Abstract
Background:
Infants of women with pregestational diabetes are at risk for developing obesity in later life. This study aimed to identify subgroups at highest risk, by studying growth profiles of offspring from women with type 1 or 2 diabetes mellitus (ODM1, ODM2) until the age of 14 y.
Methods:
Information from infant welfare centers was received for 78 ODM1 and 44 ODM2. Mean BMI SD scores (SDS) (based on 1980 nation-wide references) and height SDS (based on 2009 references) were calculated and included in a random-effects model. Values were compared to the 2009 Dutch growth study.
Results:
BMI SDS profiles differed between ODM1 and ODM2, with the highest mean BMI SDS profiles in ODM2. Other factors that affected growth profiles in these infants included the presence of maternal obesity, large for gestational age (LGA) at birth and in ODM2 a Dutch-Mediterranean origin.
Conclusion:
Offspring of women with diabetes have higher BMI SDS profiles than observed in the 2009 Dutch growth study, with the highest BMI SDS in ODM2 who are LGA at birth and have obese mothers. Preventive strategies for offspring adiposity may include pursuing lower prepregnancy maternal BMI, prevention of LGA at birth, and prevention of increased weight gain during childhood.
Similar content being viewed by others
Main
Maternal hyperglycemia in pregestational diabetes is associated with fetal hyperinsulinism and asymmetrical fetal overgrowth expressed as a higher abdominal circumference compared to head circumference, even in neonates with a normal birthweight (1,2,3). This cascade not only leads to short-term neonatal problems, but also forms the basis for problems later in life, with increased risks for obesity and associated metabolic disorders (4,5).
As adolescents, offspring from pregnancies complicated by type 1 or type 2 diabetes (DM1; DM2) are at risk for developing type 2 diabetes mellitus (6,7). Offspring growth trajectories, expressed as BMI and height trajectories, could be helpful in identifying those children at risk for obesity. For instance, in a nondiabetic cohort study, (8) an accelerated rise in BMI after the age of 2 predicted later development of DM2. Also, the cumulative incidence of DM2 has been shown to be positively related to BMI at each age from age 4 y onward (9).
Postnatal linear growth and BMI trajectories of infants from diabetic women may identify those with the greatest risk to develop diabetes and/or metabolic syndrome. Cross-sectional studies, which included offspring BMI at a single age, have shown higher BMI SD score (SDS) in offspring of women with either DM1 (7,10,11) or DM2 (6,12,13) compared to controls. A small study has shown that offspring of DM2 women have a higher BMI than offspring of DM1 women or nondiabetic controls (14). A retrospective cohort showed, however, a similar BMI between offspring of DM1 women and controls (15). Due to the cross-sectional design and methodology of these studies, growth patterns were not available. Also, differences in methodology and definitions of overweight/obesity make comparisons difficult. The few longitudinal studies performed in offspring from pregestational diabetic pregnancies have shown different growth trajectories between large for gestational age (LGA) and non-LGA offspring of DM1 pregnancies (16) and higher risks of overweight in offspring of DM1 (17) or DM2 (13).
There are no longitudinal studies comparing growth of offspring of DM1 and DM2 women, which might be crucial to formulate preventive strategies in these high-risk groups. Data on height of offspring from women with diabetes are also lacking; such data may help to identify the importance of the weight component in the equation toward possible childhood overweight/obesity. Therefore, the aim of the current study was to construct growth trajectories for offspring of women with type 1 and type 2 diabetes, expressed as height and BMI SDS trajectories, with subgroups of LGA and non-LGA offspring. This was done to assess possible differences in postnatal growth trajectories and to identify infants with the most pathological growth pattern, possibly resulting in overweight.
Methods
Patients
The study group consisted of singleton offspring of women with type 1 diabetes (ODM1) and type 2 diabetes (ODM2) who delivered in the University Medical Centre Utrecht, the Netherlands between 1990 and 2006. Women with gestational diabetes were excluded from this study. All these women were contacted in 2013 and were invited to participate by consenting to retrieve individual offspring growth charts from the Dutch infant welfare and school health centers. The parents completed a questionnaire including questions regarding maternal and paternal height, current weight, comorbidities and ethnicity; also parents provided the most recent height and weight data of the child, measured either by a health professional or themselves. The welfare centers have a high coverage and record infant weight, supine length (<2.0 y), and height (≥2.0 y) on specified dates between birth and 4 y (1, 4, 6, 9, 11, 14, 18, 24, 36, 45 mo). Thereafter, children are measured in the school health service at 5.5, 11, and 13 y, with a variance of 1–2 y around these time points. Trained health care professionals perform the measurements. Infants’ length and standing height was measured to the nearest 0.1 cm. Up to 15 mo, children were weighed naked. Older children were weighed wearing underwear only, on calibrated mechanical or electronic step scales. Weight was to the nearest 0.1 kg. Ethics approval was granted by the Medical Ethics Committee at the University Medical Center, Utrecht in the Netherlands (application number 13/179, reference number WAG/om/13/053639) on the 09 April 2013.
