Abstract
Clinical criteria for genetic testing of genes other than BRCA1/2 in epithelial ovarian cancer (EOC) still do not exist. We assessed the frequency and predictors of deleterious mutations in 19 cancer predisposition genes in high-grade serous ovarian cancer (HGSOC) in Serbia. Next-generation sequencing was used to identify germline mutations in the whole coding regions of a gene panel. Patients’ characteristics and sequencing data were summarized with descriptive statistics and compared using chi-square test. Among 131 HGSOC patients, 23 had BRCA1 (17.6%) while 5 had BRCA2 (3.8%) mutation. In addition, 9 (6.9%) pathogenic mutations were detected in other genes including BRIP1 (n = 2;1.5%), CHEK2 (n = 2;1.5%), NBN (n = 3;2.3%) and RAD51C (n = 2;1.5%). Factors that predicted for BRCA1/2 mutations were: breast and ovarian cancers in the same patient (p = 0.031), young age of EOC (p = 0.029), menstrual status (p = 0.004) and family history of cancer (p < 0.0001). However, these factors did not predict for mutations in other cancer susceptibility genes. Applying established referral criteria for genetic testing in Serbia will help identify BRCA1/2 mutation carriers but will not help identify mutations in other cancer susceptibility genes. Until better predictors emerge we should be performing wider genetic testing of EOC in order to identify all mutation carriers.
Similar content being viewed by others
Introduction
Ovarian cancer is the fifth most common cause of cancer-related deaths even though it accounts for only 3 percent of cancers in women [1, 2]. Approximately 15% of patients are presented with disease confined to the ovaries with 5-year survival being more than 90% after the surgery. A 5-year survival among patients with advanced disease (FIGO stage III–IV) is <30% [3]. The most frequent type of ovarian cancer is epithelial ovarian cancer (EOC) (85–95%) [1] with four main histological types: serous, endometrioid, mucinous and clear cell. Almost 70% of all epithelial tumors are aggressive high-grade serous carcinomas and present in advanced stages [4].
EOC is predominantly a disease of postmenopausal women and occurs usually after the age of 50 [5]. The vast majority of newly diagnosed ovarian cancers in Serbia, Vojvodina and South Great Plain, Hungary are patients with advanced disease stages, epithelial serous subtype, mostly found in women over 50 years [6]. One of the major risk factors for OC is family history and associated genetic syndromes that may indicate hereditary predisposition. About 23% of OCs have been related to hereditary conditions, and in about 65-85% of those cases the genetic change is pathogenic germline mutation in BRCA1/2 genes [7, 8]. However, more than 15% of hereditary OCs are derived from genetic conditions unrelated to BRCA genes [8]. It has been shown that additional mutations have been distributed in a larger number of other tumor suppressor genes such as RAD51C, RAD51D, BRIP1, CHEK2, NBN, ATM… [4, 8, 9].
Family history is still used as the main criterion in the models for calculation of BRCA1/2 carrier probability [10,11,12]. In many European countries, referral for genetic counseling and subsequent germline DNA testing is mainly based on the age of EOC diagnosis and family history. However, wider testing unrelated to these criteria in recent years showed that restricting testing to cases with a family history of breast or ovarian cancer results in 8-54% of mutation carriers being undetected [8, 13,14,15]. However, criteria for testing other, lower penetrance genes still do not exist since predictive factors have not yet been identified and clinical utility for most of these genes is still being evaluated.
In this study, we determined the frequencies of mutations in the panel of 19 cancer predisposition genes recommended by National Comprehensive Cancer Network (NCCN) in Serbian EOC patients [16]. Our goals were to better understand the contribution of inherited mutations in high- and moderate- penetrance genes in EOC in Serbia, and to evaluate any factors that predict for mutations in these genes. The aim was also to reevaluate the existing selection criteria for genetic testing in Serbia and to define a population specific subpanel that should be offered to patients with EOC in this Slavic population.
