Abstract
Background:
The Neonatal Resuscitation Program (NRP) recommends upper and lower limits of preductal saturations (SpO2) extrapolated from studies in infants resuscitated in room air. These limits have not been validated in asphyxia and lung disease.
Methods:
Seven control term lambs delivered by cesarean section were ventilated with 21% O2. Thirty lambs with asphyxia with meconium aspiration were randomly assigned to resuscitation with 21% O2 (n = 6), 100% O2 (n = 6), or initiation with 21% O2 followed by variable FIO2 to maintain NRP target SpO2 ranges (n = 18). Hemodynamic and ventilation parameters were recorded for 15 min.
Results:
Control lambs maintained preductal SpO2 near the lower limit of NRP target range. Asphyxiated lambs had low SpO2 (38 ± 2%), low arterial pH (6.99 ± 0.01), and high PaCO2 (96 ± 7 mm Hg) at birth. Resuscitation with 21% O2 resulted in SpO2 values below the target range with low pulmonary blood flow (Qp) compared to variable FIO2 group. The increase in PaO2 and Qp with variable FIO2 resuscitation was similar to control lambs.
Conclusion:
Maintaining SpO2 as recommended by NRP by actively adjusting inspired O2 leads to effective oxygenation and higher Qp in asphyxiated lambs with lung disease. Our findings support the current NRP SpO2 guidelines for O2 supplementation during resuscitation of an asphyxiated neonate.
Similar content being viewed by others
Main
The use of 100% oxygen was routine during resuscitation of newly born infants (1) prior to the 2010 Neonatal Resuscitation Program (NRP) guidelines (2,3,4). Pulse oximetry studies of healthy term and preterm infants who did not require resuscitation at birth demonstrated that preductal oxygen saturation (SpO2) is ~60% at birth and takes 5–10 min to reach 85–90% (5). The percentiles of SpO2 at each minute of life have been identified and the goal saturation range has been approximately defined as interquartile ranges for healthy term infants (3). Current guidelines recommend starting resuscitation with 21% oxygen in term infants. Oxygen supplementation is then guided by preductal SpO2 and adjusted to maintain SpO2 values in the goal saturation range at the corresponding minute of postnatal life (3,6,7).
However, it is important to recognize that infants with asphyxia or lung disease who needed resuscitation were excluded from these studies. Asphyxia results in hypoxemia and acidosis (8) resulting in lower SpO2 values at the time of birth (9). Furthermore, in the presence of lung disease (such as meconium aspiration) and increased alveolar–arterial oxygen gradient, 21% inspired oxygen may not be sufficient to achieve the target SpO2 values recommended by the NRP. Also, the combination of asphyxia and lung disease predisposes infants to persistent pulmonary hypertension of the newborn (10) that can lead to intra- and extrapulmonary right-to-left shunting of blood, further decreasing SpO2 (11). The effect of maintaining preductal SpO2 in the reference goal range recommended by the NRP on hemodynamics and gas exchange in the presence of perinatal asphyxia and lung disease is not known. Controversy remains as to whether a lower percentile SpO2 target (that can potentially be achieved with 21% inspired oxygen) might be as effective and potentially safer in asphyxiated neonates (12).
The aim of our study was to evaluate gas exchange and pulmonary/cerebral hemodynamics during resuscitation in an ovine model of perinatal asphyxia (induced by umbilical cord occlusion) and lung disease (through instillation of meconium through the endotracheal tube) (9) adhering to the current NRP oxygen saturation target guidelines. We compared these results with lambs resuscitated with 21 and 100% inspired oxygen. We hypothesize that adjusting inspired oxygen to achieve goal NRP SpO2 range in asphyxiated lambs with lung disease and persistent pulmonary hypertension of the newborn will result in hemodynamics and gas exchange similar to that observed in control lambs (without asphyxia or lung disease) ventilated with 21% O2 at birth.
Results
Thirty lambs were randomized, instrumented, asphyxiated, and delivered. Eighteen lambs were randomized to the variable FIO2 group to keep preductal SpO2 between 60 and 85% for the first 15 min after birth and six lambs each were randomized to receive inspired oxygen of 100 or 21% irrespective of SpO2. To generate control data, seven healthy term lambs were ventilated with 21% O2. Gestational age, birth weight, and gender distribution were similar among the groups. None of the animals required chest compressions or epinephrine. The gender distribution was equal (15 male and 15 female lambs) and no significant hemodynamic or gas exchange differences were observed between the genders.
