Abstract
Hydroxyurea (HU) is the sole approved pharmacological therapy for sickle cell disease (SCD). Higher levels of fetal hemoglobin (HbF) diminish deoxygenated sickle globin polymerization in vitro and clinically reduce the incidence of disease morbidities. Clinical and laboratory effects of HU largely result from induction of HbF expression, though to a highly variable extent. Baseline and HU-induced HbF expression are both inherited complex traits. In children with SCD, baseline HbF remains the best predictor of drug-induced levels, but this accounts for only a portion of the induction. A limited number of validated genetic loci are strongly associated with higher baseline HbF levels in SCD. For induced HbF levels, genetic approaches using candidate single-nucleotide polymorphisms (SNPs) have identified some of these same loci as being also associated with induction. However, SNP associations with induced HbF are only partially independent of baseline levels. Additional approaches to understanding the impact of HU on HbF and its other therapeutic effects on SCD include pharmacokinetic, gene expression–based, and epigenetic analyses in patients and through studies in existing murine models for SCD. Understanding the genetic and other factors underlying the variability in therapeutic effects of HU for pediatric SCD is critical for prospectively predicting good responders and for designing other effective therapies.
Similar content being viewed by others
Main
Healthy People 2020, the federal public health agenda, has set a goal of “Increase(ing) the proportion of persons with hemoglobinopathies who receive disease-modifying therapies” (1). For the vast majority of people with sickle cell disease (SCD), the Healthy People goal will be reached through increased use of hydroxyurea (HU). Critical questions surrounding its use include how this drug works to ameliorate the clinical severity of SCD and what subpopulation of children with SCD benefit most from its use. This review addresses these questions from a translational science perspective.
SCD affects an estimated 90,000 people in the United States (2), with more than 1,900 newborns detected annually through universal newborn screening (2). Infant screening, early preventive therapy, and parental guidance have largely eliminated early child mortality from SCD (3,4,5). Moreover, specialized care and ongoing preventive services have prolonged average life expectancy (6). Despite these successes, multiorgan damage and mortality accumulate by early adulthood, resulting in shortened life span (6).
HU holds expanding promise for improved clinical outcomes. More than 2 decades ago, the seminal Multicenter Study of Hydroxyurea phase III trial for adults demonstrated the striking clinical impact of HU: 40% reduction in the incidence of acute pain episodes, acute chest syndrome, and hospitalization (7). These results led to approval in 1998 of HU for use in symptomatic SCD by the US Food Drug Administration (FDA). HU remains the only FDA-approved drug for SCD, but approval does not extend to pediatric use. The approval gap for children is partially attributed to the lack of a commercial pharmaceutical sponsor. Helping to span the gap is the FDA’s recent commissioning of a pediatric study of the pharmacokinetics of HU and its relative bioavailability of a liquid formulation (http://clinicaltrials.gov/show/NCT01506544).
Clinical efficacy of HU treatment varies among individuals, although most patients with severe phenotypes benefit from its use (7,8). This review describes newly identified mechanisms for the effects of HU, including genetic regulation of fetal hemoglobin (HbF) as a disease modifier and the biologic effects of HU on blood vessels and gene regulation. These recent advances improve the prospects for prospectively assessing efficacy of HU therapy, are inspiring clinical trials for additional salutatory effects of HU, and may guide future drug development.
Clinical Effects
The profound clinical effects of HU in children with SCD have been recently reviewed (9,10,11); these are summarized here in Table 1 . Much of the work on HU in children with SCD has come from phase II and III trials trials led by Ware et al., including pivotal studies such as phase I/II trial of HU in children with sickle cell anemia (HUG KIDS) (12,13,14), Hydroxyurea Safety and Organ Toxicity Trial (HUSOFT) (15), Pediatric Hydroxyurea Phase III Clinical Trial (BABY HUG) (16,17,18) and an early pediatric trial published in 1999 (12). French investigators have also contributed insights into the impact of HU (19,20). Randomized pediatric trials with HU have demonstrated decreased occurrences of pain episodes (18), acute chest syndrome, hospitalization (8,11,18), transfusion, and splenic autoinfarction (18), along with improved quality of life (21,22). Prolonged use sustains the laboratory effects of decreased anemia, markers of hemolysis, and counts of white blood cells and platelets, in addition to increased red cell mean corpuscular volume (23). Early HU use stabilizes renal hyperfiltration (24), hyposthenuria (25), and age-dependent decrease in HbF (18). Induction of HbF is described below.
