Abstract
Background:
The potential of microRNAs (miRNAs) as bedside biomarkers in selecting newborns with hypoxic-ischemic encephalopathy (HIE) for neuroprotection has yet to be explored. Commonly, blood-based biomarker tests use plasma or serum which don’t allow evaluation of both intracellular and extracellular changes.
Methods:
We describe a technique to extract and compare expression of miRNAs from a single small 6-mm-diameter dried blood spot (DBS) stored at room temperature with those from EDTA-blood, plasma, and urine. Three miRNAs (RNU6B, let7b, and miR-21) were quantified via extraction and quantitative RT-PCR performed from a DBS and compared with levels from EDTA-blood, plasma, and urine. Secondarily, candidate miRNAs let7b, miR-21, miR-29b, miR-124, and miR-155 in DBS were evaluated as potential biomarkers for HIE.
Results:
Candidate miRNAs were extractable in all biosamples from newborns, with the highest expression in DBS. There was a good correlation between miRNAs’ levels in DBS and EDTA-blood at −80 °C. No significant difference was observed in the miRNA levels between the favorable and unfavorable outcome groups for babies with HIE.
Conclusion:
DBS may be useful for studying the potential of miRNAs as biomarkers for brain injury.
Similar content being viewed by others
Main
The lack of more effective methods in selecting babies for neuroprotection and the need for prognostication for outcomes after hypoxic-ischemic encephalopathy (HIE) pose major challenges to clinicians, patient families, and the economy. At present, there is no effective tissue biomarker at the bedside to allow selection of babies for neuroprotection within the narrow therapeutic window or for early prognostication of outcomes. A single or a panel of biomarkers with high sensitivity and specificity that is reliable and reproducible at the bedside would be extremely valuable in selecting babies for appropriate interventions. Biomarkers allow objective measurement and evaluation of biological and pathological response to therapeutic intervention (1). Biological sample (biosample)–based biomarkers hold much promise with many potential advantages; these are bedside tests, extraction is relatively quick, easy, and cheap, and the technology involved in analysis has developed rapidly with small volumes of tissue required.
In all central nervous system injuries, whether in adults, children, or newborns, biosample-based biomarker studies have been carried out mostly on blood samples as well as on urine or cerebrospinal fluid. Blood-based biomarker studies have commonly been performed on plasma or serum components (2) and have not analyzed whole blood or the cellular components. However, using plasma or serum exclusively is limited by the need for long-term cold storage (e.g., −80 °C), cellular debris contamination, hemolysis, and analysis confined to only extracellularly released components within the blood. Blood sampling in newborns can be challenging due to the small size of the infant, with technical difficulty in vascular access and/or obtaining sufficient volume of non-hemolyzed blood. Dried blood spots (DBS), typically obtained from a heel prick and allowing drops of blood to settle onto absorbent paper, have been routinely used in newborn babies in the first week of life for mass screening and early detection of multiple diseases including cystic fibrosis, congenital hypothyroidism, and inborn errors of metabolism (3). The use of DBS to assay biomarkers of brain injury in newborns has yet to be fully explored. Protein biomarkers such as inflammatory molecules, cell structures, or trophic factors have been explored in newborns with HIE, but their effectiveness as successful biomarkers in the clinical setting remains to be determined (2,4). In this study, we report our method of extracting microRNAs (miRNAs) from DBS stored at room temperature.
MiRNAs are endogenous short noncoding single-stranded RNA molecules made up of 18–22 nucleotides, which are important in the regulation of gene expression post transcription (5). They appear to function by binding to the 3′-untranslated region of messenger RNAs (mRNA) from target protein-coding genes leading to gene silencing by mRNA cleavage, translational repression, and deadenylation (6). They have been found to influence varying stages of neurogenesis, including cell differentiation, proliferation, and synaptogenesis, from in vitro and in vivo studies (7). Studies of miRNAs in mice and humans have shown that approximately 70% of identified miRNAs are expressed in brain tissue, in relation to the complexity of the nervous system and its connections (8,9,10).
