Abstract
Background
Hyperthermia after hypoxia–ischemia (HI) in newborn infants is associated with worse neurological outcomes. Loss of thermoregulation may also be associated with greater injury.
Methods
In the postnatal-day 7 (P7) rat, the effect of 5 h of graded hyperthermia (38 °C or 39 °C) immediately after unilateral HI was compared with normothermia (NT, 37 °C) and therapeutic hypothermia (TH, 32 °C). Early (negative geotaxis) and late (staircase test) behavioral testing was performed, as well as neuropathology scoring in adulthood. Separately, P7 rats were exposed to HI, and individual nesting temperatures were monitored before analysis of neuropathology at P14.
Results
Mortality increased as temperature was increased from 38 °C (0%) to 39 °C (50%) after HI. Hyperthermia also resulted in early behavioral deficits compared with NT. In adulthood, pathology scores in the thalamus, basal ganglia, cortex, and hippocampus increased as post-hypoxic temperature increased above NT. Significant global neuroprotection was seen in the TH group. However, no significant difference was seen between HI groups in the staircase test. One hour after HI, the core temperature of pups was inversely correlated with global pathology scores at P14.
Conclusion
Early temperature is a significant determinant of injury after experimental HI. Spontaneous decreases in core temperature after HI may confound neuroprotection studies.
Similar content being viewed by others
Main
Temperature control after brain injury is critical to optimizing recovery and functional outcome. In randomized controlled trials (RCTs) of therapeutic hypothermia (TH) for term newborns with encephalopathy of suspected hypoxic–ischemic origin, infants in the control (normothermia, NT) groups with elevated core temperatures experienced an increased risk for adverse outcomes (1, 2). Pyrexia in adults after traumatic brain injury (TBI), stroke, or cardiac arrest is associated with greater mortality and worse neurological outcomes (3, 4, 5). Perinatal maternal fever >38 °C is also the strongest single risk factor (odds ratio (OR) of 9.3) for cerebral palsy (6). Regardless of patient age and etiology, optimizing temperature after brain injury, and preventing hyperthermia (HT), is therefore an important therapeutic goal.
In the treatment of perinatal asphyxia and resulting hypoxic–ischemic encephalopathy (HIE), TH is the current standard of care, with better outcomes seen if cooling is commenced early within the 6-h therapeutic window (7). However, the efficacy of TH in certain settings, including the presence of severe encephalopathy or infection-induced inflammation, is still uncertain (8, 9, 10). The relationship between exposure to maternal infection and pyrexia and poor neurological outcome is well established (6). Aseptic intrapartum pyrexia is also associated with adverse outcomes (11). However, the effect of degree and duration of HT on brain injury is unknown. In general, spontaneous temperature responses after HI brain injury are also poorly understood, and may be an important part of the diagnostic and prognostic process for asphyxiated neonates (12). Early thermoregulation is particularly important with regard to asphyxiated infants in the developing world, where the prevalence of (maternal) infection is higher (13), as well as for the investigation of optimal temperature regulation before the initiation of active TH.
The objectives of the current study were twofold: first, to investigate the effects of graded increases in HT temperature after HI on long-term pathological and functional outcomes in the Vannucci rat model of unilateral HI; and second, to investigate whether early spontaneous temperature changes after HI are correlated with the degree of injury in this model.
Methods
Animals
Experimental procedures examining the effect of controlled post-HI temperature on long-term pathology and behavioral outcome were performed with postnatal-day seven (P7) Wistar rats (Charles River Laboratories, Margate, Kent, UK). The experiments were carried out under Home Office license in accordance with UK regulations and approved by the University of Bristol’s animal ethical review panel. Experiments investigating the effect of spontaneous post-HI temperatures on short-term pathology were performed with P7 Wistar rats (Charles River Laboratories, Sulzfeld, Germany) and were reviewed and approved by the University of Oslo’s animal ethics research committee. All experiments were carried out in accordance with the approved protocols as well as the ARRIVE (Animal Research: Reporting in vivo Experiments) guidelines. Pups were kept in an animal facility with their dams under a 12 h:12 h dark:light cycle at 21 °C environmental temperature. Dams had access to food and water ad libitum, and pups were checked daily for health. For long-term survival and behavioral testing, the pups were weaned on P28, and 2–3 animals were housed (split by sex) per cage until they were killed at 11 weeks of age.