Of note, although the laboratory effects of HU apply across the pediatric ages tested, many of the various clinical improvements noted for one age range have not necessarily been assessed for other ranges. For example, reduced dactylitis, hyposthenuria, and transfusions were noted in the BABY HUG trial of children enrolled at age 9–13 mo (17,18). Improved transcranial Doppler blood flow through large cerebral arteries has been demonstrated in school-aged children (26,27). Despite positive findings, some of these trials had mixed results. For example, the primary end points of the BABY HUG study were not met (18). In the Stroke with transfusions changing to hydroxyurea (SWiTCH) study for secondary stroke prevention, continued chronic transfusion was advantageous relative to HU with phlebotomy (28). Moreover, HU reduces but does not eliminate the symptoms of and morbidity in SCD. For example, the SWiTCH trial demonstrated that chronic transfusions more effectively prevented pain episodes than HU with phlebotomy (29).
Two long-term studies demonstrated substantially improved life spans from prolonged use of HU in adults, including a study based on the Multicenter Study of Hydroxyurea in Sickle Cell Anemia trial (30,31). Prospective life span data for children taking HU are not yet available due to later uptake into pediatric trials. Nonetheless, a recent retrospective study from Brazil reported improved childhood mortality for those taking HU for up to 6 y (32). Collectively, these data are increasingly persuasive about the enduring impact of HU on SCD.
The pharmacokinetics of HU appears to follow a biphenotypic metabolism in children (33). Multiple single-nucleotide polymorphisms (SNPs) are associated with two apparent pharmacokinetic profiles of HU uptake and excretion. However, these genotypes do not correlate with response by the biomarker HbF.
Fetal Hemoglobin
The clinical severity of SCD is highly variable. Children experience multiple different clinical complications of differing severity levels and frequencies. HbF is of critical importance in the major sickle subtype homozygous sickle cell disease (HbSS; and sickle (HbS)-β-zero thalassemia, herein collectively referred to as HbSS). Lower HbF levels correlate with overall severer disease manifestations (34). Unlike “adult” hemoglobin A (HbA), HbF actively inhibits the polymerization of HbS, the underlying pathophysiology of SCD. In solution, HbF concentration higher than 15% prevents sickle globin polymerization (35). The cutoff for defining lower risk of severe complications has been estimated at 20% (36).
Sharp declines of HbF during infancy occur as HbF-producing γ-globin is replaced by β-globin. This switch leads to the predominant expression of either HbA or HbS. The F-to-S switch in children affected by HbSS (37) occurs more gradually than the F-to-A switch in nonanemic children. HbF levels in toddlers with SCD stabilize by age 3 or 4 y and are generally constant throughout childhood. Despite bearing the same β-globin sickle variant, affected populations with African ancestry exhibit wide variations in HbF levels (37,38,39,40). In the United States, pediatric levels vary from 3 to 20% of total hemoglobin, compared with only 0.5–2% for nonanemic individuals. The average HbF level in the US SCD pediatric population is ~10% (36).
Use of HU
Mechanism of Action
The physiology of the HU effect is complex and can generally be generally categorized into two overlapping pathways: effects on HbF production and improved blood flow through reduced intercellular adhesion ( Figure 1 ). HU is a short-acting cytotoxic drug that induces a state of “stress erythropoiesis.” Enhanced HbF production from intermittent mild marrow toxicity is believed to stem from the steady shifting of marrow physiology to the stressed state. The marrow responds to the repetitive pharmacological injury of daily use by enhanced erythropoiesis and increased HbF production (34,41). Paradoxically, the net effect of marrow toxicity is induced HbF and stabilization of cellular hemoglobin solubility. These effects lead to decreased levels of red blood cell (RBC) membrane damage and hemolysis (34,41).
Physiological effects of hydroxyurea on sickle cell disease (SCD). Hydroxyurea has pleiotropic effects in ameliorating SCD, with complex and interacting effects of vascular and red blood cell (RBC) components. Hb, hemoglobin; HbF, fetal Hb; HbS, sickle hemoglobin; WBC, white blood cells.
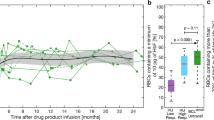
HbF induction usually occurs within the first few months after initiating HU and is reversible on cessation or diminution of dosing ( Figure 2 ). Relevance of HU induction of HbF was demonstrated through a proof-of-principle murine model for SCD. Lack of expression of human HbF precluded HU induction in those mice. In the murine model, HU itself had no effect on improving anemia or protecting organs from SCD damage. In contrast, HbF gene therapy markedly improved the blood smear, microscopic, and organ-level pathological effects of SCD (42).