Methods
Baseline maternal characteristics at pregnancy and pregnancy outcomes were retrieved from records of the UMC Utrecht. Parents provided information regarding their own current height and weight, educational status, and current height and weight for each child when they completed the written questionnaire. Birth weight (BW) SDS was calculated as follows: (BW minus mean BW for gender, parity, and gestational age)/SD for gender, parity, and gestational age, based on Dutch reference data (18). LGA was defined as a BW ≥90th percentile corrected for gestational age, gender, and parity (18). Conditional target height (cTH) of offspring was calculated based on parental height according to Hermanussen and Cole (19) and adapted to Dutch growth standards (20). Length and height of ODM1 and ODM2 were expressed as SDS for age and gender based on the Fifth Dutch Growth Study performed in 2009 (21). BMI was calculated from height and weight with the following formula: weight (kg)/(height (m))2. BMI was expressed as a SDS for the 1980 nation-wide growth study (before the onset of the so-called “obesity epidemic”), in which SDS 0 equals the age- and gender-specific mean of the 1980 Dutch reference population (22), in order to assess the degree of overweight and obesity. The latter data are presently used as the normative standard for BMI in the Netherlands. However, our data were also compared to the 2009 Dutch BMI data, to enable comparison with the current child population (23).
Values from the Fifth Dutch Growth Study and Subgroup Analyses
As mentioned above, since the BMI of Dutch children has increased from the 1980s onwards, we also calculated mean BMI SDS for children of Dutch origin participating in the 2009 (Fifth) Dutch Growth Study (23). The values of the 2009 nation-wide study were plotted in the BMI SDS graphs for visual comparison of our offspring from the diabetic pregnancies, in order to show the effect of the obesity epidemic. The ODM2 group was a heterogeneous group with a 50% of Mediterranean origin, therefore subgroup analyses of this group was performed. BMI SDS of Dutch-Mediterranean children was calculated based on a 50-50% mixed sample of Dutch-Mediterranean children participating in the 2009 (Fifth) Dutch Growth Study (23,24). These values are plotted in the BMI SDS graphs of ODM2 for visual comparison to the nation-wide BMI SDS of Dutch-Mediterranean children.
Statistical Analysis
For comparison of ODM1 and ODM2 at baseline, categorical variables were compared through the Chi-square test; continuous variables with the t-test and non-parametric variables with the Mann–Whitney U-test.
The longitudinal analyses fitted smooth, flexible curves with a random-effects model to estimate the growth trajectories of ODM1, ODM2, non-LGA ODM1, non-LGA ODM2, LGA ODM1, and LGA ODM2. Mixed model addresses the correlation of repeated height and BMI SDS measurements obtained within the same child, as well as time-independent variables (maternal age at delivery, parity, educational level, employment hours, marital status, ethnicity, breast feeding, preconceptional HbA1c, mean pregnancy HbA1c, paternal BMI, paternal ethnicity, or paternal diabetes) and accommodates to the available values in the dataset. Fixed effects were the covariates maternal diabetes type (DM1, DM2), LGA (yes, no), time (age in years), and the interaction between time and maternal diabetes type to show increases or decreases in growth over time. Random effects were intercept and time. Potential confounders were the previously mentioned time-independent variables; these were labeled as covariates in a sensitivity analysis. If the addition of a covariate to the model changed the estimate with more than 10%, we considered this a confounder. In a next step, we checked whether these potential confounders changed the model by visual inspection of the graphs.
Given the known rapid decreases in BMI SDS during the first year of life in infants born LGA, both in (non)-diabetic populations (9,13,16), we separately analyzed the growth trajectories in infancy.