Methods
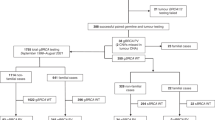
Patients: All 131 women diagnosed with EOC with the referral for genetic counseling at the Institute for Oncology and Radiology of Serbia (IORS) between May 2016 and May 2018, were included in this study. Personal and family cancer histories were obtained during pre-test genetic counseling. Clinical and pathologic data were abstracted from patients’ medical records. Besides the patients with EOC and no previous medical history of other cancers, women with bilateral disease and those with both breast and ovarian cancers were also included in the study with the aim to investigate the mutational spectra in these types of EOC as well. All patients consented for clinical research and signed informed consent for genetic testing approved by the Ethics Committee of the IORS.
NGS assay: DNA was extracted from whole blood on ABI PrismTM 6100 Nucleic Acid PrepStation using Blood-Prep Kit (Applied Biosystems, Foster City, CA) according to the manufacturer’s instructions. The coding sequences and exon/intron boundaries were enriched using Nextera DNA Library Preparation Kit in combination with TruSight® Cancer Panel (Illumina, San Diego, USA). Next-generation sequencing (NGS) was performed on Illumina MiSeq Sequencing System (Illumina) according to the manufacturer’s protocol. All clinically actionable mutations identified by NGS, as well as regions that did not meet our NGS quality metrics (minimum of 50x coverage per region), were independently sequenced and confirmed with site-specific Sanger sequencing. Secondary data analysis and base calling were performed by MiSeq Reporter Software 2.5.1 (Illumina). VCF v4.1 files generated during secondary analysis of sequencing data were imported into Illumina Variant Studio software for variant annotation and filtering.
Variant annotation: Online databases were used in order to evaluate functional significance of genetic variations: population databases (Exome Aggregation Consortium- ExAC, The Genome Aggregation Database and dbSNP), disease and locus-specific databases (ClinVar, Human Gene Mutation Database-HGMD, Leiden Open Variation Database-LOVD and BRCA Share) and sequence databases (RefSeqGene, NCBI Genome and Locus Reference Genomic-LRG). Literature search using Google scholar was also performed. In silico tools: SIFT, Polyphen2 and Align GVGD were used as a source of supporting evidence for variant annotation. Human Splice Finder 3.0 was used to identify splice-site variants most likely to affect gene splicing. To confirm and visually inspect the presence of deleterious variants we used Integrative Genomic Viewer (IGV).
Nonsense mutations, frameshift indels and splice-site mutations were defined as deleterious in case those were predicted to result in protein truncation. Missense variations were carefully analyzed for correct classification using ACMG and AMP guidelines [17]. 28 different criteria including population data, computational and predictive data, functional data, segregation data and allelic data were evaluated for every missense mutation. The accumulated criteria were than compared to rule classifications table and some of the missense mutations were further classified as deleterious (Appendix Table A1). In case there was insufficient evidence for classification, missense variations were classified as variants of uncertain significance VUS [17] (Appendix Table A2).
Statistics: Patients’ characteristics and sequencing data were summarized with descriptive statistics including medians, means and standard deviations for continuous data. 95% confidence intervals (CIs) were calculated for proportions, clinical and pathologic characteristics were compared using chi-square test (χ2) and p values < 0.05 were considered significant.
Results
This study included 131 women with EOC who were sent to Genetic Counseling Service and Laboratory for Molecular Genetics at IORS for genetic counseling and genetic testing. 4 women had bilateral ovarian cancer while 9 had both ovarian and breast cancers. None of the patients were of Ashkenazi descent. Clinical and tumor pathologic features for patients are provided in Table 1. Blood samples were taken and DNA was successfully isolated and analyzed for the presence of genetic variations in the panel of 19 genes (BRCA1, BRCA2, ATM, BRIP1, CDH1, CHEK2, MSH2, MLH1, MSH6, PMS2, EPCAM, NBN, NF1, PALB2, PTEN, RAD51C, RAD51D, STK11 and TP53) (Table 2). The mean age of OC diagnosis was 54.8 years (range, 22–76 years). All women had high-grade serous ovarian cancer (HGSOC) and the majority had stage III or IV of disease (93.1%). In all, 20.6% of patients presented with distant metastasis that included liver, lung, breast and skin. Further, 19.1% of patients reported having at least one first or second-degree relative diagnosed with breast or ovarian cancer, while 13.7% had at least one relative diagnosed with breast or ovarian cancer before the age of 50.