However, the physiological and pathophysiological role of miRNA in newborns remains to be fully determined. With organs such as the brain still developing, it is likely that the expression of miRNA in newborns is distinctive, yet related to their counterparts in adults based on their regulatory role. RNU6B belongs to a class of small nucleolar RNA and is frequently used as an “endogenous control” to normalize miRNA expression studies. Let7b and miR-21 have both been shown to be important in adult brain pathology following trauma in animal studies (11,12), ischemia/stroke (13,14), and Alzheimer’s disease (15,16). Recently, miR-29b, which is activated during neuronal maturation, has been linked to inhibition of neuronal apoptosis (17). Brain-specific miRNA 124 has been well noted to influence the nervous system in a number of conditions including nervous system development, neurodegeneration, central nervous system stress, stroke, neuro-immunity, and brain tumors (18). It has been shown to influence neurodevelopment by triggering brain-specific alternative pre-mRNA splicing (19). Additionally, miRNA 124 has been shown to predict neurological outcome following cardiac arrest in adults (20). MiRNA 155 has been shown to be involved in microglia-mediated immune response (21). To date, the role of these miRNAs in newborns remains to be determined.
We set out to prospectively (i) extract sufficient total RNA from a single DBS stored in room temperature from newborn babies admitted to the neonatal unit after suspected hypoxia-ischemia; (ii) quantify the expression of three selected miRNAs (RNU6B, Let7b, and miR-21) in DBS compared with those in EDTA-blood, plasma, and urine using quantitative RT-PCR; and (iii) explore the role of five selected miRNAs (Let7b, miR-21, miR-29b, miR-124a, and miR-155) normalized to RNU6B (putative endogenous control) as candidate biomarkers for babies with perinatal asphyxia.
Results
Study Population
Between January and August 2014, 30 newborn babies were recruited from two centers.
From the 30 newborns studied, 19 were selected for therapeutic hypothermia on the basis of standard cooling criteria (22) and 11 infants with perinatal acidosis and no encephalopathy were managed conservatively.
MRI/Clinical Outcome
Of the 30 babies, 19 had magnetic resonance imaging (MRI) performed between 5 and 35 d (median = 9 d) of life. The pattern of cerebral tissue injury noted on MRI was scored using the system described by Rutherford et al. (23). Based on MRI and/or short-term clinical outcome at discharge, 23 babies were predicted to have a favorable outcome and 7 were predicted to have an unfavorable outcome. The favorable outcome group had 10 babies with normal neurological examination at discharge and no MRI performed, 8 babies with normal MRI scans, and 5 babies with minor changes on MRI. The unfavorable outcome group comprised of five babies with severe changes in MRI, one baby who died on second day of life with multi-organ failure associated with severe hypoxia-ischemia, and a baby with severely abnormal neurological examination at discharge and having ongoing investigations for a neurologic diagnosis. Overall, 19 babies were treated with therapeutic hypothermia (n = 13 in favorable group; n = 6 in unfavorable group). Table 1 describes the baseline characteristics of all babies based on their predicted outcome group.
Total RNA Concentration
Total RNA concentration values (mean ± SEM) were 11.1 ± 1.6 ng/µl for all biosamples and 10.6 ± 2.5 ng/µl for DBS alone. The RNA quality values of 260/280 (mean ± SEM) were 1.68 ± 0.04 and 1.77 ± 0.05 for all and DBS samples, respectively ( Figure 1 ), which represent similar quality of RNA from all biofluids.
RNA extraction: quantity and quality in all four biosamples. (a) Total RNA extracted from newborns with hypoxic-ischemic encephalopathy (HIE) showed similar average total RNA concentration as measured in ng/μl. (b) Average total RNA quality in plasma, blood, urine, and dried blood spot (DBS) measured as a 260/280 ratio. NS, nonsignificant (P > 0.05) using one-way ANOVA with Tukey’s post hoc test. Data are presented as mean ± SEM.
