeficiencies and the carrier, and the AαGlu activity in fibroblasts could not distinguish between individuals with and without Pompe disease. However, this method could be useful for the diagnosis of potential patients who have two or more mutation alleles, as shown in our study. Finally, we detected no IOPD and identified three newborns with potential LOPD in this study using the combination of the AαGlu assay in DBS and fibroblasts, and GAA gene analysis. Analysis of the GAA gene showed that 32 (45.1%) and 37 (52.1%) of the 71 were homozygous and heterozygous for the pseudodeficiency alleles c.[1726G>A; 2065G>A], respectively. The frequency of homozygous c.[1726G>A; 2065G>A] in newborns with low AαGlu activity was 11.6-times higher than that of normal newborns (3.9%) [10]. Additionally, 24 newborns (33.8%, 24/71) had no mutated alleles but harbored homozygous c.[1726G>A; 2065G>A], defined as a pseudodeficiency. The other eight had one mutation and the homozygous c.[1726G>A; 2065G>A] allele. Moreover, 35 (49.3%) had one mutation and the heterozygous c.[1726G>A; 2065G>A] allele and two (subject IDs 87 and 93) had two mutated alleles with heterozygous c.[1726G>A; 2065G>A]. One subject (subject ID 84) had two mutated alleles without c.[1726G>A; 2065G>A]. These results showed that the pseudodeficiency alleles c.[1726G>A; 2065G>A] significantly affected NBS owing to high recall and false-positive rates, suggesting that a combination of GAA gene sequencing and enzyme assays is necessary for NBS in Pompe disease. Interestingly, in newborns with pseudodeficiency alleles, 70 of 71 newborns had compound homozygous or heterozygous pseudodeficiency alleles c.[1726G>A; 2065G>A] in this study. Labrousse et al. [11] analyzed the GAA genes of 107 newborns, screened from an NBS of 132,538 newborns, and detected 54 types of variants. Thirty-six of 107 newborns (33.6%) had pseudodeficiency alleles c.[1726G>A; 2065G>A]. Additionally, in Taiwan, the pseudodeficiency alleles c.[1726G>A; 2065G>A] had a significant effect on NBS.
In our study, four newborns (subject IDs 8, 84, 87, and 93) were found to have two mutations (Table 2). Subject ID 8 was diagnosed as a carrier because normal AαGlu activity in fibroblasts was observed. Subject IDs 93 and 84 were confirmed as compound heterozygotes by sequencing their parents’ genes. The c.317G>A and c.752C>T+c.761C>T of subject ID 93 were derived from father and mother, respectively. The c.752C>T+c.761C>T and c.1244C>T of subject ID 84 were derived from mother and father, respectively. Unfortunately, informed consent was not approved in subject ID 87. In compliance with diagnosis guidelines [22, 23], we diagnosed these three subjects as potential LOPD patients. As of November 2018, these patients’ ages are 2 years 7 months (subject ID 93), 3 years (subject ID 87), and 3 years 1 month (subject ID 84), and no symptoms related to Pompe disease have been observed. The use of ERT in patients with pre-symptomatic LOPD is controversial. Although ERT is necessary and effective in patients with LOPD who exhibit symptoms related to Pompe disease, further studies are needed to determine whether ERT should be applied in pre-symptomatic patients with Pompe disease.
Bodamer et al. [24] summarized an NBS for Pompe disease in Taiwan (n = 473,738) and the USA (n = 980,507). The frequencies of IOPD and potential LOPD were 1/52,637 and 1/24,933 (Taiwan) and 1/122,563 and 1/15,563 (USA), respectively. In our study, the frequencies of IOPD and potential LOPD were estimated to be less than <1/103,204 and <1/34,401, respectively. Although further studies are needed to confirm these findings, the onset frequency of IOPD and LOPD is expected to be significantly lower in Japan than those of other countries.
A literature search revealed some of the characteristics of GAA mutation profiles in Asian patients with Pompe disease. Some typical mutations were found in patients from Europe and the USA. The c.525delT mutation (p.E176Rfs*45) in Dutch patients [25], was not detected in Asian patients. The c.2560C>T (p.R854*) mutation, which is detected in many affected African or African-American patients with IOPD [26], was rarely detected in Asian patients (one allele in a Chinese patient and one allele in this study). The c.-32-13T>G mutation, which has been detected in adult Caucasians [27], also was rarely detected in Asian patients (one allele in a Chinese patient).
