Abstract
Background
Little is known about the relationship between brain volumes and neurodevelopmental outcome at 2 years of age in children with single-ventricle congenital heart disease (CHD). We hypothesized that reduced brain volumes may be associated with adverse neurodevelopmental outcome.
Methods
Volumetric segmentation of cerebral magnetic resonance imaging (MRI) scans was carried out in 44 patients without genetic comorbidities and in 8 controls. Neurodevelopmental outcome was assessed with the Bayley-III scales.
Results
Gray matter (GM), deep GM, white matter (WM), and cerebrospinal fluid (CSF) volumes were 611±59, 43±4.5, 277±30, and 16.4 ml, respectively (interquartile range (IQR) 13.1, 23.3 ml). Children undergoing neonatal cardiopulmonary bypass surgery showed smaller deep GM (P=0.005) and WM (P=0.021) volumes. Brain volumes were smaller in patients compared with controls (GM: P=0.017, deep GM: P=0.012, and WM: P=0.015), whereas CSF volumes were greater (P=0.014). Of all intracranial volumes, only CSF volume was associated with neurodevelopmental outcome, accounting for 21% (P=0.011) of variability in the cognitive composite score when combined with common risk factors in a multivariable analysis.
Conclusion
Increased CSF volume represents a significant risk factor for neurodevelopmental impairment in children with single-ventricle CHD. Later assessments are warranted to determine the prognostic role of intracranial volumes for long-term outcome.
Similar content being viewed by others
Main
Children with single-ventricle (SV) congenital heart disease (CHD) including hypoplastic left heart syndrome (HLHS) are at high risk for delayed intrauterine brain development as well as perioperative cerebral injuries (1, 2, 3). Brain injuries and delayed maturation seen in patients with complex CHD can be detected with cerebral magnetic resonance imaging (MRI), and may cause neurodevelopmental impairments (4, 5).
In addition to providing qualitative information about the presence of brain lesions, atrophy, and other abnormalities, emerging MRI techniques also allow for the quantitative measurement of global and regional brain volumes (6). The association between global and regional brain volumes and neurodevelopmental function in children with CHD has been investigated in infancy (7, 8), in adolescence (9), and in adulthood (10). Recently, we observed cerebrospinal fluid (CSF) enlargement in children with SV before Fontan completion that correlated strongly with poorer neurodevelopmental functioning at 2–3 years of age (11). In adolescence, structural abnormalities (atrophy or focal infarction) were associated with neuropsychological deficits in patients who underwent Fontan completion (12). However, at 2 years of age, which is an important time period for cognitive, language, and motor development, studies comparing brain volumetric measurements and developmental data in patients with SV CHD are lacking. Therefore, we quantified brain volumes in children with SV before Fontan completion using the Freesurfer software and compared the resulting volumes with neurodevelopmental outcome scores, in order to evaluate the relationship between brain volumes and neurodevelopment as well as growth in high-risk (SV) CHD patients during the vulnerable phase of brain development between infancy and early childhood.
Methods
Study design
This retrospective cohort study is part of a two-center collaboration (University Children’s hospital, Pediatric heart center, Giessen, Germany, Center A and University Children’s hospital of Zurich, Switzerland, Center B) investigating structural and functional neurological and neurodevelopmental status before Fontan completion in patients with SV without known underlying genetic comorbidities. Within a part of the original study population (n=47 (ref. 11)) enrolled between August 2012 and July 2015, we performed a secondary volumetric MR analysis in 44 patients and 8 controls. The volumetric analysis was not feasible in one patient receiving a 1.5 Tesla scan and in two patients with missing high-resolution three-dimensional MR sequences. Demographic characteristics from the excluded children did not differ from those included. The control patients (63% from Center A) were scanned for headache (3), afebrile, primary generalized epileptic seizures (2), suspected epilepsy, not confirmed in follow-up (1), recurrent vomiting (1), and dermatologic lesions (1). However, all had a normal cerebral MRI scan and neurocognitive development. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the local ethics committee and written informed consent provided by parents or caregivers.