MiRNA Expression
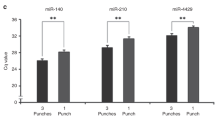
Three of the miRNAs examined (RNU6B, Let7b, and miR-21) were expressed in a majority of the biosamples (plasma, EDTA-blood, urine, and DBS) from the babies. In 4 out of 30 plasma and 8 out of 30 urine samples, RNU6B miRNA was not detectable. Correlations were carried out for the expression of RNU6B, Let7b, and miR-21 miRNAs in all biofluids with DBS. There was a significant positive correlation between DBS and EDTA-blood for RNU6B, let7b, and miR-21( Figure 2 ). There was no statistically significant correlation between the DBS with plasma or urine. The comparison of miRNA expression in different biosamples using mean cyclical threshold (Ct) values from three technical replicates for all three miRNAs analyzed demonstrated significantly lower Ct values (i.e., higher expression) in DBS than in plasma, EDTA-blood, and urine in the three selected miRNAs studied: RNU6B, Let7b, and miR-21 ( Figure 3 ). We also observed from the variable time period (range 2–191 d) in extraction of total RNA from the point of sample collection, it was still possible to extract the selected miRNAs from all samples after prolonged storage (data not shown). This was true for all biosamples; plasma, EDTA-blood, and urine stored at −80 °C and DBS stored in room temperature.
Expression of microRNAs (miRNAs) in dried blood spot (DBS) compared with plasma, blood, and urine biosamples. (a–c) Comparison of RNU6B Ct values in DBS with other biosamples. (d–f) Comparison of Let7b Ct values in DBS with other biosamples. (g–i) Comparison of miR-21 Ct values in DBS with other biosamples. Graphs b, e, and h demonstrate significant coefficient of determination (r2) values for blood and DBS in all three miRNAs: RNU6B, r2 = 0.270; Let7b, r2 = 0.503; miR-21, r2 = 0.202. *P < 0.05, **P < 0.01, †P < 0.001 indicate significance of r2 between biosamples.
Expression of microRNAs in all four biosamples. Expression of (a) RNU6B, (b) Let7b, and (c) miR-21 in all four biosamples (each performed in technical triplicates). *P < 0.05, **P < 0.01, †P < 0.001 indicate significance between biosamples; NS, nonsignificant (P > 0.05) using one-way ANOVA with Tukey’s post hoc test. Data are presented as mean ± SEM.
MiRNAs in HIE
To study the role of miRNAs in HIE, five neuro-specific miRNAs (Let7b, miR-21, miR-29b, miR-124, and miR-155) were selected. The Ct values were converted to delta Ct, which represents values normalized to a putative endogenous control RNU6B. There was no significant difference noted between the favorable and the unfavorable outcome groups in the delta Ct values of all candidate miRNAs studied, i.e., Let7b, miR-21, miR-29b, miR-124, and miR-155 in DBS ( Figure 4 ). It was also noted that delta Ct values were not different between the outcome groups in other biosamples (plasma, EDTA-blood, and urine) for Let7b and miR-21.
Comparison of normalized expression of selected candidate microRNAs (miRNAs) in dried blood spot (DBS) between favorable and unfavorable outcomes. Mean delta Ct values in DBS for various miRNAs are shown in the graphs: (a) Let7b, (b) miR-21, (c) miR-29b, (d) miR-124, and (e) miR-155. The P values did not show any significant difference between the favorable and unfavorable outcome groups in for all miRNAs. NS, nonsignificant (P > 0.05) using Student’s t test. Data are presented as mean ± SEM.
Discussion
To our knowledge, this is the first study to demonstrate that it is feasible to extract sufficient quantity and quality miRNAs in newborns from a single 6-mm-diameter DBS stored at room temperature and in quantities significantly higher than in other common biosamples, including plasma, urine, and blood for analysis of similar total RNA concentration and quality. We also showed that it was possible to extract total RNA and study the expression of miRNA from DBS stored at room temperature for up to 6 mo from the time of sample collection. The quantity and quality of RNA and all candidate miRNAs extracted from DBS were all similar to those from other biofluids after storage at −80 °C.
RNU6B is a small nucleolar miRNA and is generally low or not expressed in plasma in adults (24). On the contrary, we noted that RNU6B was expressed in most of our plasma samples apart from a small proportion. Presence of RNU6B in most plasma samples could be explained by the hemolysis noted in a significant proportion of our plasma samples (24). Furthermore, it has been noted that there is an increase in nucleated red blood cells in babies with HIE (25). We also noted RNU6B in most of our urine samples, and this may be due to cells in the urine, as uncentrifuged urine was used in this study.