Oxygenation
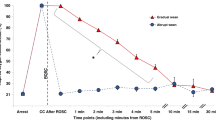
Asphyxia by umbilical cord occlusion resulted in a significant decrease in preductal SpO2 compared to the control group (38 ± 2 vs. 53 ± 1.4%, respectively). Control lambs ventilated with 21% O2 maintained preductal SpO2 in the target range recommended by NRP ( Figure 1 ). Asphyxiated lambs randomized to 21 and 100% inspired oxygen had SpO2 values below and above the NRP target range, respectively. By design, asphyxiated lambs ventilated with variable FIO2 maintained their SpO2 values within the target range ( Figure 1 ) but this required frequent titration of FIO2 ( Figure 2 ). Baseline fetal oxygen was similar in all the groups (23 ± 8 mm Hg). However, after meconium instillation and cord occlusion, the PaO2 dropped significantly in asphyxiated lambs (15 ± 12 mm Hg) but improved with resuscitation and positive pressure ventilation ( Figure 3a ). The lambs that were ventilated with 100% inspired oxygen had significantly higher PaO2 from birth until 15 min when compared to the other three groups ( Figure 3a ).
Changes in preductal oxygen saturation (SpO2) during fetal life and the first 15 min of postnatal age in control lambs (open circle) and asphyxiated term lambs ventilated with 21% O2 (gray triangle), 100% O2 (cross), and inspired oxygen (FIO2) titrated to keep the SpO2 as per Neonatal Resuscitation Program (NRP) guidelines (black square). The shaded area represents the oxygen saturation range recommended by NRP. *P <0.05 at 10 min time point by ANOVA (21% asphyxia and 100% asphyxia significantly different by Bonferroni post hoc test). PPV, positive pressure ventilation.
Titration of fraction of inspired oxygen (FIO2, closed diamond) to maintain the preductal saturation (SpO2, open triangle) between 85 and 95%. The X-axis represents fetal life, asphyxiation and resuscitation, and postnatal life in minutes. PPV, positive pressure ventilation.
Partial pressure of oxygen in preductal right carotid arterial blood (PaO2, panel a), arterial pH (panel b), and partial pressure of CO2 in arterial blood (PaCO2, panel c) during the first 15 min of postnatal age in control lambs (open circle) and asphyxiated term lambs ventilated with 21% O2 (gray triangle), 100% O2 (cross), and inspired oxygen (FIO2) titrated to keep the SpO2 as per Neonatal Resuscitation Program guidelines (black square). *P < 0.05, PaO2 in 100% O2 group is significantly higher than all other groups throughout the resuscitation period. PPV, positive pressure ventilation.
Arterial pH
Predelivery arterial pH was lower in the three asphyxiated groups (6.99 ± 0.01) when compared to controls (7.29 ± 0.09). Asphyxiated lambs ventilated with 100% inspired O2 had significantly higher pH throughout the 15 min of resuscitation when compared to the lambs ventilated with variable FIO2 ( Figure 3b ). However, there was no difference in the base deficit between the two asphyxiated groups (−6 ± 3.8 in 100% inspired oxygen vs. −7.9 ± 2 in variable FIO2).
Ventilation
Predelivery PaCO2 was similar in the three asphyxiated groups (96 ± 7 mm Hg) at the onset of ventilation and was significantly higher than controls (56 ± 9.5 mm Hg). No significant difference was noted in PaCO2 among asphyxiated lambs ( Figure 3c ). There was no difference between the ventilator rate and mean airway pressures among the three asphyxiated groups.
Systemic Hemodynamics
Baseline heart rate (HR) was similar among all the groups (154 ± 23/min) but umbilical cord occlusion caused significant bradycardia in the three asphyxiated groups (83 ± 37/min). With resuscitation by positive pressure ventilation, the HR for the first 15 min was similar between the asphyxiated lambs ventilated with variable FIO2 and controls ( Figure 4 ). Baseline fetal mean systemic blood pressure was similar among all the groups (47 ± 2.7 mm Hg). Mean systemic blood pressure in the three asphyxiated groups (52 ± 4.7 mm Hg) was significantly lower than the controls (71 ± 16.2 mm Hg) over the period of 15 min ( Figure 5a ). Left carotid blood flow was similar among all the groups (23.6 ± 2.27 ml/kg/min in asphyxiated group vs. 26.3 ± 2 ml/kg/min in controls) ( Figure 6a ).