As previously described (11), the patient characteristics were abstracted from the patient charts, socioeconomic status was evaluated according to Largo et al. (13), and anthropometric measurements were transformed into age- and gender-adjusted Z-scores (14). Microcephaly and head growth restriction were defined from Z-scores below the third and tenth percentiles, respectively. The patient characteristics are presented in Table 1. In addition, the use or duration of any of the following treatments was recorded: length of cumulative hospital stay, cumulative mechanical ventilation, and number of cardiac reinterventions.
Neurodevelopmental Assessments
Before the MRI scan, the children of the study group were assessed with the Bayley Scales of Infant and Toddler Development Version III (Bayley-III) by an experienced developmental pediatrician or neurologist (B.L. and K.W.) at each center (15). The Bayley-III scales measure cognition (Cognitive Composite Scale, CCS), language (Language Composite Scale, LCS), and motor function (Motor Composite Scale, MCS). Normative composite scores were calculated by age for each scale and compared with American test norms (mean 100 points, SD 15). For all scales, cutoff points of <85 (1 SD below normative mean) equivalent to mild to moderate impairment, and <70 (2 SD below normative mean) considered as severe impairment were used, respectively (15). All children had a neurological examination that was scored according to severity (normal, reflex or tone abnormality, reflex and tone abnormality, or cerebral palsy) (16).
Cerebral MRI and Brain Volumetry
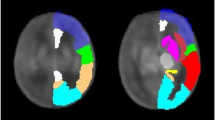
Cerebral MRI data including high-resolution three-dimensional T1-weighted images (Center A: magnetization prepared rapid acquisition gradient echo and Center B: spoiled gradient echo) were acquired before Fontan completion at a mean age of 26.7 months, SD 3.9, and in eight controls (five males, 62.5%) at a mean age of 29.8 months, SD 9.5 (P=0.21). Intracranial structural anomalies were described elsewhere (11). The three-dimensional T1 images were anatomically segmented into gray matter (GM), white matter (WM), and CSF maps, and the volumes of each tissue compartment were calculated with the Freesurfer image analysis suite (Martinos Center for Biomedical imaging, MA, USA; http://surfer.nmr.mgh.harvard.edu/) on a Linux workstation. Total brain volume was defined as intracranial volume without CSF. The Freesurfer software has been evaluated and established as a robust tool for quantification of cerebral volumes in children with CHD at 2 years of age, showing high accuracy and reliability in a recent validation study (17).
Statistical analyses
Statistical analyses were performed with IBM SPSS Statistics for Macintosh, version 23 (IBM, Armonk, NY). With the respective measures of dispersion, descriptive statistics are presented as mean, SD or median, interquartile range (IQR) for continuous variables, and as frequency and percentage for categorical variables. A Shapiro–Wilk test was used to test normality. The t-test or the Mann–Whitney U-test was applied to calculate differences between groups as appropriate based on the dispersion of the data. Significant differences between brain volume and neurodevelopment were then checked after adjusting for age, gender, and center using analysis of covariance. Correlations of brain volumes with anthropometric measures and neurologic outcome, as well as previously defined patient-related and perioperative risk factors were analyzed with a Pearson’s correlation for normally distributed variables and Spearman’s rho for non-normally distributed data. We controlled for age, center, and gender using bivariate correlations. For identification of influence of intracranial volumes on neurodevelopmental outcome, a multivariable linear regression model was used, for which all non-normally distributed interval data were logarithmically transformed (using a natural logarithm). A significance level of 0.05 was applied.
Results
Patient Characteristics
Forty-four patients (28 males, mean age 26.7 months, SD 3.9) were included in the study. The mean gestational age was 39.2 weeks, SD 1.5. Two patients (4.5%) were born at 34 1/7 and 36 3/7 gestational weeks, respectively. Twenty-three patients were treated in Center A and twenty-one in Center B. Patient characteristics are shown in Table 1. More than half of all patients (56.8%) had a prenatal diagnosis of their CHD. Cardiac diagnosis included SV, of which HLHS was the most common (56.8% for detailed cardiac diagnoses see Supplementary Table S1 online). There was a higher prevalence of HLHS at Center A (20 vs. 5), where patients were treated more frequently with the Hybrid approach (P<0.001) at a younger age for both, stage I and stage II surgery (P Stage I=0.006, P Stage II=0.001). The remainder patient characteristics did not differ between the two study centers.