There was positive correlation between EDTA-blood and DBS for all miRNAs examined. There were significantly lower Ct values for RNU6B, let7b, and miR-21 observed in DBS compared with plasma, blood, and urine, implying a higher expression of miRNAs in DBS compared with other biosamples. This may be related to the DBS representing both intra- and extracellular components of blood. There has recently been much interest in tissue biomarkers in the study of central nervous system pathology in adults (26). DBS have been routinely used in universal screening programs in newborns in the first week after birth for early detection of diseases (3). Recently, Buroker et al. (27) have successfully extracted miRNAs from two 1.5-cm-diameter DBS which had been stored at −80 °C. Bartha et al. (28) studied cytokines and tumor necrosis factor alpha from DBS by immunoaffinity chromatography in babies with neonatal encephalopathy. These samples were stored at −26 °C before analysis. We were able to extract sufficient quantity and quality of miRNAs from a single 6-mm-diameter spot from newborns stored at room temperature. One potential reason for our success is that the DBS was dried completely after collection, and studies indicate that humidity may affect miRNA preservation (29).
In our study, despite our best efforts to ensure that all blood samples were free flowing, hemolysis was noted in some of the EDTA samples, albeit minimally (data not shown). McDonald et al. (30) showed that hemolysis increased apparent concentration of some but not all miRNA levels on pre-analytical variables in extraction of miRNA. Additionally, the time from collection of the blood sample to centrifugation, and then to storage at −80 °C, was variable due to difficulty in access to the laboratory round-the-clock in our study. It has been shown that delay in centrifugation up to 6 h significantly affected the levels in some candidate miRNAs studied (31). Therefore, our miRNA extraction from plasma could have been affected due to delay in centrifuging some samples. Most studies on miRNAs have used serum or plasma. However, miRNAs can also be released during cell death as apoptotic bodies or bound to proteins such as Ago2 and lipoproteins (32). Therefore, in pathologies involving cell injury–like perinatal asphyxia, it is potentially more valuable to study whole blood in the form of DBS and not just the extracellular components found in serum/plasma. Furthermore, DBS provided highest expression of miRNA given that 10 ng of total RNA was equally loaded for all biosamples.
Limitations of our study include the variation in age after birth when the biosamples were obtained from the newborns. This was limited by age when infants were transferred from other hospitals, the time taken for parents to give informed consent, and the availability of research staff to take and process the samples. Our results did not show a variation in the expression levels of the control miRNA in relation to sampling time from birth. It is well recognized that there is a temporal variation in many biomarkers of brain injury in newborns after birth (33). In future studies, serial sampling from each baby to analyze the natural history of these biomarkers associated with varying degrees of brain injury will be carried out. Due to lack of serial testing with the same sample across various time points, it is not possible to comment on the stability of the RNA and miRNAs over time in the DBS and other biosamples from this study. Although the quality values of the total RNA extracted were not within the expected value of 2.0 when analyzed with the NanoDrop spectrophotometer, this was likely due to the circulating RNases present in the biosample which can degrade large RNAs into small RNAs (34). Li and Kowdley have shown that clinical serum samples contain mainly small RNA molecules, including miRNAs with the absence of large RNA species, such as messenger RNA and ribosomal subunits 18S and 28S when RNA quality was determined using the Agilent 2100 Bioanalyzer (34). Due to the absence of 18S and 28S subunits, it is not possible to perform RNA integrity number for determining the quality of the total RNA in the samples (34). In this study, the mirVana PARIS RNA extraction kit, which involves the strategy of combining denaturing reagents and a silica filter, was used. This RNA extraction kit has been shown to successfully extract miRNAs with least miRNA degradation and high miRNA quality in comparison with other commercially tested kits (34).
In this small cohort, we were not able to show any difference in the selected candidate miRNA expression in the unfavorable prognosis group compared with the favorable prognosis group. Larger studies exploring more candidate miRNAs at serial time points in babies presenting with perinatal asphyxia are required to understand the role of specific miRNAs’ expression in HIE.
Conclusions
We demonstrate that it is feasible to study the expression of miRNA from a very small sample of DBS obtained from newborns. MiRNA expression from DBS correlates well with those from EDTA-blood. DBS is relatively cheap, technically easier to obtain in all age groups, especially in small and sick neonates, and simpler for transportation and storage. This also eliminates potential sources of error, common in the pre-analytical stage, associated with blood collection and centrifugation. This novel method using a very small drop of blood as DBS has a potential for use in future studies to explore further the role of miRNAs as possible biomarkers in neonates.