Variation in heart rate (beats per minute) in the first 15 min of life in control lambs (open circle) and asphyxiated term lambs ventilated with 21% O2 (gray triangle), 100% O2 (cross), and inspired oxygen (FIO2) titrated to keep the SpO2 between 85 and 95% (black square). PPV, positive pressure ventilation.
Mean systemic arterial pressure (panel a) and mean pulmonary blood pressure (panel b) during the first 15 min of postnatal age in control lambs (open circle) and asphyxiated term lambs ventilated with 21% O2 (gray triangle), 100% O2 (cross), and inspired oxygen (FIO2) titrated to keep the SpO2 as per Neonatal Resuscitation Program guidelines (black square). PPV, positive pressure ventilation.
Variation in carotid blood flow (Qca, panel a) and pulmonary blood flow (Qp, panel b) in the first 15 min of postnatal age in control lambs (open circle) and asphyxiated term lambs ventilated with 21% O2 (gray triangle), 100% O2 (cross), and inspired oxygen (FIO2) titrated to maintain preductal SpO2 as per Neonatal Resuscitation Program guidelines (black square). *P <0.05 by ANOVA (21% asphyxiated and controls significantly lower than variable FIO2 asphyxia by Bonferroni post hoc test). PPV, positive pressure ventilation.
Pulmonary Hemodynamics
Pulmonary blood flow (Qp) was significantly lower in the asphyxiated 21% inspired oxygen group (42.3 ± 17 ml/kg/min) compared to variable FIO2 (65.8 ± 10 ml/kg/min) and asphyxiated 100% group (64.1 ± 13 ml/kg/min) ( Figure 6b ). Interestingly, variable FIO2 group had higher Qp than controls (51.4 ± 5 ml/kg/min). Mean pulmonary artery pressure was higher in asphyxiated lambs ventilated with variable FIO2 group (51 ± 3.4 mm Hg) when compared to asphyxiated lambs ventilated with 100% inspired O2 (42 ± 4.4 mm Hg) and 21% inspired O2 (44 ± 3.8 mm Hg) ( Figure 5b ). No difference was seen in mean pulmonary artery pressure between asphyxiated variable FIO2 group and controls. There were no significant differences in pulmonary vascular resistance among the asphyxiated lambs (data not shown).
Discussion
To our knowledge, this is the first study to evaluate pulmonary and systemic hemodynamics during resuscitation using the new preductal SpO2 target guidelines (13) in a model of perinatal asphyxia with lung disease. This study demonstrates that initiating resuscitation with 21% oxygen and titrating inspired oxygen based on preductal SpO2 in asphyxiated lambs results in hemodynamic changes similar to that achieved with room air in healthy lambs.
Baseline preductal SpO2 at initiation of resuscitation in most nonasphyxiated neonates is in the 50s and 60s (5,14,15). However, HR and SpO2 are significantly lower in severely asphyxiated neonates similar to the lambs in our study. It is encouraging to note that SpO2 and HR can be restored to “normal” range by 60 s of effective positive pressure ventilation ( Figures 1 and 5 ) with 21% inspired oxygen.
Studies evaluating inspired oxygen in asphyxiated animal models have shown that resuscitation with 21% oxygen is adequate to induce pulmonary vasodilation and “return of spontaneous circulation” (8,16,17,18,19). However, none of these animal models had lung disease and had low alveolar–arterial oxygen gradient (AaDO2). Hence resuscitation with 21% oxygen was probably adequate to achieve SpO2 in the target range. In the current study, we have used a model with meconium aspiration resulting in elevated AaDO2 (264 ± 37 in asphyxiated lambs and 69 ± 11 mm Hg in control lambs) secondary to ventilation–perfusion mismatch and pulmonary hypertension (9). Resuscitation with 21% oxygen alone in this model did not achieve target SpO2 ( Figure 1 ) and limited the increase in pulmonary blood flow ( Figure 6a ). Adjusting FIO2 significantly improved SpO2 and pulmonary blood flow compared to the 21% oxygen resuscitation.
Why was pulmonary blood flow lower in asphyxiated lambs resuscitated with 21% oxygen and controls? We have previously shown that a preductal SpO2 of approximately ≥90% is necessary to achieve optimal pulmonary vasodilation in lambs after birth (20,21). Only one asphyxiated lamb (out of six) in the 21% oxygen resuscitation group achieved SpO2 >90% ( Figure 1 ) and could have led to suboptimal pulmonary vasodilation ( Figure 6a ). In contrast, 15/18 lambs in the variable FIO2 category achieved SpO2 >90%. In addition, metabolic acidosis is known to decrease the threshold for hypoxic pulmonary vasoconstriction (22) and could have contributed to reduced pulmonary vasodilation in response to room air resuscitation in this study. We have shown in the past that maintaining PaO2 between 45 and 65 mm Hg is necessary to avoid hypoxic pulmonary vasoconstriction and does not increase pulmonary arterial contractility (23). Our control lambs had an average PaO2 of 34 ± 8 mm Hg at 10 min and reached 49 ± 6 mm Hg by 15 min. These low (albeit physiologic) PaO2 values could have contributed to lower Qp in lambs resuscitated with 21% oxygen.