Children with SV CHD are treated with a three-staged surgical palliation completed by the Fontan operation at 2–3 years of age with complete separation of passive pulmonary blood flow. Of our cohort, 24 patients were treated with Hybrid stage I procedure (catheter-guided stenting of patent arterial duct and surgical bilateral pulmonary banding) followed by Comprehensive stage II (removal of patent arterial duct stent and pulmonary artery bands, enlargement of the aortic arch, bidirectional cavopulmonary anastomosis). Six patients received classical stage I Norwood procedure (atrial septectomy, pulmonary artery to aortic arch shunt, aortic arch repair) and ten patients had other systemic-to-pulmonary shunt surgery (modified Blalock-Taussig shunt, systemic ventricular to pulmonary artery shunt, pulmonary artery banding) as stage I procedure, followed by bidirectional cavopulmonary anastomosis (Glenn procedure) as stage II procedure. Four patients did not undergo a stage I cardiac procedure because of balanced hemodynamics (reduced pulmonary artery flow due to pulmonary stenosis) during the neonatal period, and received Glenn procedure on cardiopulmonary bypass (CPB) as stage II. Ten (22.7%) patients underwent their first CPB surgery during the neonatal period at a mean age of 10.5 days, SD 7, including six patients with classical Norwood procedure, two HLHS patients, who first followed the Hybrid approach, but received a secondary surgical atrial septectomy later on, and two SV patients treated by Blalock–Taussig shunt surgery, with the need for PDA closure in one patient and atrial septectomy in the other patient. Age at stage I did not differ whether the surgery was conducted with or without CPB (P=0.13).
There was a need for re-catheterization in 14 (32.8%) patients after stage I and in 13 (29.5%) after stage II, as well as for re-operation in 13 (29.5%) patients, after stages I and II, respectively.
Growth
At birth, all growth measurements were restricted as indicated by the mean Z-scores below reference values (weight P=0.02, height P<0.001, head circumference (HC) P=0.008; Figure 1). The mean HC at birth was on the 34th percentile (mean Z-score −0.42), with seven (15.9%) patients below the 10th percentile and four (9.1%) patients with microcephaly (below the 3rd percentile).
Growth in children with single ventricle until Fontan completion. Boxplots with the median and interquartile range for Z-scores of growth indices of 44 children with single-ventricle congenital heart disease at birth (a), at stage II surgery (b), and at the time of magnetic resonance imaging (MRI) scan before Fontan completion (c). White bars represent body weight, gray bars represent height, and dark gray bars represent head circumference. Circles represent outliers. Horizontal lines show 2 SD of norm. Significant growth restriction is calculated by Student’s t-test (*P<0.05, **P<0.001).
There was a growth spurt in all growth parameters after stage II operation. However, at the age of 2–3 years, the mean body weight (33th percentile, Z-score −0.45) and height (18th percentile, Z-score −0.90) remained significantly below normative values (P values both <0.001; Figure 1), whereas HC showed a complete catch-up in growth toward reference values at that age (55th percentile, Z-score 0.13; P=0.40). The HC correlated strongly with CSF volume (r=0.45, P=0.002), but not with total brain volumes, Bayley-III scales, or with neurological abnormality (all P values >0.05; Table 3).
Neurodevelopmental Outcome
The median Bayley-III scores were all comparable to normative data: median CCS was 100 (IQR 90–105), LCS 97 (IQR 86–106), and MCS 97 (IQR 88–107; all P values >0.05). For CCS, two (4.5%) children had scores below 85 and two (4.5%) below 70. For LCS, eight (18.2%) children were below 85 and one (4.5%) was below 70. For MCS, seven (15.9%) were below 85 and three (12.6%) were below 70.
