Abstract
Background:
We previously reported that combining immediate hypothermia with immediate or 2 h delayed inhalation of an inert gas, xenon, gave additive neuroprotection in rats after a hypoxic–ischemic insult, compared to hypothermia alone. Defining the therapeutic time window for this new combined intervention is crucial in clinical practice when immediate treatment is not always feasible. The aim of this study is to investigate whether combined hypothermia and xenon still provide neuroprotection in rats after a 5 h delay for both hypothermia and xenon.
Methods:
Seven-day-old Wistar rat pups underwent a unilateral hypoxic–ischemic insult. Pups received 5 h of treatment starting 5 h after the insult randomized between normothermia, hypothermia, or hypothermia with 50% xenon. Surviving pups were tested for fine motor function through weeks 8–10 before being euthanized at week 11. Their hemispheric and hippocampal areas were assessed.
Results:
Both delayed hypothermia–xenon and hypothermia-only treated groups had significantly less brain tissue loss than those which underwent normothermia. The functional performance after 1 wk and adulthood was significantly better after hypothermia–xenon treatment as compared to the hypothermia-only or normothermia groups.
Conclusion:
Adding 50% xenon to 5 h delayed hypothermia significantly improved functional outcome as compared to delayed hypothermia alone despite similar reductions in brain area.
Similar content being viewed by others
Main
Neonatal encephalopathy (NE) affects 1–6 per 1,000 live term births worldwide (1), and is associated with high risks of death and neurodevelopmental impairment (poor outcome). Therapeutic hypothermia (HT) is standard of care in treating moderate to severe NE (1,2). This treatment reduces the risk of poor outcome from ~70% to ~50%. The recommended clinical HT protocols state that cooling should start within 6 h after birth (3,4,5). The effective time window of HT is 5–6 h in both fetal lamb (6) and postnatal day 7 (P7) rats (7) and <3 h in injured newborn pigs (8). In NE infants, better motor outcome has been found at 18 mo if cooling was started within 3 h as compared to those were cooled between 3–6 h of age (9). The inert anesthetic gas xenon has received increasing interest as a neuroprotectant over the last decade. Xenon has proven to be neuroprotective both in vivo and in vitro with minimal adverse effects (10,11,12,13,14,15,16,17,18,19,20,21). We and others have previously reported that adding immediate xenon to HT enhanced the neuroprotective effects of HT after induced hypoxia–ischemia in neonatal rats (12,13,15,20) and newborn pigs (21). In neonatal rats, xenon, when added to immediate hypothermia, either immediately or after a 2 h delay, increases neuroprotection (20). In multicentre randomized clinical trials, cooling was started after a mean delay of 4.5 h from birth (3,4,5), therefore examining HT with or without simultaneous xenon after a delay of 5 h is a time point of clinical importance. We aimed to examine whether adding a 5 h period of xenon to a 5 h period of HT after a delay of 5 h from birth improved long-term pathological and functional outcome compared to a 5 h period of HT alone administered after a delay of 5 h from birth.
Results
Behavioral Testing
Negative geotaxis test. Four pups (two each) in the NT- and HT-treated groups respectively, did not manage to complete the task, while all HT-xenon-treated rats finished the task. The mean (95% confidence interval (CI)) for the best performance time was 6.46 (4.19–8.73) seconds in NT group, 4.00 (2.72–5.27) seconds in the HT group, and 4.21 (3.49–4.9) seconds for the HT-xenon group. A linear regression model indicated a significant effect of treatment on the performance time (P = 0.032). The rats treated with HT-Xenon performed twice as fast as those which were remained at normothermia. The observed power was 69%.