The mutation profile of the GAA gene was different in each Asian country. In Japan, the first most common mutation, c.546G>T, is a leaky splice mutation that produces a normally spliced transcript and is responsible for the low expression level (approximately 10%) of the active enzyme [28]. This mutation may also correspond with the phenotype of LOPD. The c.1798C>T mutation, which was the second most common mutation in Japan, was previously reported as a common variant in Japanese patients [15]. The c.1935C>A mutation, the most common mutation in Taiwan, is known as a typical example of the founder effect, which was previously reported in Taiwanese patients [29]. The second most common mutation in Taiwan was c.2238G>C, and a patient with homozygous c.2238G>C alleles was previously reported in Taiwan [30]. The first and second most common variants were the same in Taiwan and China, potentially because Taiwan and China have a common ethnic background and because some Chinese groups have moved to Taiwan. The ratios of IOPD to LOPD (n/n) were 0.21 (11/53), 3.33 (10/3), 2.06 (68/33), and 1.49 (52/35) in Japan, Korea, Taiwan, and China, respectively. The onset frequency of IOPD in Japanese patients was significantly lower than those from Taiwan and China (one-tenth and one-seventh, respectively). This may be a characteristic of the Japanese population or a result of under-diagnosis of LOPD in the other countries. Moreover, the most common variant detected in patients with IOPD, and LOPD differed. In Japan, the most common variant in patients with LOPD (c.546G>T) was not detected in patients with IOPD. Similarly, the second most common variant in LOPD (c.2238G>C) was very rare in patients with IOPD in Taiwan and China. These findings suggested that c.546G>T and c.2238G>C may be characteristic variants in LOPD.
There was one limitation to our literature search, namely, that one mutation was detected, but other mutations were unknown in 26 of 277 families. The reason for this discrepancy may be related to the sequencing method used in these studies; indeed, in all 41 articles, exons and intron/exon boundaries of the GAA gene were amplified by PCR and sequenced. Theoretically, some mutations in the 5′, 3′, and intronic regions are not detectable. Thus, the use of NGS may increase the detection rate and generate more reliable results. Further studies are needed to evaluate the roles of patient ethnicity or characteristics.
The method for AαGlu activity assays with 4MU-αGlc used in this study could not effectively distinguish LOPD from pseudodeficiency or carriers, even if AαGlu activity was analyzed in fibroblasts. Some researchers have reported that liquid chromatography-tandem mass spectrometry (LC-MS/MS) is an effective method for AαGlu activity assays and can permit LOPD to be distinguished from pseudodeficiency or carriers [31, 32]. Therefore, in future studies, it will be necessary to develop a practical screening method using the combination of 4MU-αGlc and LC-MS/MS for analysis of DBSs (e.g., where LC-MS/MS is performed in the second and third AαGlu assays).
In conclusion, in this study, we performed NBS for Pompe disease in 103,204 newborns and detected three patients with potential LOPD, and no patients with IOPD. The GAA gene mutation locus, which we detected in the middle Kyusyu area and was reported from patients with Pompe disease in Japan and neighboring countries, was different. Therefore, there are fewer patients with IOPD in Japan than in neighboring countries, and the onset frequencies of IOPD and LOPD in Japan are different from those in the middle Kyusyu area in our study. These results may reflect the differences in genetic backgrounds between Japanese patients and those from other countries as shown by our literature study.
References
Martiniuk F, Mehler M, Pellicer A, Tzall S, La Badie G, Hobart C, et al. Isolation of a cDNA for human acid α-glucosidase and detection of genetic heterogeneity for mRNA in three α-glucosidase deficient patients. Proc Natl Acad Sci USA. 1986;83:9641–4.
Hagemans MLC, Winkel LPF, Hop WCJ, Reuser AJJ, Van Doorn PA, Van der Ploeg AT. Disease severity in children and adults with Pompe disease related to age and disease duration. Neurology. 2005;64:2139–41.
Kishnani PS, Corzo D, Nicolino M, Byrne B, Mandel H, Hwu WL, et al. Recombinant human acid alpha-glucosidase: major clinical benefits in infantile-onset Pompe disease. Neurology. 2007;68:99–109.