In a model with the factors as fixed effects and random effects (mentioned before), the models were examined using the Akaike information criterion and Bayesian information criterion. The best model fit had the lowest Akaike information criterion and Bayesian information criterion, which included a linear and square interaction of diabetes with age, with intercept and age as a random effect, in order to determine the trajectories for BMI and height SDS. Consequently, for the growth SDS points in the square model, the values of ODM1, ODM2, and both non-LGA and LGA ODM1 and ODM2 were modeled as
SDS = intercept+β0ij+β1ij(age)+β2ij(age)**2, where β0 represents the intercept, βi is the diabetes type (e.g. maternal DM1 or DM2), βj is LGA and age is offspring age in years (**2=square). The mixed model values for BMI SDS are available in Supplementary Table S1 online. Data were analyzed using IBM SPSS Statistics version 23.0 for Mac and Microsoft Excel for Mac 2011. Software prepared by the Dutch Growth Research Foundation, Growth Analyser 3.5, Rotterdam, The Netherlands, was used to calculate height SDS using the 2009 data from the Fifth Dutch Growth Study (21) and BMI SDS using the 1980 Dutch nationwide data which are used as normative standards for present day Dutch children (22).
Results
From 1990 to 2006, 150 ODM1 and 70 ODM2 were identified, of which we received parental informed consent for offspring growth charts from 78 (52%) and 44 (63%), from respectively 52 and 32 mothers. From these parents, we received a completed questionnaire for 51 (65% of responders) ODM1 and 21 (48% of responders) ODM2. Age at childbirth and pregestational BMI from mothers who participated were comparable to those of nonresponders. Children were followed for a median of 9 y (interquartile range 6 y).
Baseline Characteristics
One of the included infants had a small ventricular septum defect. All mothers were treated with insulin during their pregnancies. The average ± SD number of height and weight measurements per child between birth and 14 y of age was 8 ± 2, with 629 measurements for ODM1 and 342 for ODM2.
Therefore, we subdivided Table 1 into maternal and offspring characteristics with different numbers and certain maternal and paternal characteristics (e.g., ethnicity) valid for all pregnancies within that family. All mothers with DM1 were Caucasian as well as 96% of their partners ( Table 1 ). In contrast, 46% of mothers with DM2 were of Mediterranean descent, as were all partners in this subgroup. Maternal BMI in ODM2 was 6 points higher than ODM1. Conditional target height was significantly lower for girls in ODM2 compared to ODM1; between the boys there were no significant differences. The percentage of offspring overweight or obese at the ages 4–6, 7–10, and 11–14 y is also given in Table 1 ; these numbers are based on the International Obesity Task Force (IOTF) cut-off values (25). The women with DM1 had a higher cesarean section rate than those with DM2; the relatively lower birthweight of DM1 infants was consistent with the shorter gestational age. 46% of ODM1 were LGA and 43% of ODM2. Only two infants had a birth weight below the 10th centile (one in each group).
BMI SDS Trajectories: 1 to 12 mo
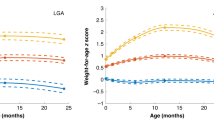
At age 1 mo, LGA ODM1 and LGA ODM2 had a higher BMI, as was to be expected giving their high birth weight. All BMI SDS trajectories showed a negative slope with decreasing BMI SDS ( Figure 1a , b ). Even though LGA ODM2 had the highest BMI SDS values, there were no statistically significant differences compared to ODM1.
Mixed model for mean BMI SDS for offspring at the age of 1 to 12 mo for ODM1 and ODM2 (a); (non)-LGA ODM1 vs. ODM2 (b); mean BMI SDS from 1 to 14 y for ODM1 vs. ODM2 with reference values from the 2009 growth study (c); (non)-LGA ODM1 vs. ODM2 with values from the 2009 growth study (d). Reference values of Dutch boys and girls were adapted from Schonbeck et al. (23), based on values from Cole & Roede (22). White triangle = ODM1 ▵, black triangle = ODM2 ▴; white circle = non-LGA ODM1 ○; black circle = LGA ODM •; white square = non-LGA ODM2 □; black square = LGA ODM ▪; dotted line = Dutch Boys 2009 ••••; dashed line = Dutch Girls 2009 −−−−.
BMI SDS trajectories for primiparous women are shown in Supplementary Table S1 online. Due to comparable results, all offspring are included in the analyses.
BMI SDS Trajectories: 1 to 14 y
BMI SDS trajectories are shown in Figure 1c , d , in which the values of the 2009 Dutch growth study are also shown. Growth trajectories of ODM1 and ODM2 were significantly different from each other (age P < 0.001; age2 P = 0.004).