Vannucci Model of Unilateral Hypoxia–Ischemia
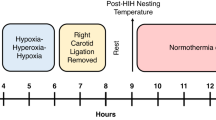
The effects of post-hypoxic temperature on neonatal HI brain injury were assessed using a modified Vannucci model of unilateral HI in P7 rat pups (9, 14, 15). On P7, pups underwent ligation of the left carotid artery under anesthesia, with 3% isoflurane in a 2:1 gas mixture of NO2/O2, via a nose cone. All pups within a litter were separated from their dam for the same period. After recovering under a heat lamp, pups were returned to the nest once all were alert and responsive. After a further 30-min recovery period with the dam, pups were exposed to 8% oxygen for 90 min at 37 °C rectal temperature in a specially designed chamber (9, 15). During hypoxia, the core temperature was continuously recorded in each chamber in “sentinel” pups carrying a rectal temperature probe (IT-21, Physitemp Instruments, Clifton, NJ). Rectal temperature was maintained within ±0.2 °C of the target using a water-filled mat (Tecotherm, Inspiration Healthcare Ltd Leicester, UK or CritiCool, MTRE, Yavne, Israel) inside the chamber. In P7 rats, rectal temperature correlates within 0.1 °C with brain temperature (16).
Controlled Post-Hypoxic Temperature
Before hypoxia, pups were randomized by litter, weight, and sex to one of five temperature treatments. In this model, NT refers to a rectal temperature of 37 °C, with standard TH treatment occurring at 32 °C (14, 15). To assess the effect of increased post-injury temperature, two other treament groups were assigned to 5 h at 38 °C or 39 °C. Immediately after hypoxia, the pups were transferred to treatment chambers at the allotted temperature. Rectal temperature was maintained within ±0.2 °C of the target, as described above. After 5 h of the allocated treatment, the pups were removed from the treatment chambers and returned to the dams (17). Control animals without the preceding ligation or hypoxia period underwent 5 h of treatment at normothermia (37 °C) on P7. A total of five groups were therefore used: Control (CON), and HI followed by 5 h at NT (NT37), therapeutic hypothermia (TH32), or HT at 38 °C (HT38) or 39 °C (HT39).
Early Neurobehavioral Testing
Negative geotaxis (i.e., active movement away from the action of gravity) is an innate neurological reflex in rats that develops in the second week after birth, before their eyes open. To quantify the function of this reflex at P14, pups were placed with their head oriented downhill (15), and the time (in seconds) taken to rotate to face uphill (a rotation of 180°) was recorded. If the pup fell from the platform or did not complete the task in 60 s, it was returned to the starting position for a maximum of four attempts. If a pup failed in all attempts, it was given a score of 60. If, on their best attempt, a pup managed to get halfway but then fell off, they were given a score of 59. To enable the ranking of all outcomes (including death) for non-parametric statistical testing between groups, pups that died after hypoxia but before negative geotaxis testing at P14 were assigned a score of 61.
Long-Term Neurobehavioral Testing of Motor Function
Beginning at 8 weeks of age (i.e., 7 weeks after the insult), forepaw fine motor dexterity was assessed using the staircase test (15). Briefly, sucrose pellets (three 45 mg pellets per step; BioServ, Frenchtown, NJ) were placed on each of the seven descending steps of either the right or the left staircase. The narrow design of the chamber was such that a rat could only reach the pellets from the left staircase with its left paw, and from the right staircase with its right paw. Thus, by baiting one staircase at a time, each forepaw is tested independently. Rats underwent one trial with each staircase baited per day, five days per week for 3 weeks. During the first 5 days of testing, rats were allowed 7.5 min with each baited staircase. After the first week, the testing period was reduced to 3.5 min. As the first two steps can be reached with the tongue, retrieval of pellets from these steps was not included in the final analysis. The number of pellets (a maximum of 15 pellets beyond the first two steps per side) retrieved during this time was recorded daily, and the average for the last 3 days was calculated. To enable the ranking of outcomes for non-parametric statistical testing between groups, rats that died after hypoxia but before 8 weeks of age were assigned a score of −1 in the staircase test.