Staircase test. The greater the number of pellets retrieved in the staircase test the better the function. The median (95% CI) number of pellets retrieved by the impaired paw (R) during the 3 test days was 5.5 (4.67–7.34) in the NT group, 6.33 (5.67–9.84) in the HT-only group, and 8.58 (6.67–10.52) in the combined HT-xenon group ( Figure 1a ). Pups treated with HT-xenon managed to retrieve significantly more pellets with the impaired paw compared to pups treated with NT (Mann–Whitney U-test, P = 0.001) or HT (P = 0.019). There was a trend that the HT-only group performed better than the NT group (Mann–Whitney U-test, P = 0.166). Adding 50% xenon enhanced the functional performance by 54% (P = 0.009, linear regression, stepwise). Gender did not influence the result. The observed power was 70%.
Panel a represents median (95% confidence interval (CI)) number of pellets retrieved with the right (impaired) paw in the rats treated with normothermia (NT), hypothermia (HT), and HT-Xenon (*P < 0.05). The panel b represents the mean (SEM) number of steps rats reached on each day. The closed diamond shape represents the normothermia group (NT), the open square represents the hypothermia (HT) group, and the gray triangle represents the HT-Xenon group.
The staircase comprises a series of 7 “steps” arranged so they present a task of increasing difficulty while the first step can be reached by the tongue. Rats had to use their paws to retrieve sugar pellets beyond the first step. The higher number of the step a rat can reach, the better its functional performance. In the HT-xenon group, rats reached beyond the first step from the second habitation day. In HT group, rats reached beyond the first step from the third habitation day while NT rats did not manage to reach the second step until the eighth habituation day ( Figure 1b ). Gender did not influence this performance.
Body Weight and Brain Area Loss
The body weight distribution at the end of the experiment (P77) was the same among all treatment groups (P = 0.466, independent-samples Kruskal–Wallis test). There was no difference in the total area of the unligated contralateral (right) hemisphere among NT (n = 15) (73.22, 69.5–75.54 mm2), HT (n = 18) (71.6, 67.76–75.5 mm2), and HT-xenon group (n = 19) (69.78, 65.84–73.1 mm2) (P = 0.559, independent-samples Kruskal–Wallis test). The median (95% CI) area loss ratio of the ligated (left/right) hemisphere was 49.02% (31.7–67.25%) in the NT-treated group, 33.19% (13.91–45.84%) in the HT-treated group and 21.47% (14.16–31.45%) in the combined HT-xenon-treated group ( Figure 2a ). There was no significant difference between HT-only and HT-xenon however both groups were less injured than the NT group with a reduction seen in the HT group (P = 0.02, Mann–Whitney U-test) and a statistically significant reduction in the HT-xenon group (P = 0.006, Mann–Whitney U-test). The observed power was 74%. The median (95%CI) left/right hippocampal area loss was 55.81% (32.73–83.16%) in the NT, 34.24% (20.36–62.42%) in the HT, and 27.04% (17.82–38.97%) in the HT-xenon group ( Figure 2b ) (P = 0.095, Kruskal Wallis test). The observed power was 68%. Adding 50% xenon to 5 h delayed HT did not improve either the total brain area loss (Mann–Whitney U-test, P = 0.75) or hippocampal area loss (P = 0.96). There was no gender effect.
Median (95% CI) area loss ratio of the left hemispheric area (panel a) and left hippocampal area (panel b) in the normothermia (NT) (n = 15), hypothermia (HT) (n = 18), and HT-Xenon (n = 19) treated animals after 10 wk survival (*P < 0.05, two sided).
The Correlation Between Area Loss and Functional Tests
There was a significant negative correlation between the brain area loss and the mean number of pellets retrieved by the impaired (right) paw over the 3 test days ( Figure 3 ) for female rats (Pearson correlation, r = −0.76, P < 0.0001) but not for male rats (r = −0.4, P = 0.063). Female rats that had less brain area loss retrieved more pellets as seen in a previous published study (22).
A significant correlation (r = −0.76, P < 0.0001) in the female rats between brain area loss ratio (the ipsilateral (L) hemisphere normalized to the contralateral (R) hemisphere) and mean pellets retrieved with the right (R, impaired) paw during the 3 test days.