Intracranial Volumes
Intracranial volumes are presented in Table 2. Compared with age-matched controls, all brain volumes were decreased in children with CHD (Table 2). Deep GM and WM volumes were reduced if stage I was performed with CPB during the neonatal period (Figure 2). Volumes for children with and without neonatal CPB were 39.7 ml, SD 4.0, and 43.5 ml, SD 4.3, for deep GM (P=0.005), and 255.5 ml, SD 21.3, and 283.5 ml, SD 28.8, for WM (P=0.021), respectively. Patients with GM or WM lesions did not have larger CSF volumes compared with those without lesions (P=0.30). Of note, all brain volumes were highly intercorrelated (GM with WM: r=0.83, GM with deep GM: r=0.71, WM with deep GM: r=0.72, all P values <0.001), whereas CSF volume did not correlate with other intracranial volumes (with GM: r=0.09, P=0.58; deep GM: r=0.04, P=0.81; WM: r=0.14, P=0.38). Of all intracranial volumes, only CSF volume correlated with neurodevelopmental outcome (Figure 3 and Table 3; CCS P≤0.001, LCS P=0.019, MCS P=0.002). In a multivariable model combined with previously identified risk factors for impaired neurocognitive function (socioeconomic status, HC at birth, number of reinterventions, length of hospital stay, and mechanical ventilation), CSF contributed up to 21% of variability in the CCS outcome (P=0.011).
Intracranial volumes in patients with and without neonatal cardiopulmonary bypass (CPB) surgery. The graphs show brain volumes before Fontan completion at 2–3 years of age, with median and interquartile range. Gray bars represent patients undergoing neonatal CPB surgery (n=10) and white bars represent those operated beyond the neonatal period (n=34). Circles represent outliers. Significant differences between patients in deep gray matter volumes and white matter volumes are displayed (*P<0.05). P values by Student’s t-test or Mann–Whitney U-test.
Association of intracranial volumes and Bayley-III Scales. The graph shows the correlation of cerebrospinal fluid (CSF) volumes and total brain volumes (TBVs) with neurodevelopmental outcome measured by the Bayley-III Scales. Results are given for the Cognitive Composite Scale (CCS) in (a), for the Language Composite Scale (LCS) in (b), and for the Motor Composite Scale (MCS) in (c). Circles represent TBV with its legend on the left and triangles represent CSF volumes with its legend on the right (italic). Linear relation represented by Spearman’s correlation coefficient. Significant correlations between CSF and all Bayley-III composite scales are represented as solid lines (P<0.05). Dashed line represents nonsignificant correlations.
Discussion
In this study, brain volumes of children with CHD and healthy controls were measured with a morphometric MRI technique at 2–3 years of age. The main finding of our study is that children with SV CHD showed reduced brain volumes and increased CSF volumes compared with healthy controls. Importantly, neurodevelopmental outcome showed Bayley-III median scores comparable to normative data. Of all intracranial volumes, only CSF volume correlated negatively with the neurodevelopmental performance. We did not find any association between brain tissue volumes (GM, deep GM, or WM) or HC with neurodevelopmental outcome scores, as previously described for infants (18) and adolescents (9) with CHD.
Complete catch-up growth of HC at time of the MRI scan most likely does not represent normal brain development, as HC only correlated strongly with CSF volume, but not with total brain matter at 2–3 years of age. HC catch-up growth in SV CHD patients is probably not a reliable parameter, as it did not correlate with neurodevelopment at that age. Thus, more sensitive brain-imaging techniques are needed to elucidate intracranial volume changes and reduction of cell mass in certain brain regions. Our findings offer new insights into the extent of CSF enlargement and the development of WM and GM structures.