Histological Preparation and Assessment
At 11 weeks of age, transcardiac perfusion with 10% phosphate-buffered (0.1 M) formaldehyde was performed under halothane/fentanyl anesthesia as previously described (15). Brains were immersion fixed and held in 4% formaldehyde until further processing. Coronal 3-mm blocks were cut through each brain using a standardized rat brain matrix (ASI Instruments, Warren, MI) and embedded in paraffin. Blocks were sectioned at 6 μm and stained with hematoxylin and eosin. The left side of the brain was examined and scored. Four areas of the brain were examined (cortex, basal ganglia, thalamus, and hippocampus) by an investigator blinded to the treatment allocation. The severity of damage was graded from 0 (no injury) to 4 (maximum injury), with 0.5 intervals for each of the four regions, giving a 9-step scale of pathology (15, 16, 17). Results were analyzed for each individual region, as well as an average of the scores from these regions, giving a global pathology score. Animals that died after hypoxia but before 11 weeks of age were assigned a pathology score of 4.5.
Spontaneous Post-Hypoxic Temperature and Short-Term Pathology
To investigate the connection between innate thermoregulatory responses after HI and later brain injury, a separate group of pups underwent HI as described above. Rectal temperatures of all pups were assessed after the end of hypoxia. Repeated rectal temperature measurements were taken from each pup hourly for up to 6 h following hypoxia, and then every 24 h until P14. At P14, rats were killed via transcardiac perfusion with saline and 10% neutral-buffered formalin under isoflurane/N2O anesthesia. Brains were harvested and kept in 10% neutral-buffered formalin until further processing. Six coronal 3 mm slices were cut through the brain using a standard rat brain matrix (ASI Instruments), and embedded in paraffin. Sections of 5 μm from the slices best representing the cortex, hippocampus, basal ganglia, and thalamus were taken, and stained with hematoxylin and eosin (H&E). Regional and global pathology scores were assessed by an investigator blinded to the treatment allocation, as described above.
Statistical Analysis
Statistical analyses were performed using SPSS software, version 22 (SPSS, Chicago, IL), and GraphPad Prism, version 6.00 (GraphPad Software, La Jolla, CA). Pups in which rectal and skin temperature was monitored were excluded because the stress of restraint at normothermia has previously been shown to have a neuroprotective effect in this model (18). Within groups, data were presented as median with 95% CIs for animals that performed the behavioral test or survived into adulthood for assessment of neuropathology. Between-group comparisons, including deaths, were carried out using the two-sided Wilcoxon–Mann–Whitney two-sample test. To assess the effect of increasing temperature above NT after HI, Spearman rank correlation analyses were performed on pathology scores and neurobehavioral scores from the NT37, HT38, and HT39 groups. A Spearman correlation analysis was also performed to investigate the effect of spontaneous temperature changes after HI with region-specific and global pathology at P14. A P-value <0.05 was generally considered to be statistically significant. If, however, multiple between-group comparisons were performed for a given outcome, the confidence intervals across treatment groups were compared, and a maximum of five comparisons were considered important. The results of these were adjusted using Bonferroni correction. Therefore, for multiple comparisons across treatment groups, a P-value <0.05/5=0.01 was considered to be statistically significant (9).
Results
Inclusions and Mortality
To determine the effects of HI followed by immediate TH, NT, or HT on long-term function and brain pathology, 126 postnatal-day 7 (P7) rat pups were randomized to a control group (CON; n=27) or to unilateral HI, followed by 5 h at one of four controlled temperatures: normothermia at 37 °C (NT37; n=34), therapeutic hypothermia at 32 °C (TH32; n=34), HT at 38 °C (HT38; n=15), or HT at 39 °C (HT39; n=16). Behavioral testing results were analyzed at P14 (negative geotaxis) and at 11 weeks of age (Montoya staircase test), followed by assessment of neuropathology after 11 weeks. During temperature treatment, one animal (2.9%) died in the NT37 group, and eight animals (50% mortality) died in the HT39 group.
Negative Geotaxis
Compared with the CON group, stepwise increases in the median time to rotate 180° at P14 were seen in the TH32, NT37, HT38, and HT39 groups (Figure 1a). For animals that performed the task (i.e., excluding deaths but including animals that performed the test but failed to complete it), the median (95% CI) time to rotate was 4 (3–6), 9 (8–10), 11.5 (6–59), 15 (5-59), and 60 (6-60) s, respectively (Table 1). Including deaths and after adjusting for multiple comparisons (Bonferroni correction), the CON group performed significantly better than all HI groups (P<0.001), and the HT39 group performed significantly worse compared with all other groups (P<0.001). No other between-group differences were statistically significant.