Discussion
We used the established “Vannucci” neonatal brain injury model (23) in this study and a proven optimal 5 h duration cooling protocol for P7 rats (7). We have previously examined the combination therapy of immediate HT and 50% xenon in rats (13) and pigs (21) and found that adding immediate xenon to immediate HT doubled the neuroprotection in both models from 35 to 70%. However, it is rarely feasible to start both active cooling and xenon treatment immediately after injury in most clinical settings. Therefore, it is of great clinical importance to investigate the therapeutic window of this combined treatment. Currently, there are two ongoing randomized clinical trials in term infants with NE using different time windows; starting xenon treatment either within 5 h (Clinicaltrials.gov NCT01545271) or within 12 h (Clinicaltrials.gov NCT00934700) after birth. If our current experimental result is transferred to a clinical NE protocol, we suggest that HT and 50% xenon should both be started before 5 h of age as in the protocol design NCT01545271.
Xenon is an N-methyl-D-aspartate (NMDA) receptor antagonist and an antiapoptotic agent which demonstrates its neuroprotective effect in both in vivo and in vitro models. The maximum safe concentration of inhaled xenon has been suggested to be less than one minimum alveolar concentration (MAC) on hippocampal slices from 7-d-old rats (24). A concentration of 50% xenon used in this study is equivalent to about 1/3MAC in rats (25) or 0.7MAC in humans (26) which is well within the range of proposed safe concentrations as well as being within the effective neonatal neuroprotective range (13,15,20).
Similar to our (7) and others’ (6) previous studies, we found that 5 h delayed HT provided nearly significant neuroprotection compared to normothermic animals. In this study, we examined the possibility of extending the therapeutic windows of HT by adding xenon. The study design focused on neurofunctional recovery as well as brain volume loss in rats surviving to adulthood. Long-term motor and cognitive outcome is the gold standard in clinical neuroprotection trials (3,4,5). The Montoya staircase test used was originally designed for testing motor deficits in adult rats. We and others have later shown that this is also effective for investigating long-term functional capacity after neonatal injury (13,27). Similarities in skilled reaching performance between rodents and primates (28) has been documented, therefore a staircase test is a valuable behavior test in this type of translational research. Additionally, by only allowing one paw to be used at a time, this test is an unbiased quantitative test that is advantageous in our unilateral model. In this study, we did not find improved brain volume by adding xenon to hypothermia as compared to hypothermia alone, however, we found a significant long-term functional improvement in the hypothermia–xenon group. The research group of F. Silverstein have demonstrated ongoing long lasting neuronal and glial degeneration and recovery in an ultrastructual study (29). Combining delayed HT and delayed xenon improved long-term functional outcome even without a corresponding increase in brain volume. Other neuroprotective studies in both rats and humans have shown that long-term functional outcome was improved despite no effect on brain infarct volume per se (30,31,32) after stroke. An improved function despite a nonsignificant improvement in brain volume has also been observed in the same rat model with erythropoietin treatment (33,34). Additionally, other studies have shown that xenon provides neuroprotection in different preclinical models. Xenon has shown protection in different types of models; an oxygen-glucose deprived neuronal-glial coculture cell model (35), a middle cerebral artery occlusion rat model (36) and a cardiac arrest pig model (37). Hence, our study findings may be transferable to wider clinical applications.
We report some limitations to our study. To reduce the number of experimental animals, we did not include a sham operation or a xenon-normothermia group in this study. From previous published results (13), a five-group design as compared to this three-group design would greatly increase the number of animals needed. It is logistically difficult for us to have more than six litters that delivered on the same day which would be required if we wished to correctly randomize into five rather than three groups. In this study, we chose a cooling temperature and duration that have previously yielded optimal protection in our rat model instead of the hypothermic temperature of 33.5 °C used clinically. We have previously used a range of different cooling temperatures and found neuroprotection in all cases; 37 to 34 °C (38), from 38.3 to 32.5 °C (39), and from 37 to 32 °C (13). We conclude that the effective time window for adding xenon to hypothermia in the neonatal rat is 5 h after the insult. Adding 50% xenon to hypothermia for a duration of 5 h after a delay of 5 h significantly improved functional outcome as compared to 5 h hypothermia alone started after a 5 h delay, despite the absence of gross morphological brain protection.