Among 131 HGSOC patients, 37 (28.2%) deleterious mutations in cancer predisposition genes were identified overall. 28 (21.4%) women had germline BRCA1/2 mutation, 23 in BRCA1 and 5 in BRCA2. In addition, 9 (6.9%) deleterious germline mutations were detected in non-BRCA1/2 predisposition genes including BRIP1 (n = 2; 1.5%), CHEK2 (n = 2; 1.5%), NBN (n = 3; 2.3%) and RAD51C (n = 2; 1.5%) (Table 2). One NBN deleterious mutation was identified in a woman who was also a carrier of CHEK2 mutation. In addition, one patient had deleterious mutations in both BRCA1 and NBN genes. No deleterious mutations were detected in ATM, CDH1, MSH2, MLH1, MSH6, PMS2, EPCAM, NF1, PALB2, PTEN, RAD51D, STK11 and TP53. Specific deleterious mutations identified and associated patients’ characteristics are provided in Appendix Table A1. Frameshift mutation c.4356delA (p.Ala1453GlnTer3) in BRCA1 was previously reported for the first time by our group in two unrelated ovarian cancer patients from Serbia and Slovenia [18].
Besides deleterious mutations, 18 (13.7%) variants of uncertain significance (VUS) were also detected. The most frequent ones were in ATM (n = 4) and PALB2 (n = 3). Two VUS were detected in BRCA2, BRIP1, PMS2, MLH1 and NBN while one of each was detected in CHEK2, MSH2 and RAD51D. Only one patient with VUS in PALB2 also had a deleterious mutation in BRCA1. VUS identified are listed in Appendix Table A2.
The prevalence of BRCA1 deleterious mutations was highest among patients who were diagnosed with OC before the age of 45. The number of mutations decreased with age with a frequency of 38.4%, 16.4 and 8% for women diagnosed at age ≤ 45 years, 46 to 60 years and older than 60 years, respectively (Table 3, Fig. 1). BRCA2 mutations were the most prevalent in the patients who were diagnosed with OC in the age range from 46 to 60 years (7.3%). The frequency of mutations in genes other than BRCA1 and BRCA2 for the patients older than 45 ranged from 1.8 to 6%, while no deleterious mutations in other genes were detected in patients diagnosed with OC under the age of 45 (Table 3, Fig. 1). Among patients who had OC before the age of 45 the only mutations that we found were in BRCA1 gene (Fig. 1).
Association between mutation carrier status and family history of breast and ovarian cancers was evaluated. 12 BRCA1 mutations (11.3%) were detected in patients who had no family history of breast or ovarian cancers (n = 106) while 11 (44%) mutations were detected among those who had family history of these cancers (n = 25). A total of 5 patients had only ovarian cancers in their families, 4 had only breast cancers and 2 mutations were detected in patients who had both breast and ovarian cancers in their families. Of 5 BRCA2 mutations, only one was detected in a patient who had breast cancer in her family. Other deleterious mutations in BRIP1, CHEK2, NBN and RAD51C were found only in cases with no family history (Table 4, Fig. 1).
Table 5 shows clinical and pathologic predictors of germline mutations in BRCA1/2 and other cancer predisposition genes detected in our cohort. Factors that significantly predicted for BRCA1/2 mutations were the existence of both breast and ovarian cancers in the same patient (p = 0.031), age of OC diagnosis (p = 0.029), menstrual status (p = 0.004) and family history of cancer (p = 0.002). The most significant association was between BRCA1/2 mutations and family history of breast or ovarian cancers diagnosed < 50 years among first/second-degree relatives (p < 0.0001). When other genes were analyzed as a single group, no factors predicted for deleterious mutations.
Discussion
Identification of mutation status in epithelial ovarian cancer (EOC) has both prognostic and predictive importance. It was shown that BRCA1/2 mutation carriers with EOC have longer progression-free (PFS) and overall survival (OS) compared to non-carriers [19, 20]. The identification of mutations in OC associated genes may guide treatment decisions since some of the mutations may be strong predictors of response rate, PFS and OS for some therapies [21]. BRCA1/2 mutation carriers have higher sensitivity both to platinum chemotherapy [22] and poly (ADP-ribose) polymerase (PARP) inhibitors [23, 24].