Interestingly, neonatal CPB was associated with a significant reduction of deep GM and WM volumes. Our data suggest that neonatal CPB surgery might have led to a reduction of brain volumes at 2 years of age before the Fontan completion. It is well known that deep and periventricular cerebral regions are highly susceptible to chronic hypoxia–ischemia during the first months of life (19). Neuropathological studies revealed that brain disturbances in children with complex CHD before surgery are dominated by cerebral WM injury (20, 21). Brain abnormalities in CHD patients, similar to those in preterm infants, originate and evolve over the last trimester of gestation and are caused by hypoxia–ischemia, are primarily chronic, and involve a complex amalgam of destructive and developmental disturbance, affecting the WM and multiple neuronal/axonal structures (22). Moreover, subplate neurons, a transient population of neurons beneath the cortical plate, develop rapidly in the last trimester in the human brain (23). These neuronal cells have an important role in the development of thalamocortical, corticocortical, and commissural cortical fibers (24). Subplate neurons have been shown to be vulnerable to hypoxia–ischemia in a neonatal rat model (25). Various preoperative cerebral lesions are found to be accentuated after CPB (26). Furthermore, the shorter postoperative care after hybrid approach or no neonatal surgery in cases of SV with balanced hemodynamic compared with a CPB operation is less invasive for the newborn patient. Open-heart surgery in the neonatal period is characterized by numerous blood transfusions, hemodynamic and coagulation imbalances, inflammatory responses, catecholamine infusions, longer mechanical ventilation, and pain and sedation medication among other risk factors. It is well known that cerebral ischemia/reperfusion (27), oxidative stress combined with the propensity for impaired vascular autoregulation in the immature brain (28), infection/inflammatory processes, and certain medication (e.g., fentanyl and volatile anesthetics (5, 29)) can cause neonatal brain injury. Postponing the time of CPB from the more critical neonatal time period toward infancy at 4–6 months of age might result in higher maturational stage of the developing brain and may contribute to a lessened susceptibility of these structures toward perioperative cerebral damage (30). The adverse effect of neonatal CPB on the immature brain as a potential risk factor for consecutive neurodevelopmental outcome has been suggested by Sakamoto et al (31).
In contrast, Beca et al. (32) have shown that new WM injury occurred in the same rate in infants undergoing surgery with and without CPB. However, the predominant cell death pathway for immature brain cells is apoptosis and autophagy (33). Conventional MRI will not be able to detect this consecutive brain cell loss that might have occurred during stage I operation in the neonatal period.
A downside of the hybrid approach might be the prolongation of the intrauterine conditions by stenting the duct and maintaining the retrograde perfusion through the hypoplastic aortic arch. Depending on the size of the aortic arch, the brain perfusion is not improved. This may result in maturation delay of the white and subcortical GM.
Increased CSF and smaller brain volumes are indicative of impairment in brain growth and brain development. Both changes have been shown in patients with SV during fetal life (2, 3), in newborns (7), and in adults (10). Our finding that increased CSF volumes are significantly associated with poorer neurodevelopmental outcome is consistent with recent findings by Owen et al. (7), who reported a relationship between poor behavioral state regulation, greater CSF volumes, and reduced deep GM in cyanotic CHD in neonates with complex CHD before bypass surgery.
Impaired prenatal brain development with a disproportionate increase in CSF volumes (1, 2, 3), and parenchymal as well as metabolic abnormalities (1), has been observed in children with CHD during the third trimester. In this period of rapid brain growth, the maturing oligodendrocytes are highly sensitive to hypoxia/ischemia. Animal studies have shown that chronic hypoxia/ischemia is one of the key pathogenic factors of microstructural dysmaturation with disruption of neuronal connectivity (34). Hence, periventricular WM injury might contribute to secondary ventriculomegaly (35). The delay in brain maturation of ~4–5 weeks in children with HLHS and other complex CHD at term (4) might further increase brain vulnerability against hypoxic–ischemic injuries (36, 37). Premyelinating immature oligodendrocytes are vulnerable during the last trimester, characterized by extensive oligodendrocyte migration and maturation.