Figure 1d shows BMI SDS trajectories in non-LGA and LGA subgroups. LGA ODM2 infants had the highest BMI SDS in early childhood with a gradual increase and subsequently the highest BMI SDS in early adolescence, possibly showing a pathway to pediatric overweight. LGA ODM1 showed a steady increase in BMI SDS with lower values than LGA ODM2, with a significantly different BMI SDS trajectory (P = 0.04). The trajectories of non-LGA ODM1 and non-LGA ODM2 followed the same pattern as that of their LGA counterparts, albeit at a lower level. The BMI SDS trajectory of non-LGA ODM1 was significantly lower than that in non-LGA ODM2 (P = 0.01). The same analysis was performed in primiparous women, thus including only one infant of each woman, which showed similar results (Supplementary Table S1 online).
Comparison to 2009 (Fifth) Dutch Growth Study
It was not possible to calculate statistically significant differences between offspring of diabetic pregnancies and the 2009 nation-wide study. However, visual comparison of the growth trajectories was possible as the mean BMI SDS values for the 2009 Dutch study are plotted in the graphs. In infancy, length of ODM1 was slightly below and ODM2 was slightly above the Dutch nation-wide study, which is represented as SDS 0. Up to age 14, height SDS was similar to the 2009 nation-wide growth study in both ODM1 and ODM2 (Supplementary Figure S1c online, SDS 0).
BMI SDS of ODM1 was close to that of the Dutch growth study population in early childhood and showed a gradual increase from mid–childhood onwards, slightly above the 2009 growth study. ODM2 showed a gradual increase in BMI SDS from the first year onwards, resulting in a BMI SDS in early adolescence that was higher than the 2009 growth study ( Figure 1c ).
The BMI SDS trajectory of non-LGA ODM1 was lower than the 2009 growth study. LGA ODM1 was slightly above the 2009 growth study in early childhood with a continuing increase and reaching higher values than Dutch children. Both non-LGA and LGA ODM2 have higher values than the Dutch growth study ( Figure 1d ).
Covariates maternal age at delivery, parity, educational level, employment hours, marital status, ethnicity, breast feeding, preconceptional HbA1c, mean pregnancy HbA1c, paternal BMI, paternal ethnicity, or paternal diabetes did not influence any of the models and were therefore excluded as confounders.
Ethnicity in ODM2
Even though maternal ethnicity was a non-significant confounder in the mixed model for the whole group, we performed a subgroup analysis in ODM2 because of a heterogeneity of ethnicities within this group and known differences between Dutch and Dutch-Mediterranean offspring (24,26).
There were no differences in height for Dutch and Dutch-Mediterranean ODM1 and ODM2 ( Figure 2a ).
Mixed model for only ODM2 with mean height SDS of Dutch and Mediterranean (non)-LGA ODM2 (a) ; and BMI SDS of Dutch and Mediterranean (non)-LGA ODM2 and the 2009 growth study (reference population) (b) . Reference values from Dutch boys and girls were adapted from Schonbeck et al. (23), Mediterranean data from van Dommelen et al. (24) ; based on values from Cole and Roede (22). White diamond = non-LGA Dutch ODM2 ◊; black diamond = LGA Dutch ODM ◆; white circle = non-LGA Mediterranean ODM2 ○; black circle = LGA Mediterranean ODM2 •; white triangle = ODM2 Mediterranean Δ; black triangle = ODM2 Dutch ▴; dotted line = Dutch Boys 2009 •••; dashed + dot line = Dutch Girls 2009 −−•; dashed line = Mediterranean Boys 2009 −−−; cross = Mediterranean girls 2009→.
LGA Dutch-Mediterranean ODM2 showed the highest increase in BMI SDS from early childhood until early adolescence, however there were no statistically significant differences in BMI SDS trajectories between LGA and non-LGA Dutch and Dutch-Mediterranean ODM2 ( Figure 2b ). Even non-LGA Dutch-Mediterranean children have a BMI SDS trajectory slightly above their nation-wide counterparts. Values from the nation-wide studies are all lower in their mean BMI SDS trajectories, indicating that ODM2 have a risk of becoming overweight with BMI SDS reaching up to +3SDS in adolescence.
Maternal BMI
Addition of maternal BMI to the models of growth trajectories in ODM1 and ODM2 resulted in an increase of BMI SDS trajectories in the non-LGA and LGA ODM1 and in a decrease in the non-LGA ODM2 subgroups ( Figure 3a , b ). A separate subgroup analysis of ODM1 from mothers with a prepregnancy BMI below 25 kg/m2 or above 25 to 30 kg/m2 is shown in Figure 3c and a similar analysis of ODM2 from mothers with a BMI from 25 to 30 kg/m2 or above 30 kg/m2 is shown in Figure 3d . The growth trajectories according to maternal BMI did not differ much in the ODM1, but ODM2 from a mother with a BMI > 30 had a considerable higher BMI SDS than those from a mother with a BMI 25–30 kg/m2.