Most of the European countries still struggle to define adequate criteria for genetic testing of EOC. There is also a difficulty in defining specific gene panel for EOC that will enable balancing clinical utility with cost effectiveness of genetic testing. In that manner, analysis of frequency of deleterious mutations within relevant predisposition genes correlated with specific patients’ characteristics may have significant contribution. Thus, we aimed to identify predictors for deleterious mutations, to narrow down the criteria for genetic testing and to define gene panel that should be offered for genetic testing of EOC in Serbia. This is the first study reporting germline sequence variations in BRCA1/2 and other cancer predisposition genes in high-grade serous ovarian cancer (HGSOC) patients from Serbia. We found that 21.4% of HGSOC patients had germline BRCA1/2 deleterious mutations and an additional 6.9% had a mutation in other cancer predisposition genes. In total, 28.2% of patients had a deleterious mutation in at least one of the cancer predisposition genes. The overall frequency of germline BRCA1/2 mutations in OC considerably differs between previously published data. The reason for this discrepancy probably lies within the differences between genetic structures of the investigated populations, inclusion criteria or different technologies used for genetic analyses. Taken together, data shows that germline BRCA1/2 mutations are found in 10-15% of women with EOC unselected for other criteria [5, 25]. The results of our study are in accordance with those who reported that, for the patients with HGSOC, BRCA1/2 mutation frequency rises up to even 22% [26, 27].
Mutations in other cancer susceptibility genes such as RAD51C, RAD51D and BRIP1, account for additional 2.5% of unselected ovarian cancer cases [8, 25]. Verifying that deficiencies in homologous recombination pathway are central in ovarian carcinogenesis, high frequency of mutations was also reported in genes including CHEK2, ATM, NBN, PALB2, RAD50 and MRE11A [28]. The most frequent deleterious mutations after BRCA1 and BRCA2 in our study were found in NBN (3/131, 2.3%). This gene has been described as moderately penetrant for breast cancer, but most data on its penetrability and associations was obtained for specific 657del5, mutation frequently found in people of Slavic origin [29, 30]. Two out of three mutations we detected in NBN were precisely 657del5 mutations which was somewhat expected since Serbian population is of Slavic origin. Even though it was previously reported that NBN is unlikely to contribute substantially to OC risk [9] this does not change the fact that it accounts for a proportion of EOC cases in Serbia. Besides NBN, we also detected deleterious mutations in BRIP1, RAD51C and CHEK2 (1.9% for each gene). BRIP1 was previously shown to confer 2.6 times higher risk for OC [9] while RAD51C confers even up to 5 time higher risk for OC [31]. We previously showed the lack of deleterious mutations in RAD51C in hereditary breast cancer in Serbia [32], but current results encourage us to make further efforts in defining RAD51C mutation spectra in OC in this population. There are conflicting reports regarding CHEK2 and its association with OC. Some studies report that there is no significant contribution [33] while others say that common variants at 22q12.1 are in fact associated with risk of serous OC which puts CHEK2 as a plausible target susceptibility gene [34]. We detected two missense I157T CHEK2 mutations in our cohort. This missense mutation was associated with increased breast cancer risk among Finnish, Polish and German populations with a frequency of 2.2%-7.4% [35, 36]. Interestingly, this particular mutation was at first associated with ovarian cystadenomas, borderline ovarian tumors, and low-grade invasive cancers but not high-grade OC [37]. Our study implies that the association of this missense mutation in CHEK2 with HGSOC pathogenesis should be further investigated.