Methods
Animal Preparation
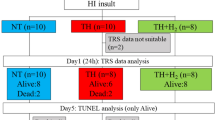
All animal procedures were approved and in accordance with UK Home Office guidelines. Seven-day-old Wistar rat pups (Han strain, B&K Universal Limited, Hull, UK, n = 72) were anesthetized for a total duration of ≤5 min via a nose cone using 65% N2O, 32% O2, and 3% Isoflurane during permanent left common carotid ligation. Pups were then kept with the dams for a minimum of 30 min before exposure to 8% oxygen in an airtight chamber at 36 °C (rectal temperature) with CO2 levels <3% for 90 min (23). This combined procedure produces a unilateral stroke-like injury on the left hemisphere. The right hemisphere is exposed to hypoxia but not subjected to ischemic injury and therefore can be used as a control. Pups were returned to the dams for 5 h after the insult. They were then randomized and received the assigned postinsult treatment for a further 5 h. The three groups were: (i) normothermia (NT, 37 °C) in air, (ii) HT (32 °C) in air, and (iii) HT (32 °C) in 50% xenon +30%O2+20%N2. The gas mixture of xenon, oxygen, and nitrogen was delivered via a specially designed closed circuit gas recirculation system attached to the chamber (40).
Seventy-two rats from six litters were used. Thirteen rats died before behavioral testing: five during the carotid ligation procedure, three during the hypoxic–ischemic insult, two during the 5 h treatment period, and three during the survival period. Six rats were used as temperature monitoring animals only. The brain tissue was damaged during the tissue processing stage in one rat. In summary, 20 rats were excluded and 52 remained for final analysis.
Behavioral Testing
Negative geotaxis test. At the age of P14, rats were placed facing down at an inclined (45° angle) rough surface. Each rat was given three 60-s trials. The time of each pup to turn 180°, facing uphill, in each trial was recorded. The fastest of three trials for each pup was used in the final analysis. If a pup could not perform, i.e., turning 180° uphill or fell off the plane in all three trials, the results were noted but not included in the analysis.
Staircase test. A Montoya staircase test, also known as the skilled reaching test, was performed in adult rats aged 8–10 wk (41). To maximize their performance, the rats were habituated to the testing room, the box, the sugar pellets, and the researchers during the first two and half weeks (5 d a week) of the 3 wk testing period. According to our local animal facility rules, rats were not allowed to be starved before testing. In the first week, both broken rat chews (ordinary rat food) and chocolate-flavored sugar pellets were baited in the staircase. Three sugar pellets were placed on each step. This was used to establish a positive association between the food reward and the task. Additionally, rats can be neophobic toward food. By mixing the familiar rat chews with sugar pellets, one reduces their anxiety toward the novel food. The staircase design allows only one paw to be used at a time which is an important design in our unilateral brain injury model. Rats were kept in the staircase for 7 min for habituation for each side during the first week and 3.5 min for each side during the following 2 wk. In the second and third week, only sugar pellets were baited. The final testing days were the last 3 d of the third week. The number of pellets retrieved and the number of steps reached were recorded for each paw. The staircase is arranged so that the retrieval of food from each step becomes progressively more challenging as a test of motor function and co-ordination. As in humans, the corticospinal tract is largely crossed in rats. Hence, the left side of the brain predominantly controls the right side of the body. We produced unilateral injury on the left side of the brain resulting in an impaired function of the right paw compared to the left paw.