Mixed model for offspring mean BMI SDS for non-LGA vs. LGA ODM1 and the effect of maternal BMI on the growth trajectories (a); similar for non-LGA vs. LGA ODM2 (b). Offspring mean BMI SDS for ODM1 for mothers with a BMI ≤ 25 kg/m2 and 25–30 kg/m2(c); similar for ODM2 according to a maternal BMI of BMI 25–30 kg/m2 and > 30 kg/m2 (d); White triangle = ODM1 non-LGA* Δ; black triangle = ODM1 LGA* ▴; white diamond = ODM2 non-LGA* ◊; black diamond = ODM2 LGA* ◆; white circle = ODM1 and mothers with BMI ≤ 25 kg/m2 ○; black circle = ODM1 and mothers with BMI >25/≤ 30 kg/m2 •; white square = ODM2 and mothers with BMI >25/≤30 kg/m2 □; black square = ODM2 and mothers with BMI > 25 kg/m2 ▪.*corrected for maternal BMI.
Length and Height
Length SDS plateaued in the first year of life (Supplementary Figure S1a,b online). Differences between ODM1 and ODM2 were not significant (NS, Supplementary Figure S1b online).
Height of ODM1 and ODM2 were similar (Supplementary Figure S1c online). LGA offspring in both ODM1 and ODM2 were slightly taller than the non-LGA groups, although differences were small (NS; Supplementary Figure S1d online).
Discussion
This is the first exploratory study comparing growth trajectories of BMI and height SDS of offspring of women with type 1 and type 2 diabetes mellitus. At birth, almost 50% of neonates in both groups were LGA but thereafter growth trajectories differed significantly with highest BMI SDS in ODM2. While non-LGA ODM1 showed a BMI pattern similar to the 2009 nation-wide study, BMI SDS increased steadily in LGA ODM1. Highest values were found in ODM2 that were LGA at birth, which is in line with current literature (16,17). There were no significant differences in glucose control during pregnancy between the two types of maternal diabetes (HbA1C), but maternal BMI was significantly higher in women with type 2 diabetes. Subgroup analyses of ODM2 based on ethnicity showed the highest BMI SDS values for Dutch-Mediterranean ODM2 who were born LGA. Height SDS for both ODM1 and ODM2 were comparable to those of the 2009 Dutch Growth Study, which indicates that only weight in these infants is increased and not height.
Previous studies on growth of offspring of women with pregestational diabetes either analyzed only ODM1 (16,17) or combined different types of diabetes (27). There are a number of cross-sectional studies, but these lack a uniform definition for overweight/obesity and differences in statistical methodology preclude adequate comparison (7,10,11,28,29,30). There are only two longitudinal growth studies in infants of women with pre-existing diabetes. In one study, it was found that maternal BMI and being LGA at birth were predictors of childhood overweight in ODM1 (16). The other longitudinal study was performed in children who were born to First Nation Canadian inhabitants with pediatric-onset type 2 diabetes. In this diabetes-prone population, 89% of infants were already overweight or obese at an early age and remained so until the age of 19 y (13). Our longitudinal data expressed in growth trajectories could be a first step in providing parents and health care workers with a gross estimation of when they should provide special attention to offspring from pregestational diabetic women in order to possibly prevent adiposity.
Addition of maternal BMI to the model did not change the BMI SDS trajectories, but it did shift the intercept slightly upwards in LGA and non-LGA ODM1 and resulted in a downward shift in the non-LGA ODM2, suggesting that maternal BMI does influence offspring growth in this population. Subgroup analyses according to maternal BMI did not reveal major differences in growth profiles in ODM1, but obese women with type 2 had children with a higher BMI SDS. In other words, a normal or slightly increased BMI (25–30 kg/m2) in women with diabetes, results in an offspring growth trajectory that is relatively close to the Dutch 2009 reference group (23). In contrast, maternal obesity, which was the case in about half of ODM2, results in a higher growth trajectory with more childhood obesity.
Developmental Origins of Health and Disease (DOHaD) states that intrauterine overnutrition leads to programming of adaptations of the fetal metabolism to cope with an adverse postnatal environment, which is enriched with suitable nutrients (31,32). These adaptations cause accumulation of adipose tissue and (relative) hyperglycemia leading to pathological changes in appetite and energy regulation in offspring (4). These epigenetic changes affect both ODM1 and ODM2, because not only genetic susceptibility to overweight, but also epigenetics and parental lifestyle habits may echo into the child and influence growth trajectories in their development. Environmental factors might possibly have a larger effect in ODM2 because of a higher maternal BMI. Unfortunately, there are no studies investigating lifestyle and eating habits in nurturing of ODM and subsequent development of overweight/obesity.