Two patients in our cohort had more than one pathogenic mutation. Patient with both CHEK2 and NBN mutations (missense and frameshift respectively) was diagnosed with stage IV of EOC in the age of 63 and had no previous family history of malignant diseases. Patient with both BRCA1 and NBN mutations (nonsense and splice-site respectively) had similar characteristics. She was diagnosed with stage III EOC in the age of 69 and had no previous family history of malignant diseases. We compared the phenotypes of these patients with those who had only one mutation in either CHEK2 or NBN. There were no apparent phenotypic differences between the patient that had only one CHEK2 missense mutation and the patient who had additional NBN frameshift mutation. The only difference was that the patient with CHEK2 only mutation had slightly earlier age of onset of EOC (in the age of 49). We had similar results comparing patient with BRCA1 and NBN mutations and the patient with only NBN mutation. Patient with frameshift NBN mutation only, was diagnosed with stage III OC in the age of 74 and had no family history of malignant diseases. In all of these cases, it seems that additional NBN mutation did not have significant effect on the age of disease onset. However, in order to investigate the precise role of NBN as the modifier of penetrability it will be necessary to collect more cases with these rare, specific genotypes and the same mutation types so proper conclusions can be drawn.
Family history of breast and/or ovarian cancers is a well-established criterion for genetic testing. It is shown that the prevalence of BRCA1/2 mutations is higher among those who have affected relatives (mean probability 26.4% (95% CI 20.5-32.3)) [5]. On the other hand, it has been reported that the mean probability of finding germline BRCA1/2 mutations in OC patients without a positive family history is significantly lower- 6.2% (95% CI 3.2–9.1) [38]. Personal medical history also plays important role in this calculation. It was shown in the studies by Pal et al. [39] and Alsop et al. [22] that there is a significant association of carrier probability with personal history of breast cancer. Personal medical history predicted for BRCA1/2 mutations in our study as well (p = 0.031), showing higher frequency of mutations among those who had either bilateral disease or both ovarian and breast cancers compared to those who have been diagnosed with ovarian cancer only. This result was similar with the previously published data indicating bilateral disease and the existence of breast cancer together with ovarian cancer as important criteria for genetic testing [39, 40]. However, this was not the case for mutations in other genes in our study (p = 0.401).
Besides personal medical history, family history was a strong predictor for BRCA1/2 carrier probability in our study as well. Those patients that had at least one first or second-degree relative with breast or ovarian cancer and those patients with at least one first or second-degree relative < 50 years with breast or ovarian cancer had higher frequency of BRCA1/2 mutations compared to those without family history (p = 0.0007, p < 0.0001 respectively). Of 12 mutations detected among these patients, 11 (91.7%) were in BRCA1, while only 1 (8.3%) was in BRCA2. All patients with positive family history and detected BRCA1/2 mutations came from families with at least one first or second-degree relative diagnosed with breast or ovarian cancer before the age of 50. Even though these results somewhat concur with previous reports, our study also shows that negative family history will not safely exclude all germline BRCA1/2 mutations and that more than 10% of BRCA1/2 mutation carriers would not be identified if this criterion would have been strictly applied. In addition, all mutations we detected in other genes were found in patients that had no family history of cancers. Thus, family history should still be the indicator of mutation carrier probability in HGSOC in Serbia but it should not be a restricting factor for including patients in genetic screening.
Age of onset of OC is an important criterion for genetic counseling and testing. It was shown that the highest probability of finding germline BRCA1/2 mutations was in OC patients diagnosed with the disease between the ages 40 and 50 (mean 19.7% (95% CI 15.1-24.3)) followed by the age group between 50 and 60 (mean 14.8% (95% CI 7.8-21.7)). In women with OC and younger than 40, mutations were less frequent (10% (95% CI 3.2-16.9)). The frequency was less than 10% for those older than 60 [8, 41, 42]. In our study cohort, on the other hand, patients who were younger than 45 had the highest number of deleterious mutations. All mutations that we detected in this age group were found in BRCA1 (38.4% (95% CI 20.2–59.4)). Frequency of BRCA1 mutations decreased with the age in the groups 45-60 and more than 60 years (16.4 and 8% respectively). BRCA2 mutations were the most frequent in the 45-60 years age group- 7.3% (95%CI 2.0–17.6), which indicates that BRCA2 mutation carriers hardly differ from women with sporadic OC. Mutations in other genes were found only in OC patients older than 60 years of age in our cohort. Our results show that the age criterion is important for predicting BRCA1 mutations in HGSOC. However, this criterion, as well as family history, should only be taken as indicator of carrier probability since its strict application will result in not identifying germline BRCA2 mutations as well as mutations in other OC susceptibility genes such as BRIP1, NBN, RAD51C and CHEK2. We also found more mutations in BRCA1/2 genes in premenopausal women (p = 0.004) which was in accordance with the younger age of the mutation carriers.