In addition to ischemic–hypoxic brain injury, an altered hemodynamic state with a central venous pressure increase after stage II procedure may lead to diffused brain injury and CSF increase (38) in some patients with SV CHD. At pivotal interacting structures between the cardiovascular circulation and the CSF spaces (i.e., cerebral arteries, capillaries of the plexus choroideus (CSF secretion), absorption sites at the arachoid granulations and venous sinuses, and autonomic brain regulatory nuclei in the deep GM) (39) altered hemodynamics may contribute to isolated CSF space enlargement in children with complex CHD. This may result in an imbalance in cardiac impulse distribution and disturbance of the cerebral autoregulation (deficient ‘Windkessel effect’) (38). Increased mean venous pressure after stage II procedure may lead to disturbed CSF resorption and may contribute to chronic congestion as suggested by the pulsation theory (39). Studies measuring intracranial pulse waves may be able to elucidate the interaction of the complex hemodynamic state and brain growth in the future. Whether the increase in CSF volume is the result of underlying diffused brain injury due to microstructural changes needs to be further investigated with more sophisticated imaging techniques or animal studies.
Methodological Considerations
The favorable neurodevelopmental outcome compared with former outcome studies of children with complex heart disease highlights the recent progress in well-adapted surgical treatment and perioperative care of children with CHD of SV physiology. However, these results should be interpreted with caution as it has been shown that the Bayley-III Scales overestimate cognitive outcome compared with the Bayley Scales of Infant Development-II (40), confounding direct comparisons of neurodevelopmental outcomes assessed with the Bayley-III to those reported previously with the Bayley-II.
Limitations to our study arise from the non-randomized study design. Criteria for the choice of the surgical approach may influence the outcome depending on the surgeon’s decision to allocate certain patients to certain surgical procedures.
Our study is further limited by the small size and inhomogeneity of our patient population, particularly with regard to cardiac diagnoses and surgical procedures. Given the small sample size, some apparently significant correlations might have occurred by chance. We used a retrospective study design and studied intracranial volumes at 2–3 years of age, which precludes the observation of volumetric changes over time and their association with anthropometric data at birth. The cross-sectional study design limits the validity of intracranial volumes as predictor of further neurodevelopmental outcome. Further, we did not examine regional volumes or shapes of different brain structures, which may be relevant for future analyses.
Conclusions
Brain volumes of children with SV CHD before the Fontan completion are smaller compared with controls. Those undergoing CPB surgery during the neonatal period had reduced deep GM and WM volumes. There was no difference in brain volumes with regard to the type of SV morphology. Increased CSF volumes seem to represent a risk factor and an early marker for neurodevelopmental impairment in children with SV CHD. Altered brain volumes may result from diffused microstructural brain injury, underscoring the importance of volumetric measurements as a sensitive tool for the assessment of brain development. Assessments of our patient’s neurodevelopmental performance at school age will determine the long-term clinical impact of these early imaging findings.
References
Limperopoulos C, Tworetzky W, McElhinney DB et al, Brain volume and metabolism in fetuses with congenital heart disease evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation 2010; 121: 26–33.
Brossard-Racine M, Plessis AJ, du, Vezina G et al, Prevalence and spectrum of in utero structural brain abnormalities in fetuses with complex congenital heart disease. Am J Neuroradiol 2014; 35: 1593–9.
Mlczoch E, Brugger P, Ulm B et al, Structural congenital brain disease in congenital heart disease: results from a fetal MRI program. Eur J Paediatr Neurol 2013; 17: 153–60.
Licht DJ, Shera DM, Clancy RR et al, Brain maturation is delayed in infants with complex congenital heart defects. J Thorac Cardiovasc Surg 2009; 137: 529–36discussion 536–7.
Andropoulos DB, Ahmad HB, Haq T et al, The association between brain injury, perioperative anesthetic exposure, and 12-month neurodevelopmental outcomes after neonatal cardiac surgery: a retrospective cohort study. Paediatr Anaesth 2014; 24: 266–74.
Fischl B, Salat DH, Busa E et al, Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002; 33: 341–55.
Owen M, Shevell M, Donofrio M et al, Brain volume and neurobehavior in newborns with complex congenital heart defects. J Pediatr 2014; 164: 1121–7 e1.