Recently, Kapadia et al. performed a novel clinical study to evaluate inspired oxygen titrated based on preductal SpO2 in preterm neonates (24). Low oxygen strategy in this study was similar to the variable FIO2 arm in the current study. Compared to 100% oxygen resuscitation, low O2 strategy was associated with lower bronchopulmonary dysplasia. Our study demonstrates that 21% oxygen alone may not be adequate to induce pulmonary vasodilation in asphyxiated neonates with lung disease. We also demonstrate that adjusting FIO2 based on preductal SpO2 leads to pulmonary vasodilation similar to 100% oxygen resuscitation with considerably less oxygen exposure.
Resuscitation with 100% oxygen did not result in higher pulmonary blood flow compared to resuscitation with variable FIO2. Clinical and translational studies (23,25) have demonstrated increased oxidative stress following resuscitation with 100% oxygen leading to increased pulmonary vasoconstriction (23). We speculate that resuscitation with variable FIO2 resulted in an optimal balance between oxygen-mediated pulmonary vasodilation and oxygen free radical-induced vasoconstriction.
Adjusting FIO2 to achieve target SpO2 requires additional personnel and equipment. In the current study, an additional neonatal provider (a total of four resuscitators) was required to closely monitor SpO2 and adjust FIO2. Absence of adequate providers during resuscitation may limit personnel availability to perform this function. In addition, many delivery suites/birthing rooms in level I nurseries in the United States and nurseries in developing countries are not equipped with oxygen blenders and pulse oximeters. Current clinical literature (26,27) and results from our study clearly indicate that resuscitation and effective ventilation of the lungs with 21% oxygen improves HR and systemic blood pressure and decreases pulmonary vascular resistance in a model of severe asphyxia, acidosis, and lung disease. Based on these results, if resources are limited during resuscitation of a severely asphyxiated neonate, the initial focus should be on effective ventilation of the lungs with room air and monitoring HR.
Recent evidence suggests that initiation of resuscitation and ventilation of the lungs before clamping the cord results in stable cerebral blood flow and influence SpO2 and oxygen delivery to the brain (28,29). Further studies to evaluate the impact of delayed cord clamping and ventilation before cord clamping on SpO2 during resuscitation are warranted.
There are several limitations to this study. All lambs were intubated prior to delivery. Mask ventilation and the process of emergent intubation after delivery may result in lower SpO2 values and were not evaluated in this study. The severity of lung disease, pulmonary hypertension, and hypoxemic respiratory failure was moderate (with oxygenation index in the mid-20s). Very severe persistent pulmonary hypertension of the newborn and profound hypoxemia may result in much higher oxygen requirement. A fixed positive end expiratory pressure of 5 cm H2O was adapted in this study. A higher positive end expiratory pressure or use of sustained inflation may have established functional residual capacity earlier leading to lower FIO2 requirement (30). Finally, this model only evaluates short-term acute lung injury. Long-standing asphyxial insult and lung injury, as seen in meconium aspiration, may lead to vascular remodeling and fixed pulmonary hypertension. Finally, we did not euthanize the lambs at the end of 15 min of resuscitation to evaluate oxidant injury to the lungs (as we did in our previous study) (8). As the lambs were part of a different study to evaluate optimal SpO2 target at term gestation, we continued to ventilate them for 6 h.
In conclusion, adjusting FIO2 to maintain preductal SpO2 in the target range recommended by NRP, in asphyxiated lambs with lung disease, results in pulmonary hemodynamics similar to that observed in control nonasphyxiated lambs with normal lungs. However, in resource-limited and emergent situations, effective ventilation of the lungs with 21% oxygen leading to increased HR may be a reasonable approach as well. Clinical studies evaluating the efficacy of these recommendations are warranted.