In conclusion, applying the existent referral criteria for genetic testing such as the age of onset and personal/family history of breast and ovarian cancers will help to identify potential BRCA1/2 mutation carriers in HGSOC in Serbia. However, restricting genetic testing to those who fulfill these criteria might result in not identifying a subset of germline BRCA2 mutation carriers as well as carriers of mutations in other OC susceptibility genes (BRIP1, NBN, RAD51C and CHEK2). The lack of predictive factors for mutations in other cancer susceptibility genes presents a challenge in identifying these carriers in OC in Serbia. Until better predictors emerge, the results of our study show that we should be more careful in defining criteria for genetic testing and that it will be necessary to continue performing wider genetic testing of OC, outside these criteria, in order to define population specific gene panel. Future studies should be focused on the clinical follow up of these patients in order to identify the value of detected genetic variations in terms of disease prognosis and prediction to therapy.
References
Roett MA, Evans P. Ovarian cancer: an overview. Am Fam Physician. 2009;80:609–16.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30.
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49.
Toss A, Tomasello C, Razzaboni E, Contu G, Grandi G, Cagnacci A, et al. Hereditary ovarian cancer: not only BRCA 1 and 2 genes. Biomed Res Int. 2015;2015:341723.
Arts-De Jong M, De Bock GH, Van Asperen CJ, Mourits MJE, De Hullu JA, Kets CM. Germline BRCA1/2 mutation testing is indicated in every patient with epithelial ovarian cancer: a systematic review. Eur J Cancer. 2016;61:137–45.
Aljoša Mandić, László Thurzó, Dejan Ninčić, Milica Živaljević, Tihomir Dugandžija, Róbert Berkecz. Epidemiological data of ovarian cancer in Vojvodina and South Great Plain region in Hungary in 2007-2012 period: CrossBiomark IPA PROJECT HUSRB/1203/214/091. Arch Oncol. 2013;21:97–100.
Novaković S, Milatović M, Cerkovnik P, Stegel V, Krajc M, Hočevar M, et al. Novel BRCA1 and BRCA2 pathogenic mutations in Slovene hereditary breast and ovarian cancer families. Int J Oncol. 2012;41:1619–27.
Walsh T, Casadei S, Lee MK, Pennil CC, Nord AS, Thornton AM, et al. Mutations in 12 genes for inherited ovarian, fallopian tube, and peritoneal carcinoma identified by massively parallel sequencing. Proc Natl Acad Sci. 2011;108:18032–7.
Ramus SJ, Song H, Dicks E, Tyrer JP, Rosenthal AN, Intermaggio MP, et al. Germline mutations in the BRIP1, BARD1, PALB2, and NBN genes in women with ovarian cancer. J Natl Cancer Inst. 2015;107:1–8.
Berry DA, Iversen ES, Gudbjartsson DF, Hiller EH, Garber JE, Peshkin BN, et al. BRCAPRO validation, sensitivity of genetic testing of BRCA1/BRCA2, and prevalence of other breast cancer susceptibility genes. J Clin Oncol. 2002;20:2701–12.
Antoniou AC, Pharoah PPD, Smith P, Easton DF. The BOADICEA model of genetic susceptibility to breast and ovarian cancer. Br J Cancer. 2004;91:1580–90.
Evans DGR, Eccles DM, Rahman N, Young K, Bulman M, Amir E, et al. A new scoring system for the chances of identifying a BRCA1/2 mutation outperforms existing models including BRCAPRO. J Med Genet. 2004;41:474–80.
Malander S, Ridderheim M, Måsbäck A, Loman N, Kristoffersson U, Olsson H, et al. One in 10 ovarian cancer patients carry germ line BRCA1 or BRCA2 mutations: results of a prospective study in Southern Sweden. Eur J Cancer. 2004;40:422–8.