Brain Area Loss
Pups were weighed, anesthetized, and sacrificed at P77 using cross heart perfusion–fixation with 4% formaldehyde. Brains were subsequently stored in 4% formaldehyde for 1 wk and sectioned using 3 mm coronal planes (six blocks per brain). The blocks were paraffin processed. Slides of 6 µm thickness were prepared and stained with hematoxylin and eosin. These slides were assessed by an observer blinded to the treatment allocations using Image J software (National Institute of Health, Bethesda, MD). Left and right hemispheric brain and hippocampal areas were measured using a customized computerized programme from slides cut from two adjacent middle blocks of each brain (including cortex, hippocampus, thalamus, and basal ganglia). In brief, stained slides were scanned at a resolution of 1,200 dots per inch, 24-bit color using a flat bed scanner (Epson V30, Epson (UK) Ltd, Hemel Hempstead, UK). These images were first converted to eight-bit gray scale (red channel) and then to a binary picture. The threshold of each image was set to 200 (arbitrary units) automatically. If necessary, the threshold of the image was manually adjusted to present an outline of the measured area by comparing the binary image with the 24-bit color image. The brain area loss ratio on the ipsilateral side (left) was calculated using the following equation: Brain area loss % = (1−(remaining area on the ipsilateral side)/(brain area on the contralateral side)) × 100
The hippocampal area loss ratio on the ipsilateral side was calculated as: Hippocampal area loss % = (1−(remaining hippocampal area on the ipsilateral side)/(hippocampal area on the contralateral side)) × 100
A larger brain area loss ratio represents more severe brain injury (7). By using this method, we were able to reliably measure gross morphology change such as cavitations or atrophy. We were unable to distinguish between pan-necrosis and selective neuronal loss or digitally measure the area of basal ganglia and thalamus accurately on the hematoxylin and eosin-stained slides. Every brain image was assessed in random order on two occasions a few weeks apart and the mean of these two values was used in the final calculation.
Statistical Analysis
For nonparametric data, median (95% CI) was used for presentation, Mann–Whitney U-test was used to define the difference in the two groups. Independent-samples Kruskal–Wallis test was used to compare multiple groups (post hoc Bonferroni rules applied in determining significance) and Pearson correlation was used to compare the correlation between relative hemispheric area loss and functional outcome assessed as number of pellets retrieved. A Univariance linear model was used to calculate observed power. IBM SPSS statistics version 19 was used for the above statistical analyses.
Statement of Financial Support
We would like to thank Sport Aiding Medical Research for Kids (SPARKS UK), the Norwegian Research Council (Oslo, Norway) and Laerdal Foundation (Stavanger, Norway) for supporting our research.
Disclosure
None.
References
Wyatt JS, Gluckman PD, Liu PY, et al.; CoolCap Study Group. Determinants of outcomes after head cooling for neonatal encephalopathy. Pediatrics 2007;119:912–21.
Kattwinkel J, Perlman JM, Aziz K, et al. Part 15: neonatal resuscitation: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2010;122(18 Suppl 3):S909–19.
Azzopardi DV, Strohm B, Edwards AD, et al.; TOBY Study Group. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med 2009;361:1349–58.
Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet 2005;365:663–70.
Shankaran S, Laptook AR, Ehrenkranz RA, et al.; National Institute of Child Health and Human Development Neonatal Research Network. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med 2005;353:1574–84.
Gunn AJ, Gunn TR, de Haan HH, Williams CE, Gluckman PD. Dramatic neuronal rescue with prolonged selective head cooling after ischemia in fetal lambs. J Clin Invest 1997;99:248–56.
Sabir H, Scull-Brown E, Liu X, Thoresen M. Immediate hypothermia is not neuroprotective after severe hypoxia-ischemia and is deleterious when delayed by 12 hours in neonatal rats. Stroke 2012;43:3364–70.
Karlsson M, Tooley JR, Satas S, et al. Delayed hypothermia as selective head cooling or whole body cooling does not protect brain or body in newborn pig subjected to hypoxia-ischemia. Pediatr Res 2008;64:74–8.