Preventive strategies that might combat childhood obesity in this population should include striving for a lower maternal prepregnancy BMI, especially in women with type 2 diabetes. To pursue normal maternal prepregnancy BMI might also contribute to prevent childhood obesity, given that families are better accustomed to a healthier lifestyle. Mothers with a higher maternal BMI are, for example, known to impose less dietary restrictions on offspring (33). Another important aspect is the prevention of LGA at birth. This seems difficult at present and data indicate an increase rather than a decrease in LGA infants of these women (34). This may among others be explained by the better periconceptional glucose control of the present resulting in better placentation (35), increased maternal weight gain during pregnancy (36), and poorer glucose control in the third trimester of pregnancy, since women are not routinely admitted to hospital anymore (37). Strict dietary and maternal weight gain monitoring during pregnancy is likely to be the most effective preventive measure at this moment. Follow-up studies have shown that accelerated weight gain in young infants is strongly related to development of obesity and metabolic syndrome in later life (9). Given the relationship between maternal diabetes and overweight/obesity in their offspring, child welfare centers and pediatricians should monitor the weight trajectories in these infants closely, including dietary and lifestyle advices. Priority should be given to infants of (obese) women with type 2 diabetes and—in the Netherlands—especially to those of Dutch-Mediterranean background.
Strengths and Limitations
Our data on growth trajectories of offspring of pregestational diabetic pregnancies could not be compared with a control group (data from uncomplicated pregnancies from the same institution). We were, however, able to compare growth trajectories with those obtained from data dating before the obesity epidemic, namely the 1980 Dutch population study (SDS of 0) and these served as the basis for SDS calculation. Also, we could visually compare our data to the 2009 Dutch Growth Study on healthy children of Dutch and Mediterranean origin (21,23,24). It was not possible to calculate whether differences between our mixed model trajectories were significantly different from the most recent Dutch nation-wide study.
Growth trajectories in the current study were based on the measurements at several ages with differences in the number of available measurements at the different ages. However, the mixed model technique builds the curve based on the available values, which gives a custom model that fits all available values. Although the growth trajectories as depicted in the figures were all different, statistical significance was not always reached which may probably be due to the low numbers of infants included. Furthermore, Tanner stages and the onset of puberty was unknown in our study population. Correction for the onset of puberty was therefore not possible.
The parental informed consent for offspring growth charts was 52–63% of all women contacted, which is comparable to that in retrospective cross-sectional studies (response rate of 46 to 66% (38,39,40)). Due to the respective nature of this study, with children born up to 14 y earlier, such a relatively low response rate might be inevitable. The response regarding the supplementary questionnaires was low. This may possibly be due inadequate knowledge of the Dutch language in women with type 2 diabetes and/or a busy lifestyle of working parents. Selection bias is probably minimal, since it is unlikely that the parental response rate would be associated with their children’s body characteristics.
We did not include women with gestational diabetes in this study given the relatively short duration of gestational diabetes during pregnancy. This group will be subject of a subsequent study.
In conclusion, BMI SDS trajectories of offspring of women with type 1 or type 2 diabetes born in a single tertiary centre, differed despite a similar percentage of LGA infants at birth. Risk factors for a higher BMI SDS profile were: type 2 diabetes of the mother (especially in those of Dutch-Mediterranean background), maternal obesity and being LGA at birth. Some of these risk factors (type 2 diabetes and maternal obesity) are likely to be inter-related. Prevention of childhood obesity in these offspring might be achieved by prepregnancy prevention of overweight in women with type 2 diabetes, prevention of excessive weight gain in pregnancy and by close follow-up of weight gain of the infants during childhood with dietary and lifestyle advices. The latter seems especially important in infants of women with type 2 diabetes who were LGA at birth. Studies on lifestyle and nutrition in offspring of women with diabetes are thus far lacking, but are important to further unravel the causes of childhood obesity in this population.
Statement of Financial Support
No financial assistance was received in support of this study.
Disclosure
There are no disclosures.
References
Wong SF, Chan FY, Oats JJ, McIntyre DH. Fetal growth spurt and pregestational diabetic pregnancy. Diabetes Care 2002;25:1681–4.
Lim EL, Burden T, Marshall SM, Davison JM, Blott MJ, Waugh SJ, Taylor R. Intrauterine growth rate in pregnancies complicated by type 1, type 2 and gestational diabetes. Obstetric Medicine 2009;2:21–5.