Behavioral testing. Individual performance in the negative geotaxis test at P14 (a) and the staircase test at 11 weeks of age (b). Error bars show median (95% CI) speed of rotation (a) or pellets retrieved (b) for animals that survived to perform the task in the control (black triangles), HT32 (white circles), NT37 (light gray circles), HT38 (dark gray circles), and HT39 (hatched diamonds) groups. In the HT39 group, n=8 animals died during temperature treatment (50% mortality). *Adjusting for multiple comparisons, the control group performed significantly better than all other groups (P<0.001 for all) in both the negative geotaxis task and the staircase test, and the HT39 group performed significantly worse compared to all other groups in the negative geotaxis task only (P<0.001). No other between-group differences were statistically significant.
Staircase Testing and Fine Motor Control
Compared with the CON group, fewer pellets were retrieved from the lowest five steps of the staircase on the right side in the TH32, NT37, HT38, and HT39 groups (Figure 1b). For animals that survived to perform the task, the median (95% CI) number of pellets retrieved across the groups was 9.3 (6.7–9.7), 5.7 (5–6.7), 5.7 (5–5.7), 4.7 (1.7–6), and 5.5 (2.7–11), respectively (Table 1). Including deaths and adjusting for multiple comparisons, the CON group performed significantly better than all HI groups (P<0.001). To determine the effect of HT on functional outcomes, the NT37 group (n=33 survivors, n=1 death) was compared with the two HT groups (n=23 survivors, n=8 deaths) in both the negative geotaxis and staircase tests. Including deaths, animals in the normothermia group were significantly faster in the negative geotaxis test at P14 (P=0.007).
Global and Regional Pathology Scoring
The median global pathology score increased with increasing temperature after HI (Table 1). Immediate hypothermia in the TH32 group significantly reduced the global pathology score compared with that in the NT37 group (P=0.01; Figure 2a), as well as providing significant neuroprotection in the cortex (P=0.004), thalamus (P=0.04), and hippocampus (P=0.009; Figure 2b). Including deaths and adjusting for multiple comparisons, the global pathology score increased significantly from the NT37 group to the HT38 group (P=0.003), and from the HT38 group to the HT39 group (P<0.001). Non-parametric Spearman correlation analysis showed a significant correlation between post-HI temperature in the NT37, HT38, and HT39 groups and global pathology (r=0.70, P<0.001). A similar effect of increasing temperature on injury was seen in regional pathology scores from the cortex, basal ganglia, thalamus, and hippocampus (P<0.001 for all; Table 1).
Pathology scoring. (a) Scatter plot of global pathology score across all five groups. Error bars show median (95% CI) of global pathology scores from survivors in the control (black triangles), HT32 (white circles), NT37 (light gray circles), HT38 (dark gray circles), and HT39 (hatched diamonds) groups. *Denotes significant difference (Wilcoxon–Mann–Whitney test) between groups (P<0.01), including death as an outcome and adjusted for multiple comparisons. †Indicates an animal that died during temperature treatment. (b) Floating bar plot (median with 95% CI) of regional pathology scores in survivors in the TH32 (n=34, white), NT37 (n=33, light gray), HT38 (n=15, dark gray), and HT39 (n=8, hatched) groups. Compared with normothermia, hypothermia provided significant neuroprotection in the cortex (P=0.004), thalamus (P=0.04), and hippocampus (P=0.009).
Spontaneous Post-HI Temperature and Short-Term Pathology
Owing to the significant influence of early post-hypoxic temperature on long-term outcome, further experiments were performed in the Vannucci model to determine whether spontaneous core temperature after HI could predict later injury. A total of 28 animals received HI on P7, followed by regular rectal temperature measurements and pathology analysis at P14. Two animals (7.1%) died during the survival period, or were killed due to weight loss over two consecutive days. One other animal died as a result of an injury sustained during temperature measurements, and was excluded from the analysis. Immediately after HI, the core temperature (median, 95% CI) was 36.6 °C (36.3–37 °C), which fell to 34.2 °C (33.2–34.4 °C) 1 h after HI, and recovered to 35.6 °C (35.2–36.1 °C) over the next 24 h (Figure 3). By P14, the median temperature was 36 °C (35.5–36.2 °C). The core temperature 1 h after HI correlated significantly with the global pathology score at P14 (n=27, Spearman r=−0.55, P=0.003; Figure 4). Including deaths, animals whose temperature 1 h after HI was below the 95% CI of the median (<32.2 °C) had a significantly worse outcome (P=0.007) compared those with a temperature above the 95% CI of the median (>34.4 °C).