Van der Ploeg AT, Clemens PR, Corzo D, Escolar DM, Florence J, Groeneveld G, et al. A randomized study of alglucosidase alfa in late-onset Pompe's disease. N Engl J Med. 2010;15:1396–406.
Kishnani PS, Corzo D, Leslie ND, Gruskin D, Van der Ploeg A, Clancy JP, et al. Early treatment with alglucosidase alpha prolongs long-term survival of infants with Pompe disease. Pedia Res. 2009;66:329–35.
Prater SN, Banugaria SG, DeArmey SM, Botha EG, Stege EM, Case LE, et al. The emerging phenotype of long-term survivors with infantile Pompe disease. Genet Med. 2012;14:800–10.
Chien Y-H, Lee N-C, Thurberg BL, Chiang S-C, Zhang XK, Keutzer J, et al. Pompe Disease in Infants: improving the prognosis by newborn screening and early treatment. Pediatrics. 2009;124:e1116.
Yang C-F, Liu H-C, Hsu T-R, Tsai F-C, Chiang S-F, Chiang C-C, et al. A large-scale nationwide newborn screening program for Pompe disease in Taiwan: towards effective diagnosis and treatment. Am J Med Genet A. 2014;164A:54–61.
Kroos MA, Mullaart RA, Van Vliet L, Pomponio RJ, Amartino H, Kolodny EH, et al. p.[G576S; E689K]: pathogenic combination or polymorphism in Pompe disease? Eur J Hum Genet. 2008;16:875–9.
Kumamoto S, Katafuchi T, Nakamura K, Endo F, Oda E, Okuyama T, et al. High frequency of acid α-glucosidase pseudodeficiency complicates newborn screening for glycogen storage disease type II in the Japanese population. Mol Genet Metab. 2009;97:190–5.
Labrousse P, Chien Y-H, Pomponio RJ, Keutzer J, Lee N-C, Akmaev VR, et al. Genetic heterozygosity and pseudodeficiency in the Pompe disease newborn screening pilot program. Mol Genet Metab. 2010;99:379–83.
Shigeto S, Katafuchi T, Okuda Y, Nakamura K, Endo F, Okuyama T, et al. Improved assay for differential diagnosis between Pompe disease and acid α-glucosidase pseudodeficiency on dried blood spots. Mol Genet Metab. 2011;103:12–7.
Kishnani PS, Steiner RD, Bali D, Berger K, Byrne BJ, Case L, et al. Pompe disease diagnosis and management guideline. Genet Med. 2006;8:267–88.
Yoshida S. Genetic testing of lysosomal storage diseases by next-generation sequencer. Sci Rep Chemo-Sero-Ther Res Inst. 2015;24:14–31. https://www.kaketsuken.org/pdf/003-reimei-24.pdf
Tsujino S, Huie M, Kanazawa N, Sugie H, Goto Y, Kawai M, et al. Frequent mutations in Japanese patients with acid maltase deficiency. Neuromuscul Disord. 2000;10:599–603.
Landrum MJ, Lee JM, Benson M, Brown G, Chao C, Chitipiralla S, et al. ClinVar: public archive of interpretations of clinically relevant variants. Nucleic Acids Res. 2016;44:D862–D868.
Adzhubei I, Jordan DM, Sunyaev SR. Predicting functional effect of human missense mutations using PolyPhen-2. Curr Protoc Hum Genet. 2013;76:7.20.1–41.
Desmet F-O, Hamroun D, Lalande M, Collod-Beroud G, Claustres M, Beroud C. Human splicing finder: an online bioinformatics tool to predict splicing signals. Nucleic Acids Res. 2009;37:e67.
Schwarz JM, Cooper DN, Schuelke M, Seelow D. MutationTaster2: mutation prediction for the deep-sequencing age. Nat Methods. 2014;11:361–2.
Hijikata A, Raju R, Keerthikumar S, et al. Mutation@A Glance: an integrative Web application for analyzing mutations from human genetic diseases. DNA Res. 2010;17:197–208.
Qiu J-J, Wei M, Zhang W-M, Shi H-P. Clinical and molecular genetic study on two patients of the juvenile form of Pompe disease in China. Chin J Pedia. 2007;45:760–4.
Eto Y ed. Pompe disease (glycogen storage disease type II) diagnosis and management guideline for Japanese (revised edition). (2013) (En Medix Co. Ltd, Tokyo, Japan).