Methods
This study was approved by the Institutional Animal Care and Use Committee (IACUC) at State University of New York at Buffalo. Time-dated pregnant ewes (139–142 d gestation; term 145 d) (New Life Pastures, Attica, NY) were sedated, intubated, and ventilated with 2% isoflurane. Cesarean section was performed and the fetal lamb was partially exteriorized. Jugular and carotid lines were placed on the right side for access, preductal arterial blood gas sampling, and blood pressure monitoring. Catheters were placed in the main pulmonary artery and left atrium for pressure monitoring as previously described (20,21). Fetal lambs were asphyxiated by umbilical cord occlusion and meconium (5 ml/kg of 20% meconium suspended in ewe amniotic fluid) was instilled into their endotracheal tube as previously described (9). The lambs were then delivered, ventilated, and blood gases were obtained at fetal (predelivery), delivery, 1 min, 2 min, 5 min, 10 min, and 15 min. Thirty lambs were randomized into three groups (using sealed envelopes at 1:1:3 ratio): (i) FIO2 1; (ii) FIO2 0.21, irrespective of SpO2; and (iii) initial FIO2 of 0.21, titrated to keep preductal SpO2 in the target range recommended by NRP (31). In group 3, attempts were made to maintain preductal SpO2 between 60 and 65% in the first minute, 65 and 70% in the second minute, 70 and 75% in the third minute, 75 and 80% in the fourth minute, 80 and 85% in the fifth minute, and 85 and 95% between 5 and 15 min of postnatal age as per NRP guidelines. If preductal SpO2 was outside this range, inspired oxygen was adjusted by 5% every 15 s by an attending neonatologist/neonatology fellow. Initial ventilation settings were peak inspiratory pressure of 30 cm H2O, positive end expiratory pressure – 5 cm H2O and rate – 40/min. Subsequent ventilation was adjusted using blood gas values.
The randomization described in the previous paragraph was part of a larger study where the variable FIO2 group was further managed between 15 min and 6 h of postnatal age with different SpO2 ranges (85–89%, 90–94%, and 95–99%). The second portion of the experiment (from 15 min to 6 h) is not described in this manuscript. Lambs were ventilated for 6 h and then sacrificed. For controls, seven healthy (nonasphyxiated) term lambs without lung disease were ventilated with 21% O2 irrespective of SpO2. Pulmonary and systemic hemodynamic parameters were compared between the groups.
Statement of Financial Support
This work was supported by American Academy of Pediatrics, Neonatal Resuscitation Program (to S.L. and M.R.), Elk Grove Village, IL and 1R01HD072929-0 (to S.L.), National Institute of Child Health and Human Development, Bethesda, MD.
Disclosure
No financial ties to products in the study or potential/perceived conflicts of interest.
References
Kattwinkel J. Textbook of Neonatal Resuscitation. 5th edn. Elk Grove Village, IL: American Academy of Pediatrics, 2006.
Kattwinkel J. Textbook of Neonatal Resuscitation. 6th edn. Elk Grove Village, IL: American Academy of Pediatrics, 2011.
Kattwinkel J, Perlman JM, Aziz K, et al. Part 15: neonatal resuscitation: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2010;122:Suppl 3:S909–19.
Perlman JM, Wyllie J, Kattwinkel J, et al.; Neonatal Resuscitation Chapter Collaborators. Part 11: neonatal resuscitation: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation 2010;122:16 Suppl 2:S516–38.
Dawson JA, Kamlin CO, Vento M, et al. Defining the reference range for oxygen saturation for infants after birth. Pediatrics 2010;125:e1340–7.
Saugstad OD. Delivery room management of term and preterm newly born infants. Neonatology 2015;107:365–71.
Saugstad OD ; International Liason Committee on Resuscitation. New guidelines for newborn resuscitation–a critical evaluation. Acta Paediatr 2011;100:1058–62.
Lakshminrusimha S, Steinhorn RH, Wedgwood S, et al. Pulmonary hemodynamics and vascular reactivity in asphyxiated term lambs resuscitated with 21 and 100% oxygen. J Appl Physiol (1985) 2011;111:1441–7.
Lakshminrusimha S, Mathew B, Nair J, et al. Tracheal suctioning improves gas exchange but not hemodynamics in asphyxiated lambs with meconium aspiration. Pediatr Res 2015;77:347–55.
Lapointe A, Barrington KJ. Pulmonary hypertension and the asphyxiated newborn. J Pediatr 2011;158:Suppl 2:e19–24.
Nair J, Lakshminrusimha S. Update on PPHN: mechanisms and treatment. Semin Perinatol 2014;38:78–91.