Methods
Infant Recruitment and Selection
The study received Research and Ethics approval (REC 13/LO/1738) from National Research Ethics Committee, London, and informed written consent was obtained from the parents of recruited participants. Newborn babies were recruited from two tertiary neonatal centers in London; the Royal London Hospital and Homerton University Hospital. Babies were recruited to the study if (i) they were ≥36 wk of gestation, fulfilled standard cooling criteria (22), and were selected for mild hypothermia for neuroprotection by the attending clinicians (cooled group) or (ii) infants who were admitted to the neonatal unit for conservative management with suspected milder hypoxia-ischemia on the basis of metabolic acidosis but did not fulfill the criteria for cooling treatment (non cooled—mild group). Infants with multiple congenital abnormalities or confirmed inborn error of metabolism were excluded.
Biosamples
The biosamples chosen for determining miRNA expression levels were plasma, EDTA-blood, urine, and DBS collected at a median time of 18–19 h of age in both groups and after the infants in the cooled group had reached their target core temperature (33.5 °C). Of the 1.5 ml of blood obtained from the newborn, one drop was placed on absorbent filter paper (Whatman 903 protein saver card, GE Healthcare Life Sciences, Little Chalfont, UK) creating a DBS. This was allowed to dry at room temperature and was then stored in a polythene bag with a packet of desiccant to sustain desiccation. The remaining blood was transferred into tubes with sprayed-coated K2EDTA (Fisher Scientific, Loughborough, UK) and then centrifuged at 15,000g for 10 min to separate the plasma and blood components (EDTA-blood). The plasma, EDTA-blood, and urine collected from a bag or a catheter sample were all stored at −80 °C until further processed.
RNA Extraction
MirVana PARIS isolation kit (Ambion, Life Technologies, Paisley, UK) was used to extract total RNA from 50 µl of plasma, 50 µl of urine, and 1 µl of blood sample withdrawn using a 23G needle, and a single 6-mm-diameter chad from the center of DBS was collected using a clean single hole puncher. Total RNA isolation was carried out according to manufacturer’s instruction. Briefly, biosamples were suspended in cell disruption buffer to a final volume of 300 µl and vortexed for 10 s. The blood in the DBS was resuspended by pipetting up and down using a 1-ml pipette tip, then transferring the supernatant to a clean tube. After the samples were mixed with denaturing solution, acid phenol:chloroform mixture was added, vortexed, then centrifuged at 15,000g for 5 min at room temperature. The top aqueous layer was carefully collected and mixed with 100% ethanol. The total RNA was collected in a filter cartridge, washed, and eluted using 100 µl of 95 °C preheated elution buffer. Using the NanoDrop 1000 spectrophotometer (Labtech International, East Sussex, UK), the concentration and quality of the total RNA in all samples were analyzed and recorded. All RNA samples were stored at −80 °C until further processed.
TaqMan miRNA Assay
TaqMan microRNA assays (Life Technologies) for RNU6B, let7b, miR-21, miR-29b, miR-124, and miR-155 were used, which involved the reverse transcription of miRNA followed by a singleplex TaqMan assay reaction performed using real-time PCR amplification. Briefly, 10 µg of total RNA was reverse transcribed using the TaqMan MicroRNA Reverse Transcription kit (Applied Biosystems, Life Technologies) which contained 1× RT buffer, dNTPs with dTTP, RNase inhibitor, and MultiScribe Reverse Transcriptase enzyme, and reaction was carried out in a thermal Cycler at 16 °C for 30 min, at 42 °C for 30 min, at 85 °C for 5 min, and at 4 °C for holding. The quantitative PCR amplification was performed using the TaqMan Fast Universal PCR Master Mix (2×), No AmpErase UNG (Applied Biosystems, Life Technologies), TaqMan small RNA assay, and RT reaction product. The reaction samples (20 µl) were loaded in triplicates into a 96-well plate. Using the Applied Biosystems 7500 Fast Real-time PCR system, the thermal cycling conditions were hold at 50 °C for 2 min, then at 95 °C for 10 min, and then 40 cycles of denature at 95 °C for 15 s and anneal/extend at 60 °C for 1 min. The results were represented as Ct values. Ct values denote the cyclical threshold in PCRs, in which lower Ct values indicate higher expression as a result of amplification in earlier cycles due to abundance of the nucleic acid in the sample.