Spontaneous drop in core temperature after hypoxia–ischemia (HI). Median (IQR) rectal temperature after HI (n=26–28 per time point). One hour after HI the core temperature dropped significantly, and increased over the subsequent 24 h. *Denotes significant difference (Wilcoxon-matched pairs signed rank test) compared to the preceding time point (P<0.001).
Post-hypoxia–ischemia (HI) temperature and global pathology. One hour after HI, the core temperature of P7 rats is significantly correlated (Spearman r=−0.55, P=0.003) with the global pathology score at P14. Including deaths, animals whose temperature 1 h after HI was below the 95% CI of the median (<32.2 °C) had a significantly worse outcome compared with those with a temperature above the 95% CI of the median (>34.4 °C; P=0.007). † Indicates an animal that died during the survival period.
Discussion
In the current era of cooling therapy for infants with HIE, a search for the optimal TH protocol is still ongoing. This is exemplified by the “longer and deeper” clinical trial, which was stopped early because of a lack of benefit from longer (120 h vs. 72 h) or deeper (32 °C vs. 33.5 °C) cooling in neonates with moderate or severe HIE. These findings were subsequently supported by preclinical data in a range of animal models (9, 19, 20). To further elucidate some of the effects of temperature changes after HI, we investigated the effect of graded HT after HI on functional outcomes and neuropathology, as well as whether early spontaneous temperature responses after an HI insult are correlated with later injury. An elevation in temperature of 2 °C for 5 h after HI resulted in 50% mortality, and caused a significant early behavioral deficit in the negative geotaxis task. Within all studied regions (cortex, basal ganglia, thalamus, and hippocampus), increasing temperature in the range above normothermia (37–39 °C) was correlated with significantly greater pathology scores in adulthood. In nesting animals monitored after HI, pups with larger drops in core temperature early (1 h) after hypoxia had greater global pathology scores 1 week after the insult.
The current study extends and confirms findings in previous experimental work and clinical reports showing the detrimental effects of post-insult HT on the neonatal brain (1, 2, 21, 22). HT is likely to exacerbate a number of the pathological processes thought to underlie the mechanism of brain injury associated with HI, many of which are potentially ameliorated by TH (23). For instance, increased metabolic rate due to post-asphyxial pyrexia, which may occur as a result of systemic inflammation, seizures, infection, or iatrogenic causes (i.e., overheating), is likely to accelerate and exacerbate the secondary energy failure associated with greater neurological injury after HI (1). In agreement with this, prevention of a +1.5 °C HT after kainate-induced seizures in the Vannucci rat model of unilateral HI has previously been shown to be neuroprotective (21). HT of 39 °C has also been shown to produce a fivefold increase in pro-apoptotic caspase-3 activation 24 h after HI compared with NT (36.5–37 °C) (22). HT may cause severe edema following ischemia, and in an adult stroke model, HT of +2 °C disrupted the integrity of the blood–brain barrier (24, 25). Preventing HT early after perinatal asphyxia should therefore remain an important clinical goal.
To investigate spontaneous post-asphyxial temperature responses experimentally, we exposed P7 animals to an HI insult, and recorded their nesting rectal temperatures daily until P14. One hour after the end of hypoxia, the median core temperature was 1.2 °C lower in HI rats compared with that in healthy P7 rats, and increased over the next 24 h (9). This is a similar response to that seen in spontaneously breathing moderately asphyxiated infants, who experience a drop in core temperature of around 1.5 °C more than healthy controls after birth, and also recover their core temperature more slowly (12). In our model, a significant negative correlation between temperature 1 h after hypoxia and global pathology score at P14 was seen. This was particularly evident in those with a very low temperature 1 h after hypoxia (<32.2 °C; n=8, 25% mortality). As hypothermia at 32 °C is significantly neuroprotective in this model (9, 14), it is unlikely that the lower temperatures were damaging per se, and that this instead reflects a greater degree of initial injury. Similarly, animals with a temperature within the upper end of the normal range for P7 rats in our laboratory (>34.4 °C; n=7) (9) had a significantly lower median global pathology compared to those with a temperature <32.2 °C, suggesting that animals who experienced minimal post-HI temperature changes sustained a milder injury.