Thoresen M, Tooley J, Liu X, et al. Time is brain: starting therapeutic hypothermia within three hours after birth improves motor outcome in asphyxiated newborns. Neonatology 2013;104:228–33.
Banks P, Franks NP, Dickinson R. Competitive inhibition at the glycine site of the N-methyl-D-aspartate receptor mediates xenon neuroprotection against hypoxia-ischemia. Anesthesiology 2010;112:614–22.
David HN, Haelewyn B, Rouillon C, et al. Neuroprotective effects of xenon: a therapeutic window of opportunity in rats subjected to transient cerebral ischemia. FASEB J 2008;22:1275–86.
Dingley J, Tooley J, Porter H, Thoresen M. Xenon provides short-term neuroprotection in neonatal rats when administered after hypoxia-ischemia. Stroke 2006;37:501–6.
Hobbs C, Thoresen M, Tucker A, Aquilina K, Chakkarapani E, Dingley J. Xenon and hypothermia combine additively, offering long-term functional and histopathologic neuroprotection after neonatal hypoxia/ischemia. Stroke 2008;39:1307–13.
Homi HM, Yokoo N, Ma D, et al. The neuroprotective effect of xenon administration during transient middle cerebral artery occlusion in mice. Anesthesiology 2003;99:876–81.
Ma D, Hossain M, Chow A, et al. Xenon and hypothermia combine to provide neuroprotection from neonatal asphyxia. Ann Neurol 2005;58:182–93.
Martin JL, Ma D, Hossain M, et al. Asynchronous administration of xenon and hypothermia significantly reduces brain infarction in the neonatal rat. Br J Anaesth 2007;98:236–40.
Natale G, Cattano D, Abramo A, et al. Morphological evidence that xenon neuroprotects against N-methyl-DL-aspartic acid-induced damage in the rat arcuate nucleus: a time-dependent study. Ann N Y Acad Sci 2006;1074:650–8.
Petzelt C, Blom P, Schmehl W, Müller J, Kox WJ. Prevention of neurotoxicity in hypoxic cortical neurons by the noble gas xenon. Life Sci 2003;72:1909–18.
Rajakumaraswamy N, Ma D, Hossain M, Sanders RD, Franks NP, Maze M. Neuroprotective interaction produced by xenon and dexmedetomidine on in vitro and in vivo neuronal injury models. Neurosci Lett 2006;409:128–33.
Thoresen M, Hobbs CE, Wood T, Chakkarapani E, Dingley J. Cooling combined with immediate or delayed xenon inhalation provides equivalent long-term neuroprotection after neonatal hypoxia-ischemia. J Cereb Blood Flow Metab 2009;29:707–14.
Chakkarapani E, Dingley J, Liu X, et al. Xenon enhances hypothermic neuroprotection in asphyxiated newborn pigs. Ann Neurol 2010;68:330–41.
Bona E, Hagberg H, Løberg EM, Bågenholm R, Thoresen M. Protective effects of moderate hypothermia after neonatal hypoxia-ischemia: short- and long-term outcome. Pediatr Res 1998;43:738–45.
Rice JE 3rd, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol 1981;9:131–41.
Brosnan H, Bickler PE. Xenon neurotoxicity in rat hippocampal slice cultures is similar to isoflurane and sevoflurane. Anesthesiology 2013;119:335–44.
Koblin DD, Fang Z, Eger EI 2nd, et al. Minimum alveolar concentrations of noble gases, nitrogen, and sulfur hexafluoride in rats: helium and neon as nonimmobilizers (nonanesthetics). Anesth Analg 1998;87:419–24.
Cullen SC, Eger EI 2nd, Cullen BF, Gregory P. Observations on the anesthetic effect of the combination of xenon and halothane. Anesthesiology 1969;31:305–9.
Tomimatsu T, Fukuda H, Endoh M, et al. Effects of neonatal hypoxic-ischemic brain injury on skilled motor tasks and brainstem function in adult rats. Brain Res 2002;926:108–17.