Soegaard M, Kjaer SK, Cox M, Wozniak E, Hogdall E, Hogdall C, et al. BRCA1 and BRCA2 mutation prevalence and clinical characteristics of a population-based series of ovarian cancer cases from denmark. Clin Cancer Res. 2008;14:3761–7.
Eccles DM, Balmaña J, Clune J, Ehlken B, Gohlke A, Hirst C, et al. Selecting Patients with ovarian cancer for germline BRCA mutation testing: findings from guidelines and a systematic literature review. Adv Ther. 2016;33:129–50.
NCCN. Evidence-based cancer guidelines, oncology drug compendium, oncology continuing medical education. https://www.nccn.org/. Accessed 10 Jul 2018.
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J., et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–24.
Krivokuca A, Dragos VS, Stamatovic L, Blatnik A, Boljevic I, Stegel V., et al. Novel BRCA1 splice-site mutation in ovarian cancer patients of Slavic origin. Fam Cancer. 2017;17:179–85.
Bolton KL, Chenevix-Trench G, Goh C, Sadetzki S, Ramus SJ, Karlan BY, et al. Association between BRCA1 and BRCA2 mutations and survival in women with invasive epithelial ovarian cancer. JAMA. 2012;307:382.
Candido-dos-Reis FJ, Song H, Goode EL, Cunningham JM, Fridley BL, Larson MC, et al. Germline mutation in BRCA1 or BRCA2 and ten-year survival for women diagnosed with epithelial ovarian cancer. Clin Cancer Res. 2015;21:652–7.
George A, Kaye S, Banerjee S. Delivering widespread BRCA testing and PARP inhibition to patients with ovarian cancer. Nat Rev Clin Oncol. 2017;14:284–96.
Alsop K, Fereday S, Meldrum C, deFazio A, Emmanuel C, George J, et al. BRCA mutation frequency and patterns of treatment response in brca mutation–positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J Clin Oncol. 2012;30:2654–63.
Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014;15:852–61.
Fong PC, Yap TA, Boss DS, Carden CP, Mergui-Roelvink M, Gourley C, et al. Poly(ADP)-ribose polymerase inhibition: frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. J Clin Oncol. 2010;28:2512–9.
Norquist BM, Harrell MI, Brady MF, Walsh T, Lee MK, Gulsuner S, et al. Inherited mutations in women with ovarian carcinoma. JAMA Oncol. 2016;2:482.
Zhang S, Royer R, Li S, McLaughlin JR, Rosen B, Risch HA, et al. Frequencies of BRCA1 and BRCA2 mutations among 1,342 unselected patients with invasive ovarian cancer. Gynecol Oncol. 2011;121:353–7.
Bell D, Berchuck A, Birrer M, Chien J, Cramer DW, Dao F, et al. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–15.
Minion LE, Dolinsky JS, Chase DM, Dunlop CL, Chao EC, Monk BJ. Hereditary predisposition to ovarian cancer, looking beyond BRCA1/BRCA2. Gynecol Oncol. 2015;137:86–92.
Ziółkowska I, Mosor M, Nowak J. Regional distribution of heterozygous 657del5 mutation carriers of theNBS1 gene in Wielkopolska province (Poland). J Appl Genet. 2006;47:269–72.
Górski B, Dębniak T, Masojć B, Mierzejewski M, Mędrek K, Cybulski C, et al. Germline 657del5 mutation in the NBS1 gene in breast cancer patients. Int J Cancer. 2003;106:379–81.
Coulet F, Fajac A, Colas C, Eyries M, Dion-Minière A, Rouzier R, et al. Germline RAD51C mutations in ovarian cancer susceptibility. Clin Genet. 2013;83:332–6.
Krivokuca A, Yanowski K, Rakobradovic J, Benitez J, Brankovic-Magic M. RAD51C mutation screening in high-risk patients from Serbian hereditary breast/ovarian cancer families. Cancer Biomark. 2015;15:775–81.