Tarnopolsky M, Katzberg H, Petrof BJ, Sirrs S, Sarnat HB, Myers K, et al. Pompe disease: diagnosis and management. Evidence-based guidelines from a Canadian expert panel. Can J Neurol Sci. 2016;43:472–85.
Bodamer OA, Scott R, Giugliani R. Newborn screening for Pompe disease. Pediatrics. 2017;140:S4–13.
Ausems MGEM, Verbiest J, Hermans MMP, Kroos MA, Beemer FA, Wokke JHJ, et al. Frequency of glycogen storage disease type II in the Netherlands: implications for diagnosis and genetic counseling. Eur J Hum Genet. 1999;7:713–6.
Becker JA, Vlach J, Raben N, Nagaraju K, Adams EM, Hermans MM, et al. The African origin of the common mutation in African American patients with glycogen-storage disease type II. Am J Hum Genet. 1998;62:991–4.
Huie ML, Chen AS, Tsujino S, Shanske S, DiMauro S, Engel AG, et al. Aberrant splicing in adult-onset glycogen storage disease type II (GSDII): molecular identification of an IVS1 (-13T->G) mutation in a majority of patients and a novel IVS10 (+1GT->CT) mutation. Hum Mol Genet. 1994;3:2231–6.
Tsuburaya RS, Monma K, Oya Y, Nakayama T, Fukuda T, Sugie H, et al. Acid phosphatase-positive globular inclusions is a good diagnostic marker for two patients with adult-onset Pompe disease lacking disease-specific pathology. Neuromuscul Disord. 2012;22:389–93.
Shieh J-J, Lin C-Y. Frequent mutation of Chinese patients with infantile type of GSD II in Taiwan: evidence for a founder effect. Hum Mutat. 1998;11:306–12.
Wan L, Lee C-C, Hsu C-M, Hwu W-L, Yang C-C, Tsai C-H, Tsai F-J. Identification of eight novel mutations of the acid α-glucosidase gene causing the infantile or juvenile form of glycogen storage disease type II. J Neurol. 2008;255:831–8.
Mashima R, Sakai E, Kosuga M, Okuyama T. Levels of enzyme activities in six lysosomal storage diseases in Japanese neonates determined by liquid chromatography-tandem mass spectrometry. Mol Genet Metab Rep. 2016;9:6–11.
Lin N, Huang J, Violante S, Orsini JJ, Caggana M, Hughes EE, et al. Liquid chromatography-tandem mass spectrometry assay of leukocyte acid α-glucosidase for post-newborn screening evaluation of Pompe disease. Clin Chem. 2017;63:842–51.
Acknowledgements
This study was supported in part by a Grant-in-Aid for Research on Rare and Intractable Diseases, Health and Labor Sciences Research; a Grant-in-Aid for Pediatric Research from the Ministry of Health, Labor and Welfare; and a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology. We are grateful to Ms. Fumiko Nozaki, Ms. Naomi Yano, Ms. Ayako Tateishi, Ms. Emi Harakawa, Ms. Yasuyo Sakamoto, and Ms. Matsumi Harada for their technical support in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the Ethics Committee of Kumamoto University (approval no. 1537). Written informed consent was obtained from the parents or legal guardians of newborns.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Momosaki, K., Kido, J., Yoshida, S. et al. Newborn screening for Pompe disease in Japan: report and literature review of mutations in the GAA gene in Japanese and Asian patients. J Hum Genet 64, 741–755 (2019). https://doi.org/10.1038/s10038-019-0603-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s10038-019-0603-7
This article is cited by
-
Global carrier frequency and predicted genetic prevalence of patients with pathogenic sequence variants in autosomal recessive genetic neuromuscular diseases
Scientific Reports (2024)
-
Current status of newborn screening for Pompe disease in Japan
Orphanet Journal of Rare Diseases (2021)
-
Molecular study of Pompe disease in Egyptian infants
Egyptian Journal of Medical Human Genetics (2021)
-
Phenotypic implications of pathogenic variant types in Pompe disease
Journal of Human Genetics (2021)
-
Long-Term Observation of the Safety and Effectiveness of Enzyme Replacement Therapy in Japanese Patients with Pompe Disease: Results From the Post-marketing Surveillance
Neurology and Therapy (2019)