Watanabe K, Matsui M, Matsuzawa J et al, Impaired neuroanatomic development in infants with congenital heart disease. J Thorac Cardiovasc Surg 2009; 137: 146–53.
von Rhein M, Buchmann A, Hagmann C et al, Brain volumes predict neurodevelopment in adolescents after surgery for congenital heart disease. Brain 2014; 137: 268–76.
Cordina R, Grieve S, Barnett M, Lagopoulos J, Malitz N, Celermajer DS . Brain volumetrics, regional cortical thickness and radiographic findings in adults with cyanotic congenital heart disease. NeuroImage Clin 2014; 4: 319–25.
Knirsch W, Mayer KN, Scheer I et al, Structural cerebral abnormalities and neurodevelopmental status in single ventricle congenital heart disease before Fontan procedure. Eur J Cardiothorac Surg 2016; 51: 740–6.
Bellinger DC, Watson CG, Rivkin MJ et al, Neuropsychological status and structural brain imaging in adolescents with single ventricle who underwent the Fontan procedure. J Am Heart Assoc 2015; 4: e002302.
Largo RH, Pfister D, Molinari L, Kundu S, Lipp A, Duc G . Significance of prenatal, perinatal and postnatal factors in the development of AGA preterm infants at five to seven years. Dev Med Child Neurol 1989; 31: 440–56.
WHO Multicentre Growth Reference Study Group. WHO child growth standards based on length/height, weight and age. Acta Paediatr Suppl 1992; 450 ((Suppl 2006)): 76–85.
Bayley N. Bayley Scales of Infant and Toddler Development Manual, 3rd edn. San Antonio, TX: The Psychological Corporation, 2006.
Hajnal BL, Sahebkar-Moghaddam F, Barnwell AJ, Barkovich AJ, Ferriero DM . Early prediction of neurologic outcome after perinatal depression. Pediatr Neurol 1999; 21: 788–93.
Mayer KN, Latal B, Knirsch W et al, Comparison of automated brain volumetry methods with stereology in children aged 2 to 3 years, 2016 (http://link.springer.com/article/10.1007%2Fs00234-016-1714-x).
Hangge PT, Cnota JF, Woo JG et al, Microcephaly is associated with early adverse neurologic outcomes in hypoplastic left heart syndrome. Pediatr Res 2013; 74: 61–7.
Raman L, Kong X, Gilley JA, Kernie SG . Chronic hypoxia impairs murine hippocampal development and depletes the postnatal progenitor pool by attenuating mTOR signaling. Pediatr Res 2011; 70: 159–65.
Glauser TA, Rorke LB, Weinberg PM, Clancy RR . Congenital brain anomalies associated with the hypoplastic left heart syndrome. Pediatrics 1990; 85: 984–90.
Hinton RB, Andelfinger G, Sekar P et al, Prenatal head growth and white matter injury in hypoplastic left heart syndrome. Pediatr Res 2008; 64: 364–9.
Volpe JJ . Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol 2009; 8: 110–24.
Kostović I, Judaš M . The development of the subplate and thalamocortical connections in the human foetal brain. Acta Paediatr 2010; 99: 1119–27.
Bystron I, Blakemore C, Rakic P . Development of the human cerebral cortex: Boulder Committee revisited. Nat Rev Neurosci 2008; 9: 110–22.
McQuillen PS, Sheldon RA, Shatz CJ, Ferriero DM . Selective vulnerability of subplate neurons after early neonatal hypoxia-ischemia. J Neurosci 2003; 23: 3308–15.
Mahle WT, Tavani F, Zimmerman RA et al, An MRI study of neurological injury before and after congenital heart surgery. Circulation 2002; 106: I109–14.
Volpe JJ . Brain injury in the premature infant: overview of clinical aspects, neuropathology, and pathogenesis. Semin Pediatr Neurol 1998; 5: 135–51.
Menke J, Michel E, Hillebrand S, Von Twickel J, Jorch G . Cross-spectral analysis of cerebral autoregulation dynamics in high risk preterm infants during the perinatal period1. Pediatr Res 1997; 42: 690–9.