Hammoud NM, Visser GH, Peters SA, Graatsma EM, Pistorius L, de Valk HW. Fetal growth profiles of macrosomic and non-macrosomic infants of women with pregestational or gestational diabetes. Ultrasound Obstet Gynecol 2013;41:390–7.
McMillen IC, Rattanatray L, Duffield JA, et al. The early origins of later obesity: pathways and mechanisms. Adv Exp Med Biol 2009;646:71–81.
Silverman BL, Metzger BE, Cho NH, Loeb CA. Impaired glucose tolerance in adolescent offspring of diabetic mothers. Relationship to fetal hyperinsulinism. Diabetes Care 1995;18:611–7.
Dabelea D, Hanson RL, Lindsay RS, et al. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes 2000;49:2208–11.
Weiss PA, Scholz HS, Haas J, Tamussino KF, Seissler J, Borkenstein MH. Long-term follow-up of infants of mothers with type 1 diabetes: evidence for hereditary and nonhereditary transmission of diabetes and precursors. Diabetes Care 2000;23:905–11.
Eriksson JG, Osmond C, Kajantie E, Forsén TJ, Barker DJ. Patterns of growth among children who later develop type 2 diabetes or its risk factors. Diabetologia 2006;49:2853–8.
Eriksson JG, Forsén T, Tuomilehto J, Osmond C, Barker DJ. Early adiposity rebound in childhood and risk of Type 2 diabetes in adult life. Diabetologia 2003;46:190–4.
Lindsay RS, Nelson SM, Walker JD, et al. Programming of adiposity in offspring of mothers with type 1 diabetes at age 7 years. Diabetes Care 2010;33:1080–5.
Rodrigues S, Ferris AM, Peréz-Escamilla R, Backstrand JR. Obesity among offspring of women with type 1 diabetes. Clin Invest Med 1998;21:258–66.
Ezenwaka C, Kalloo R, Offiah N, Eckel J. Development of impaired glucose tolerance and diabetes in follow-up offspring of Caribbean patients with type 2 diabetes: analysis of 5-year follow-up study. Arch Physiol Biochem 2006;112:158–65.
Mendelson M, Cloutier J, Spence L, Sellers E, Taback S, Dean H. Obesity and type 2 diabetes mellitus in a birth cohort of First Nation children born to mothers with pediatric-onset type 2 diabetes. Pediatr Diabetes 2011;12(3 Pt 2):219–28.
Hunter WA, Cundy T, Rabone D, et al. Insulin sensitivity in the offspring of women with type 1 and type 2 diabetes. Diabetes Care 2004;27:1148–52.
Manderson JG, Mullan B, Patterson CC, Hadden DR, Traub AI, McCance DR. Cardiovascular and metabolic abnormalities in the offspring of diabetic pregnancy. Diabetologia 2002;45:991–6.
Rijpert M, Evers IM, de Vroede MA, de Valk HW, Heijnen CJ, Visser GH. Risk factors for childhood overweight in offspring of type 1 diabetic women with adequate glycemic control during pregnancy: Nationwide follow-up study in the Netherlands. Diabetes Care 2009;32:2099–104.
Hummel S, Pflüger M, Kreichauf S, Hummel M, Ziegler AG. Predictors of overweight during childhood in offspring of parents with type 1 diabetes. Diabetes Care 2009;32:921–5.
Visser GH, Eilers PH, Elferink-Stinkens PM, Merkus HM, Wit JM. New Dutch reference curves for birthweight by gestational age. Early Hum Dev 2009;85:737–44.
Hermanussen M, Cole J. The calculation of target height reconsidered. Horm Res 2003;59:180–3.
van Dommelen P, Schönbeck Y, van Buuren S. A simple calculation of the target height. Arch Dis Child 2012;97:182.
Schönbeck Y, Talma H, van Dommelen P, et al. The world’s tallest nation has stopped growing taller: the height of Dutch children from 1955 to 2009. Pediatr Res 2013;73:371–7.
Cole TJ, Roede MJ. Centiles of body mass index for Dutch children aged 0-20 years in 1980–a baseline to assess recent trends in obesity. Ann Hum Biol 1999;26:303–8.
Schonbeck Y, Talma H, van Dommelen P, et al. Increase in prevalence of overweight in Dutch children and adolescents: a comparison of nationwide growth studies in 1980, 1997 and 2009. PLoS One 2011;6:e27608.
van Dommelen P, Schönbeck Y, HiraSing RA, van Buuren S. Call for early prevention: prevalence rates of overweight among Turkish and Moroccan children in The Netherlands. Eur J Public Health 2015;25:828–33.
Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 2000;320:1240–3.
Schönbeck Y, van Dommelen P, HiraSing RA, van Buuren S. Trend in height of Turkish and Moroccan children living in the Netherlands. PLoS One 2015;10:e0124686.
Vohr BR, Lipsitt LP, Oh W. Somatic growth of children of diabetic mothers with reference to birth size. J Pediatr 1980;97:196–9.
Morgan K, Rahman M, Atkinson M, et al. Association of diabetes in pregnancy with child weight at birth, age 12 months and 5 years–a population-based electronic cohort study. PLoS One 2013;8:e79803.
Clausen TD, Mathiesen ER, Hansen T, et al. Overweight and the metabolic syndrome in adult offspring of women with diet-treated gestational diabetes mellitus or type 1 diabetes. J Clin Endocrinol Metab 2009;94:2464–70.
Wroblewska-Seniuk K, Wender-Ozegowska E, Szczapa J. Long-term effects of diabetes during pregnancy on the offspring. Pediatr Diabetes 2009;10:432–40.
Barker DJ. The origins of the developmental origins theory. J Intern Med 2007;261:412–7.
El Hajj N, Schneider E, Lehnen H, Haaf T. Epigenetics and life-long consequences of an adverse nutritional and diabetic intrauterine environment. Reproduction 2014;148:R111–20.
Gubbels JS, Kremers SP, Stafleu A, et al. Association between parenting practices and children’s dietary intake, activity behavior and development of body mass index: the KOALA Birth Cohort Study. Int J Behav Nutr Phys Act 2011;8:18.
Lahmann PH, Wills RA, Coory M. Trends in birth size and macrosomia in Queensland, Australia, from 1988 to 2005. Paediatr Perinat Epidemiol 2009;23:533–41.
Kuc S, Wortelboer EJ, Koster MP, de Valk HW, Schielen PC, Visser GH. Prediction of macrosomia at birth in type-1 and 2 diabetic pregnancies with biomarkers of early placentation. BJOG 2011;118:748–54.
Fisher SC, Kim SY, Sharma AJ, Rochat R, Morrow B. Is obesity still increasing among pregnant women? Prepregnancy obesity trends in 20 states, 2003-2009. Prev Med 2013;56:372–8.
Glinianaia SV, Tennant PW, Bilous RW, Rankin J, Bell R. HbA(1c) and birthweight in women with pre-conception type 1 and type 2 diabetes: a population-based cohort study. Diabetologia 2012;55:3193–203.
Schaefer-Graf UM, Pawliczak J, Passow D, et al. Birth weight and parental BMI predict overweight in children from mothers with gestational diabetes. Diabetes Care 2005;28:1745–50.
Nehring I, Chmitorz A, Reulen H, von Kries R, Ensenauer R. Gestational diabetes predicts the risk of childhood overweight and abdominal circumference independent of maternal obesity. Diabet Med 2013;30:1449–56.
Nilsson C, Carlsson A, Landin-Olsson M. Increased risk for overweight among Swedish children born to mothers with gestational diabetes mellitus. Pediatr Diabetes 2014;15:57–66.
Acknowledgements
We would like to thank all the parents and our trial manager Bianca Silvius for their cooperation in making this study possible. We also thank biostatistician Paula van Dommelen PhD for her help providing additional BMI data from the Fifth Dutch Growth Study. Finally, we thank the following GG&GD (infant welfare centres) for their help: Utrecht, Midden Nederland, Gelderland Midden, Gooi & Vechtstreek, Rivierenland, Gelre-Ijssel, IJselland, Zuid-Holland, West-Brabant, Brabant Zuidoost, Groningen, Flevoland, Hart voor Brabant, and Nijmegen.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Figure S1
(TIFF 2672 kb)
Supplementary Table 1
(DOCX 14 kb)
Rights and permissions
About this article
Cite this article
Hammoud, N., de Valk, H., van Rossem, L. et al. Growth and BMI during the first 14 y of life in offspring from women with type 1 or type 2 diabetes mellitus. Pediatr Res 81, 342–348 (2017). https://doi.org/10.1038/pr.2016.236
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2016.236
This article is cited by
-
Long-term BMI and growth profiles in offspring of women with gestational diabetes
Diabetologia (2018)
-
Intergenerational Effects of Health Issues Among Women of Childbearing Age: a Review of the Recent Literature
Current Nutrition Reports (2018)
-
A healthier future requires policy informed by lifespan science
Pediatric Research (2017)