Overall, temperature responses to HI appear to be biphasic, with late and early responses corresponding with the latent (1–6 h after the insult) and secondary (6–24+ h after the insult) phases of HI injury (26). Our data from P7 rats, as well as historical data in asphyxiated neonates (12), suggest a greater relative early hypothermia in those with greater injury. In line with this, early post-HI reductions in cerebral blood flow and temperature production were directly linked to suppressed cerebral metabolism as a result of injury in a fetal sheep model of perinatal asphyxia (27). Similarly, in a bilateral carotid artery occlusion piglet model of neonatal HI, the severity of injury was associated with the degree of suppression of cerebral metabolism during the insult (28). Early suppression of metabolism is largely mediated by adenosine as well as by the effect of hypoxia itself (27, 29), with later changes due to endogenous neurosteroid and inflammatory mediators such as allopregnanolone and platelet-activating factor (30, 31, 32). However, the decrease in temperature in rats with more severe injury after HI may be due to direct hypothalamic damage and a failure to defend normal temperature, rather than due to a physiological response to HI. In contrast to the early response, greater injury may then result in later HT. For instance, recent data using magnetic resonance spectroscopy (MRS) thermometry around the 3rd and 7th days of life showed that those with severe HIE had higher cerebral temperatures compared with those with moderate HIE (33). This HT is likely to be associated with the hypermetabolism and cerebral hyperperfusion seen during the secondary phase of injury, which occurs alongside a systemic inflammatory response (26), and could also partly explain why pyrexia in the 72 h after birth in infants with HIE is associated with worse outcomes (1, 2). Temperature during the secondary phase is likely to be controlled by more “traditional” cytokine mediators produced both peripherally and centrally as a part of the inflammatory response to injury (31, 34). Therefore, depending on the timing of the temperature measurement, more severe injury may be associated with either relative hypothermia (early) or HT (late).
This study does have some limitations. Our primary goal was to investigate the effect of HT after HI, with a standard neuroprotective TH protocol as a control. Interestingly, neither early (negative geotaxis) nor late (staircase testing) testing was sensitive enough to detect the between-group differences in pathology seen in adulthood, which is a potential limitation. Although it is a good test of unilateral fine motor deficits in this model, we have previously seen that performance in the staircase test does not always correlate with global pathology (35). This is why it is important to perform long-term studies when assessing neuroprotective strategies preclinically, and to include both behavioral and pathology scoring. Although inadvertent HT may increase the speed and degree of damage to the brain (1, 2), in the Western world it is unlikely that asphyxiated infants would spend many hours with significant HT without clinical intervention. However, the negative effects of HT on outcome are still relevant for treatment in developing countries, where infection and pyrexia rates are greater (13). With regard to the statistical analysis, the result of enforced HT at 39 °C for 5 h after HI (i.e., 50% mortality) required the assignment of behavioral test and pathology scores to animals that died so that they could be included in the analysis to represent the negative effect of HT. This is potentially analogous to the combined “death and disability” outcomes used in trials of infants with HIE (8). We also assumed that death was worse than survival with maximum injury, and assigned scores appropriately. As a result, non-parametric statistical analyses based on group ranks were used throughout to prevent the magnitude of the assigned score from skewing the results. Another limitation is the lack of a sham control group in study 2, and as such we are unable to isolate the effects of anesthesia and surgery on temperature regulation compared to the effect of HI. We did not directly compare the outcomes of nesting hypothermia with active TH treatment either, as the regular temperature measurements taken in the nesting group constitute a stress that may alter outcomes (18). Future work will investigate how TH or HT affects later temperature regulation, including diurnal changes, as well as whether shorter periods of HT before the onset of TH negate any of the beneficial therapeutic effects of cooling. To optimize and personalize TH treatment, it will be important to discover whether individual temperature responses after HI could guide the most beneficial cooling temperature (9). However, based on the recent failure of the “longer and deeper” trial, as well as recent data from the rat, piglet, and fetal sheep (9, 19, 20), it is likely that deeper or longer cooling will not result in greater neuroprotection. Current cooling protocols (i.e., 33.5 °C for 72 h), plus controlled passive hypothermia before active TH (as practiced in many neonatal units), are likely to be close to optimal for generalized recommendations.