Whishaw IQ, Alaverdashvili M, Kolb B. The problem of relating plasticity and skilled reaching after motor cortex stroke in the rat. Behav Brain Res 2008;192:124–36.
Skoff RP, Bessert D, Barks JD, Silverstein FS. Plasticity of neurons and glia following neonatal hypoxic-ischemic brain injury in rats. Neurochem Res 2007;32:331–42.
Dhawan J, Benveniste H, Luo Z, Nawrocky M, Smith SD, Biegon A. A new look at glutamate and ischemia: NMDA agonist improves long-term functional outcome in a rat model of stroke. Future Neurol 2011;6:823–34.
Ji S, Kronenberg G, Balkaya M, et al. Acute neuroprotection by pioglitazone after mild brain ischemia without effect on long-term outcome. Exp Neurol 2009;216:321–8.
Mark VW, Taub E, Perkins C, Gauthier L, Uswatte G. MRI infarction load and CI therapy outcomes for chronic post-stroke hemiparesis. Restor Neurol Neurosci 2008;26:13–33.
Fan X, van Bel F, van der Kooij MA, Heijnen CJ, Groenendaal F. Hypothermia and erythropoietin for neuroprotection after neonatal brain damage. Pediatr Res 2013;73:18–23.
Iwai M, Stetler RA, Xing J, et al. Enhanced oligodendrogenesis and recovery of neurological function by erythropoietin after neonatal hypoxic/ischemic brain injury. Stroke 2010;41:1032–7.
Bantel C, Maze M, Trapp S. Neuronal preconditioning by inhalational anesthetics: evidence for the role of plasmalemmal adenosine triphosphate-sensitive potassium channels. Anesthesiology 2009;110:986–95.
David HN, Leveille F, Chazalviel L, et al. Reduction of ischemic brain damage by nitrous oxide and xenon. J Cereb Blood Flow Metab 2003;23:1168–73.
Schmidt M, Marx T, Glöggl E, Reinelt H, Schirmer U. Xenon attenuates cerebral damage after ischemia in pigs. Anesthesiology 2005;102:929–36.
Yager J, Towfighi J, Vannucci RC. Influence of mild hypothermia on hypoxic-ischemic brain damage in the immature rat. Pediatr Res 1993;34:525–9.
Thoresen M, Bågenholm R, Løberg EM, Apricena F, Kjellmer I. Posthypoxic cooling of neonatal rats provides protection against brain injury. Arch Dis Child Fetal Neonatal Ed 1996;74:F3–9.
Chakkarapani E, Thoresen M, Hobbs CE, Aquilina K, Liu X, Dingley J. A closed-circuit neonatal xenon delivery system: a technical and practical neuroprotection feasibility study in newborn pigs. Anesth Analg 2009;109:451–60.
Montoya CP, Campbell-Hope LJ, Pemberton KD, Dunnett SB. The “staircase test”: a measure of independent forelimb reaching and grasping abilities in rats. J Neurosci Methods 1991;36:219–28.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, X., Dingley, J., Scull-Brown, E. et al. Adding 5 h delayed xenon to delayed hypothermia treatment improves long-term function in neonatal rats surviving to adulthood. Pediatr Res 77, 779–783 (2015). https://doi.org/10.1038/pr.2015.49
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2015.49
This article is cited by
-
Hydrogen and therapeutic gases for neonatal hypoxic–ischemic encephalopathy: potential neuroprotective adjuncts in translational research
Pediatric Research (2021)
-
Rectal temperature in the first five hours after hypoxia–ischemia critically affects neuropathological outcomes in neonatal rats
Pediatric Research (2018)
-
Non-Additive Effects of Delayed Connexin Hemichannel Blockade and Hypothermia after Cerebral Ischemia in Near-Term Fetal Sheep
Journal of Cerebral Blood Flow & Metabolism (2015)