Wyckoff MH. Respiratory and cardiovascular support in the delivery room. In: Bancalari E, Polin RA, eds. The Newborn Lung. 2nd edn. Philadelphia, PA: Elsevier Saunders, 2012:247–64.
Kattwinkel J, Perlman JM, Aziz K, et al.; American Heart Association. Neonatal resuscitation: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Pediatrics 2010;126:e1400–13.
Dawson JA, Kamlin CO, Wong C, et al. Oxygen saturation and heart rate during delivery room resuscitation of infants <30 weeks’ gestation with air or 100% oxygen. Arch Dis Child Fetal Neonatal Ed 2009;94:F87–91.
Dawson JA, Morley CJ. Monitoring oxygen saturation and heart rate in the early neonatal period. Semin Fetal Neonatal Med 2010;15:203–7.
Proschan MA, Waclawiw MA. Practical guidelines for multiplicity adjustment in clinical trials. Control Clin Trials 2000;21:527–39.
Solevåg AL, Dannevig I, Nakstad B, Saugstad OD. Resuscitation of severely asphyctic newborn pigs with cardiac arrest by using 21% or 100% oxygen. Neonatology 2010;98:64–72.
Linner R, Werner O, Perez-de-Sa V, Cunha-Goncalves D. Circulatory recovery is as fast with air ventilation as with 100% oxygen after asphyxia-induced cardiac arrest in piglets. Pediatr Res 2009;66:391–4.
Perez-de-Sa V, Cunha-Goncalves D, Nordh A, et al. High brain tissue oxygen tension during ventilation with 100% oxygen after fetal asphyxia in newborn sheep. Pediatr Res 2009;65:57–61.
Lakshminrusimha S, Russell JA, Steinhorn RH, et al. Pulmonary hemodynamics in neonatal lambs resuscitated with 21%, 50%, and 100% oxygen. Pediatr Res 2007;62:313–8.
Lakshminrusimha S, Swartz DD, Gugino SF, et al. Oxygen concentration and pulmonary hemodynamics in newborn lambs with pulmonary hypertension. Pediatr Res 2009;66:539–44.
Rudolph AM, Yuan S. Response of the pulmonary vasculature to hypoxia and H+ ion concentration changes. J Clin Invest 1966;45:399–411.
Lakshminrusimha S, Russell JA, Steinhorn RH, et al. Pulmonary arterial contractility in neonatal lambs increases with 100% oxygen resuscitation. Pediatr Res 2006;59:137–41.
Kapadia VS, Chalak LF, Sparks JE, Allen JR, Savani RC, Wyckoff MH. Resuscitation of preterm neonates with limited versus high oxygen strategy. Pediatrics 2013;132:e1488–96.
Vento M, Asensi M, Sastre J, García-Sala F, Pallardó FV, Viña J. Resuscitation with room air instead of 100% oxygen prevents oxidative stress in moderately asphyxiated term neonates. Pediatrics 2001;107:642–7.
Saugstad OD, Ramji S, Soll RF, Vento M. Resuscitation of newborn infants with 21% or 100% oxygen: an updated systematic review and meta-analysis. Neonatology 2008;94:176–82.
Vento M, Saugstad OD. Resuscitation of the term and preterm infant. Semin Fetal Neonatal Med 2010;15:216–22.
Bhatt S, Alison BJ, Wallace EM, et al. Delaying cord clamping until ventilation onset improves cardiovascular function at birth in preterm lambs. J Physiol 2013;591:Pt 8:2113–26.
Lakshminrusimha S, Van Meurs K. Better timing for cord clamping is after onset of lung aeration. Pediatr Res 2015;77:615–7.
Klingenberg C, Sobotka KS, Ong T, et al. Effect of sustained inflation duration; resuscitation of near-term asphyxiated lambs. Arch Dis Child Fetal Neonatal Ed 2013;98:F222–7.
Morin FI. Hyperventilation, alkalosis, prostaglandins and the pulmonary circulation of the newborn. Appl Physiol 1986;61:2088–94.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rawat, M., Chandrasekharan, P., Swartz, D. et al. Neonatal resuscitation adhering to oxygen saturation guidelines in asphyxiated lambs with meconium aspiration. Pediatr Res 79, 583–588 (2016). https://doi.org/10.1038/pr.2015.259
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2015.259
This article is cited by
-
A family of NICU graduates!
Pediatric Research (2018)
-
Effect of various inspired oxygen concentrations on pulmonary and systemic hemodynamics and oxygenation during resuscitation in a transitioning preterm model
Pediatric Research (2018)