Baysal BE, DeLoia JA, Willett-Brozick JE, Goodman MT, Brady MF, Modugno F, et al. Analysis of CHEK2 gene for ovarian cancer susceptibility. Gynecol Oncol. 2004;95:62–9.
Lawrenson K, Iversen ES, Tyrer J, Weber RP, Concannon P, Hazelett DJ, et al. Common variants at the CHEK2 gene locus and risk of epithelial ovarian cancer. Carcinogenesis. 2015;36:1341–53.
Cybulski C, Górski B, Huzarski T, Byrski T, Gronwald J, Debniak T, et al. Effect of CHEK2 missense variant I157T on the risk of breast cancer in carriers of other CHEK2 or BRCA1 mutations. J Med Genet. 2009;46:132–5.
Bogdanova N, Enβen-Dubrowinskaja N, Feshchenko S, Lazjuk GI, Rogov YI, Dammann O, et al. Association of two mutations in theCHEK2 gene with breast cancer. Int J Cancer. 2005;116:263–6.
Szymanska-Pasternak J, Szymanska A, Medrek K, Imyanitov EN, Cybulski C, Gorski B, et al. CHEK2 variants predispose to benign, borderline and low-grade invasive ovarian tumors. Gynecol Oncol. 2006;102:429–31. S
Vicus D, Finch A, Cass I, Rosen B, Murphy J, Fan I, et al. Prevalence of BRCA1 and BRCA2 germ line mutations among women with carcinoma of the fallopian tube. Gynecol Oncol. 2010;118:299–302.
Pal T, Permuth-Wey J, Betts JA, Krischer JP, Fiorica J, Arango H, et al. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer . 2005;104:2807–16.
Alsop K, Fereday S, Meldrum C, deFazio A, Emmanuel C, George J, et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J Clin Oncol. 2012;30:2654–63.
Janavičius R. Founder BRCA1/2 mutations in theEurope: implications for hereditary breast-ovarian cancer prevention and control. EPMA . 2010;1:397–412.
Song H, Cicek MS, Dicks E, Harrington P, Ramus SJ, Cunningham JM, et al. The contribution of deleterious germline mutations in BRCA1, BRCA2 and the mismatch repair genes to ovarian cancer in the population. Hum Mol Genet. 2014;23:4703–9.
Loveday C, Turnbull C, Ramsay E, Hughes D, Ruark E, Frankum JR, et al. Germline mutations in RAD51D confer susceptibility to ovarian cancer. Nat Genet. 2011;43:879–82.
Bonadona V, Bonaïti B, Olschwang S, Grandjouan S, Huiart L, Longy M, et al. Cancer Risks Associated With Germline Mutations in MLH1, MSH2, and MSH6 Genes in Lynch Syndrome. JAMA. 2011;305:2304.
Hearle N, Schumacher V, Menko FH, Olschwang S, Boardman LA, Gille JJP, et al. Frequency and Spectrum of Cancers in the Peutz-Jeghers Syndrome. Clin Cancer Res. 2006;12:3209–15.
Mai PL, Best AF, Peters JA, DeCastro RM, Khincha PP, Loud JT, et al. Risks of first and subsequent cancers among TP53 mutation carriers in the National Cancer Institute Li-Fraumeni syndrome cohort. Cancer. 2016;122:3673–81.
Acknowledgements
This study was supported by a grant from the Ministry of Education, Science and Technological development of the Republic of Serbia (Grant number 41026)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Krivokuca, A., Boljevic, I., Jovandic, S. et al. Germline mutations in cancer susceptibility genes in high grade serous ovarian cancer in Serbia. J Hum Genet 64, 281–290 (2019). https://doi.org/10.1038/s10038-019-0562-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s10038-019-0562-z
This article is cited by
-
Targeted next-generation sequencing of 21 candidate genes in hereditary ovarian cancer patients from the Republic of Bashkortostan
Journal of Ovarian Research (2023)
-
BRIP1, RAD51C, and RAD51D mutations are associated with high susceptibility to ovarian cancer: mutation prevalence and precise risk estimates based on a pooled analysis of ~30,000 cases
Journal of Ovarian Research (2020)