In conclusion, we describe early behavioral deficits and increased global neuropathological injury in adulthood after unilateral HI and HT in the neonatal rat. In addition, in this model, early spontaneous hypothermia was correlated with later injury, which may confound studies of TH neuroprotection if not adequately controlled for.
References
Wyatt JS, Gluckman PD, Liu PY et al. Determinants of outcomes after head cooling for neonatal encephalopathy. Pediatrics 2007;119:912–21.
Laptook AR, McDonald SA, Shankaran S et al. Elevated temperature and 6- to 7-year outcome of neonatal encephalopathy. Ann Neurol 2013;73:520–8.
Greer DM, Funk SE, Reaven NL, Ouzounelli M, Uman GC . Impact of fever on outcome in patients with stroke and neurologic injury: a comprehensive meta-analysis. Stroke 2008;39:3029–35.
Bao L, Chen D, Ding L, Ling W, Xu F . Fever burden is an independent predictor for prognosis of traumatic brain injury. PLoS One 2014;9:e90956.
Zeiner A, Holzer M, Sterz F et al. Hyperthermia after cardiac arrest is associated with an unfavorable neurologic outcome. Arch Intern Med 2001;161:2007–12.
Grether JK, Nelson KB . Maternal infection and cerebral palsy in infants of normal birth weight. JAMA 1997;278:207–11.
Thoresen M, Tooley J, Liu X et al. Time is brain: starting therapeutic hypothermia within three hours after birth improves motor outcome in asphyxiated newborns. Neonatology 2013;104:228–33.
Edwards AD, Brocklehurst P, Gunn AJ et al. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ 2010;340:c363.
Wood T, Osredkar D, Puchades M et al. Treatment temperature and insult severity influence the neuroprotective effects of therapeutic hypothermia. Sci Rep 2016;6:23430.
Osredkar D, Sabir H, Falck M et al. Hypothermia does not reverse cellular responses caused by lipopolysaccharide in neonatal hypoxic-ischaemic brain injury. Dev Neurosci 2015;37:390–7.
Lieberman E, Lang J, Richardson DK, Frigoletto FD, Heffner LJ, Cohen A . Intrapartum maternal fever and neonatal outcome. Pediatrics 2000;105:8–13.
Burnard ED, Cross KW . Rectal temperature in the newborn after birth asphyxia. BMJ 1958;2:1197–9.
Fleiss B, Tann CJ, Degos V et al. Inflammation-induced sensitization of the brain in term infants. Dev Med Child Neurol 2015;57:17–28.
Bona E, Hagberg H, Loberg EM, Bagenholm R, Thoresen M . Protective effects of moderate hypothermia after neonatal hypoxia-ischemia: short- and long-term outcome. Pediatr Res 1998;43:738–45.
Hobbs C, Thoresen M, Tucker A, Aquilina K, Chakkarapani E, Dingley J . Xenon and hypothermia combine additively, offering long-term functional and histopathologic neuroprotection after neonatal hypoxia/ischemia. Stroke 2008;39:1307–1313.
Thoresen M, Bagenholm R, Loberg EM, Apricena F, Kjellmer I . Posthypoxic cooling of neonatal rats provides protection against brain injury. Arch Dis Child Fetal Neonatal Ed 1996;74:F3–9.
Sabir H, Scull-Brown E, Liu X, Thoresen M . Immediate hypothermia is not neuroprotective after severe hypoxia-ischemia and is deleterious when delayed by 12 hours in neonatal rats. Stroke 2012;43:3364–70.
Thoresen M, Bagenholm R, Loberg EM, Apriccna F . The stress of being restrained reduces brain damage after a hypoxic-ischaemic insult in the 7-day-old rat. Neuroreport 1996;7:481–44.
Alonso-Alconada D, Broad KD, Bainbridge A et al. Brain cell death is reduced with cooling by 3.5 degrees C to 5 degrees C but increased with cooling by 8.5 degrees C in a piglet asphyxia model. Stroke 2015;46:275–8.
Davidson JO, Wassink G, Yuill CA, Zhang FG, Bennet L, Gunn AJ . How long is too long for cerebral cooling after ischemia in fetal sheep? J Cereb Blood Flow Metab 2015;35:751–8.