Outcomes
MR images were independently rated by a neuroradiologist (J.E.) and a neonatologist with expertise (O.K.). The pattern of MRI injury was classified into two groups using the system described by Rutherford et al., which has prognostic value in infants who have undergone therapeutic hypothermia (23). Infants with an unfavorable outcome had a severe pattern of injury, including reversed or abnormal signal intensity bilaterally on T1- and or T2-weighted sequences in the posterior limb of the internal capsule, multifocal or widespread abnormal signal intensity in the basal ganglia and thalami, or severe widespread white matter lesions including infarction, hemorrhage, and long T1 and T2. Infants with MRIs predictive of a favorable outcome had either normal images or less severe patterns of injury that are associated with normal or mildly abnormal neurodevelopmental outcome. Consensus was reached in cases of disagreement. Infants who died prior to MRI were classified in the unfavorable outcome group. Infants who were not cooled were classified as having a favorable outcome if they had a normal neurologic examination at 24 h of age and were discharged home on suck feeds. These infants did not have MRI.
Statement of Financial Support
This study was supported by a grant from the Barts Charity, London, UK.
Disclosure
All the authors have no conflict of interest to disclose in relation to this work.
References
Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther 2001;69:89–95.
Ramaswamy V, Horton J, Vandermeer B, Buscemi N, Miller S, Yager J. Systematic review of biomarkers of brain injury in term neonatal encephalopathy. Pediatr Neurol 2009;40:215–26.
UK Newborn Screening Programme Centre. Guidelines for newborn blood spot sampling, 2012. (http://newbornbloodspot.screening.nhs.uk/bloodspotsampling).
Gazzolo D, Abella R, Marinoni E, et al. New markers of neonatal neurology. J Matern Fetal Neonatal Med 2009;22:Suppl 3:57–61.
Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 2009;136:215–33.
Huntzinger E, Izaurralde E. Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat Rev Genet 2011;12:99–110.
Motti D, Bixby JL, Lemmon VP. MicroRNAs and neuronal development. Semin Fetal Neonatal Med 2012;17:347–52.
Sempere LF, Freemantle S, Pitha-Rowe I, Moss E, Dmitrovsky E, Ambros V. Expression profiling of mammalian microRNAs uncovers a subset of brain-expressed microRNAs with possible roles in murine and human neuronal differentiation. Genome Biol 2004;5:R13.
Cao X, Yeo G, Muotri AR, Kuwabara T, Gage FH. Noncoding RNAs in the mammalian central nervous system. Annu Rev Neurosci 2006;29:77–103.
Cherubini E, Gustincich S, Robinson H. The mammalian transcriptome and the cellular complexity of the brain. J Physiol 2006;575:319–20.
Liu L, Sun T, Liu Z, et al. Traumatic brain injury dysregulates microRNAs to modulate cell signaling in rat hippocampus. PLoS One 2014;9:e103948.
Sandhir R, Gregory E, Berman NE. Differential response of miRNA-21 and its targets after traumatic brain injury in aging mice. Neurochem Int 2014;78:117–21.
Long G, Wang F, Li H, et al. Circulating miR-30a, miR-126 and let-7b as biomarker for ischemic stroke in humans. BMC Neurol 2013;13:178.
Buller B, Liu X, Wang X, et al. MicroRNA-21 protects neurons from ischemic death. FEBS J 2010;277:4299–307.
Lehmann SM, Krüger C, Park B, et al. An unconventional role for miRNA: let-7 activates Toll-like receptor 7 and causes neurodegeneration. Nat Neurosci 2012;15:827–35.
Leidinger P, Backes C, Deutscher S, et al. A blood based 12-miRNA signature of Alzheimer disease patients. Genome Biol 2013;14:R78.
Kole AJ, Swahari V, Hammond SM, Deshmukh M. miR-29b is activated during neuronal maturation and targets BH3-only genes to restrict apoptosis. Genes Dev 2011;25:125–30.
Sun Y, Luo ZM, Guo XM, Su DF, Liu X. An updated role of microRNA-124 in central nervous system disorders: a review. Front Cell Neurosci 2015;9:193.