McPherson C, Haslam M, Pineda R, Rogers C, Neil JJ, Inder TE . Brain injury and development in preterm infants exposed to fentanyl. Ann Pharmacother 2015; 49: 1291–7.
Knirsch W, Liamlahi R, Dave H, Kretschmar O, Latal B . Neurodevelopmental outcome of children with hypoplastic left heart syndrome at one and four years of age comparing hybrid and norwood procedure. Ann Thorac Cardiovasc Surg 2016; 22: 375–7.
Sakamoto T. Current status of brain protection during surgery for congenital cardiac defect. Gen Thorac Cardiovasc Surg 2016;64:72–84..
Beca J, Gunn JK, Coleman L et al, New white matter brain injury after infant heart surgery is associated with diagnostic group and the use of circulatory arrest. Circulation 2013; 127: 971–979.
Hagberg H, Mallard C, Rousset CI, Wang Xiaoyang . Apoptotic mechanisms in the immature brain: involvement of mitochondria. J Child Neurol 2009; 24: 1141–6.
McClendon E, Chen K, Gong X et al, Prenatal cerebral ischemia triggers dysmaturation of caudate projection neurons. Ann Neurol 2014; 75: 508–24.
Ment LR, Schwartz M, Makuch RW, Stewart WB . Association of chronic sublethal hypoxia with ventriculomegaly in the developing rat brain. Dev Brain Res 1998; 111: 197–203.
Dimitropoulos A, McQuillen PS, Sethi V et al, Brain injury and development in newborns with critical congenital heart disease. Neurology 2013; 81: 241–8.
Sethi V, Tabbutt S, Dimitropoulos A et al, Single-ventricle anatomy predicts delayed microstructural brain development. Pediatr Res 2013; 73: 661–7.
Min KJ, Yoon SH, Kang J-K . New understanding of the role of cerebrospinal fluid: offsetting of arterial and brain pulsation and self-dissipation of cerebrospinal fluid pulsatile flow energy. Med Hypotheses 2011; 76: 884–6.
Luciano M, Dombrowski S . Hydrocephalus and the heart: interactions of the first and third circulations. Cleve Clin J Med 2007; 74 (Suppl 1): S128–31.
Johnson S, Moore T, Marlow N . Using the Bayley-III to assess neurodevelopmental delay: which cut-off should be used? Pediatr Res 2014; 75: 670–4.
Acknowledgements
We thank all children and their families for joining our study. We specially thank the entire MRI staff in Zurich and Giessen, our MR technician Ali Rad in Giessen, the anesthesiologists, our study nurse Gabriela Staub, and the very helpful technical support by Beat Werner during data acquisition and analysis. We also thank Juergen Bauer for his valuable help with the database.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Statement of Financial Support
This study was funded by Kinderherzen e.V., Bonn, Germany, and Mäxi Foundation, Zurich, Switzerland.
Disclaimer
Both sponsors had no influence on study design, the collection, analysis, and interpretation of data, the writing of the paper, and the decision to submit the paper for publication.
Supplementary material is linked to the online version of the paper at
Supplementary information
Rights and permissions
About this article
Cite this article
Heye, K., Knirsch, W., Latal, B. et al. Reduction of brain volumes after neonatal cardiopulmonary bypass surgery in single-ventricle congenital heart disease before Fontan completion. Pediatr Res 83, 63–70 (2018). https://doi.org/10.1038/pr.2017.203
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2017.203
This article is cited by
-
Altered white matter connectivity in children with congenital heart disease with single ventricle physiology
Scientific Reports (2023)
-
Association of cerebellar volume with cognitive and motor function in adults with congenital heart disease
Neurological Sciences (2023)
-
Infants with congenital heart defects have reduced brain volumes
Scientific Reports (2021)
-
Brain volumes in adults with congenital heart disease correlate with executive function abilities
Brain Imaging and Behavior (2021)
-
Postoperative brain volumes are associated with one-year neurodevelopmental outcome in children with severe congenital heart disease
Scientific Reports (2019)