Yager JY, Armstrong EA, Jaharus C, Saucier DM, Wirrell EC . Preventing hyperthermia decreases brain damage following neonatal hypoxic-ischemic seizures. Brain Res 2004;1011:48–57.
Fukuda H, Tomimatsu T, Kanagawa T et al. Postischemic hyperthermia induced caspase-3 activation in the newborn rat brain after hypoxia-ischemia and exacerbated the brain damage. Biol Neonate 2003;84:164–171.
Wassink G, Gunn ER, Drury PP, Bennet L, Gunn AJ . The mechanisms and treatment of asphyxial encephalopathy. Front Neurosci 2014;8:40.
Noor R, Wang CX, Shuaib A . Hyperthermia masks the neuroprotective effects of tissue plaminogen activator. Stroke 2005;36:665–9.
Dietrich WD, Alonso O, Halley M, Busto R . Delayed posttraumatic brain hyperthermia worsens outcome after fluid percussion brain injury: a light and electron microscopic study in rats. Neurosurgery 1996;38:533–41.
Hassell KJ, Ezzati M, Alonso-Alconada D, Hausenloy DJ, Robertson NJ . New horizons for newborn brain protection: enhancing endogenous neuroprotection. Arch Dis Child Fetal Neonatal Ed 2015;100:F541–52.
Jensen EC, Bennet L, Hunter CJ, Power GC, Gunn AJ . Post-hypoxic hypoperfusion is associated with suppression of cerebral metabolism and increased tissue oxygenation in near-term fetal sheep. J Physiol 2006;572:131–9.
Thoresen M, Penrice J, Lorek A et al. Mild hypothermia after severe transient hypoxia-ischemia ameliorates delayed cerebral energy failure in the newborn piglet. Pediatr Res 1995;37:667–70.
Hunter CJ, Bennet L, Power GG et al. Key neuroprotective role for endogenous adenosine A1 receptor activation during asphyxia in the fetal sheep. Stroke 2003;34:2240–5.
Ephgrave K, Kremer T, Broadhurst K, Cullen J . The role of platelet-activating factor in conscious, normotensive endotoxemia. J Surg Res 1997;68:170–4.
Romanovsky AA, Almeida MC, Aronoff DM et al. Fever and hypothermia in systemic inflammation: recent discoveries and revisions. Front Biosci 2005;10:2193–216.
Hirst JJ, Palliser HK, Yates DM, Yawno T, Walker DW . Neurosteroids in the fetus and neonate: potential protective role in compromised pregnancies. Neurochem Int 2008;52:602–10.
Wu TW, McLean C, Friedlich P et al. Brain temperature in neonates with hypoxic-ischemic encephalopathy during therapeutic hypothermia. J Pediatr 2014;165:1129–34.
Zampronio AR, Soares DM, Souza GE . Central mediators involved in the febrile response: effects of antipyretic drugs. Temperature 2015;2:506–21.
Liu X, Dingley J, Scull-Brown E, Thoresen M . Adding 5h delayed xenon to delayed hypothermia treatment improves long-term function in neonatal rats surviving to adulthood. Pediatr Res 2015;77:779–83.
Acknowledgements
We thank Professor Lars Walløe for his assistance with the statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Statement of financial support
This study was supported by the Wellcome Trust (UK), the Laerdal Foundation for Acute Medicine (Norway), and the Norwegian Medical Research Council (Grant No. 214356). T.W. was also supported by the University of Oslo.
Rights and permissions
About this article
Cite this article
Wood, T., Hobbs, C., Falck, M. et al. Rectal temperature in the first five hours after hypoxia–ischemia critically affects neuropathological outcomes in neonatal rats. Pediatr Res 83, 536–544 (2018). https://doi.org/10.1038/pr.2017.51
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2017.51
This article is cited by
-
Rectal temperature after hypoxia-ischemia predicts white matter and cortical pathology in the near-term ferret
Pediatric Research (2024)
-
Does sex materially modulate responses to therapeutic hypothermia?
Pediatric Research (2023)
-
Body temperature, heart rate and long-term outcome of cooled infants: an observational study
Pediatric Research (2022)
-
Variability and sex-dependence of hypothermic neuroprotection in a rat model of neonatal hypoxic–ischaemic brain injury: a single laboratory meta-analysis
Scientific Reports (2020)