Makeyev EV, Zhang J, Carrasco MA, Maniatis T. The MicroRNA miR-124 promotes neuronal differentiation by triggering brain-specific alternative pre-mRNA splicing. Mol Cell 2007;27:435–48.
Gilje P, Gidlöf O, Rundgren M, et al. The brain-enriched microRNA miR-124 in plasma predicts neurological outcome after cardiac arrest. Crit Care 2014;18:R40.
Cardoso AL, Guedes JR, Pereira de Almeida L, Pedroso de Lima MC. miR-155 modulates microglia-mediated immune response by down-regulating SOCS-1 and promoting cytokine and nitric oxide production. Immunology 2012;135:73–88.
Azzopardi D, Brocklehurst P, Edwards D, et al.; TOBY Study Group. The TOBY Study. Whole body hypothermia for the treatment of perinatal asphyxial encephalopathy: a randomised controlled trial. BMC Pediatr 2008;8:17.
Rutherford M, Ramenghi LA, Edwards AD, et al. Assessment of brain tissue injury after moderate hypothermia in neonates with hypoxic-ischaemic encephalopathy: a nested substudy of a randomised controlled trial. Lancet Neurol 2010;9:39–45.
Kirschner MB, Kao SC, Edelman JJ, et al. Haemolysis during sample preparation alters microRNA content of plasma. PLoS One 2011;6:e24145.
Walsh BH, Boylan GB, Murray DM. Nucleated red blood cells and early EEG: predicting Sarnat stage and two year outcome. Early Hum Dev 2011;87:335–9.
Jové M, Portero-Otín M, Naudí A, Ferrer I, Pamplona R. Metabolomics of human brain aging and age-related neurodegenerative diseases. J Neuropathol Exp Neurol 2014;73:640–57.
Buroker NE, Ning XH, Zhou ZN, et al. Circulating miRNAs from dried blood spots are associated with high altitude sickness. J Med Diagn Meth 2013;2:125.
Bartha AI, Foster-Barber A, Miller SP, et al. Neonatal encephalopathy: association of cytokines with MR spectroscopy and outcome. Pediatr Res 2004;56:960–6.
Patnaik SK, Mallick R, Yendamuri S. Detection of microRNAs in dried serum blots. Anal Biochem 2010;407:147–9.
McDonald JS, Milosevic D, Reddi HV, Grebe SK, Algeciras-Schimnich A. Analysis of circulating microRNA: preanalytical and analytical challenges. Clin Chem 2011;57:833–40.
Page K, Guttery DS, Zahra N, et al. Influence of plasma processing on recovery and analysis of circulating nucleic acids. PLoS One 2013;8:e77963.
Essandoh K, Fan GC. Role of extracellular and intracellular microRNAs in sepsis. Biochim Biophys Acta 2014;1842:2155–62.
Chalak LF, Sánchez PJ, Adams-Huet B, Laptook AR, Heyne RJ, Rosenfeld CR. Biomarkers for severity of neonatal hypoxic-ischemic encephalopathy and outcomes in newborns receiving hypothermia therapy. J Pediatr 2014;164:468–74.e1.
Li Y, Kowdley KV. Method for microRNA isolation from clinical serum samples. Anal Biochem 2012;431:69–75.
Acknowledgements
We are very grateful to the families who participated in this study and the medical and nursing staff on the neonatal units for their support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ponnusamy, V., Kapellou, O., Yip, E. et al. A study of microRNAs from dried blood spots in newborns after perinatal asphyxia: a simple and feasible biosampling method. Pediatr Res 79, 799–805 (2016). https://doi.org/10.1038/pr.2015.276
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2015.276
This article is cited by
-
MicroRNA therapeutic targets in neonatal hypoxic–ischemic brain injury: a narrative review
Pediatric Research (2023)
-
Biomarkers of hypoxic–ischemic encephalopathy: a systematic review
World Journal of Pediatrics (2023)
-
Neuronal let-7b-5p acts through the Hippo-YAP pathway in neonatal encephalopathy
Communications Biology (2021)
-
MicroRNA in dried blood spots from patients with Aagenaes syndrome and evaluation of pre-analytical and analytical factors
Pediatric Research (2021)