Fetal hemoglobin (HbF) levels of a teenager with homozygous sickle hemoglobin (HbSS) on hydroxyurea (HU). Before HU use, this teenager had two to three hospitalizations for pain each year. She had no admissions for 1.7 y after beginning HU. Her baseline HbF was 2.4%, and maximum recorded HbF level was 16.9%. She acknowledged intermittent adherence in the years 2 and 3, during which time she had two admissions for acute pain episodes. Blue diamonds refer to HbF data points.
HU appears to influence RBC–endothelial interactions. Decreased expression of RBCs, white blood cells, and endothelial integrins and other adhesion molecules probably improves microvascular blood flow and reduces proinflammatory cell–cell interactions (43,44). Microvascular effects of SCD and HU appeared to be replicated using an interesting microfluidic model of blood flow and endothelialized microfluidic channels (44). Whole-blood samples from SCD lead to microvascular occlusion and thrombosis. Blood samples from patients with SCD had diminished velocity and greater tendency to obstruct in the microchannels. These effects nearly normalized using blood from patients on HU (44). HU may be associated with reduced generation of microparticles, suggesting a reduction in markers of inflammation and thrombosis (45).
HU may reduce cellular adhesion in general and/or adhesion provoked by infection or inflammation. Integrins and other cell surface glycoproteins regulate neutrophil migration and RBC flow through endothelial interactions. In a murine model for SCD and pneumococcal pneumonia and sepsis, HU provided some protection by decreasing the recruitment of neutrophils into infected lungs. Mice genetically engineered to lack E-selectin were not protected by HU (46). This finding strengthens the view that HbF-independent effects of HU include decreasing leukocyte–endothelial adhesion.
HU may also stimulate nitric oxide (NO) production as an NO donor or through stimulation of intermediates (discussed below). As a potent vasodilator, NO repletion contributes to improved vascular health in SCD ( Figure 1 ) (47). Along with decreased “sticky” interaction between blood cells and the endothelium, enhanced NO-induced local vasodilation may also benefit blood flow ( Figure 1 ) (48,49). However, questions have arisen regarding these effects of NO (50). In all, decreased pathology from damaged RBCs and pathological interactions between RBCs and endothelial cells appear to synergistically reduce clinical signs, symptoms, and morbidities of the disease ( Table 1 ). The ameliorative effects of HU appear to persist for as long as it is taken and the pharmacokinetics are maintained.
HbF Response to HU
The individual extent of HU-induced HbF is highly variable. Standard pediatric dosing of HU adjusts for dose-dependent myelotoxicity (14,33). Under these conditions, HU generally induces HbF by an additional 8–18% relative to the baseline levels (14,33,51,52). In contrast with the biomarker glycosylated Hb (HbA1c) for diabetes, no absolute HbF target exists. Nonetheless, peak attained HbF levels remain fairly constant in childhood (23). No absolute limit to the therapeutic amount of HbF induction has been described. For example, people of Southeast Asia or Saudi Arabia with SCD have baseline HbF levels averaging 16–20%. HU induction raises their levels 1.5- to 2-fold, associated with further diminution of their already tempered clinical symptoms (53).
Children with SCD generally have higher baseline HbF levels than adults and more pronounced HbF response to HU (14,54). Factors responsible for differences may include the need for highly regenerative marrow RBC precursors and, for HU, normal renal function for prompt excretion. Adults normally experience age-dependent decreased marrow cellularity. In SCD, disease-related marrow infarcts and other age-related physiological effects could exacerbate normal marrow regression. Age-related diminution of response to HU increases the likelihood that genetic studies using pediatric populations may reveal more precise basic biologic insights.
Genetic Analysis of HU-Induced HbF
Analyses of HbF regulation are crucial to understanding the spectrum of SCD severity, variability of HU response, and design of novel therapies. In addition to the established observations of ethnic variability of HbF levels, several key observations drive the rationale for identifying genetic components of HU induction of HbF in US populations of SCD:
- 1
-
2
HbF induction from HU therapy is also a heritable trait (55);
-
3
Genome-wide SNP studies in normal nonanemic adults identified a few major loci associated with variation of low HbF levels. These regions are both cis and trans of the β-globin gene locus (56,57);
-
4
These same loci are associated with baseline HbF in people with SCD in the United States (56,58,59,60,61,62). Additional loci have been identified but are not yet replicated;
-
5
A modest correlation in children exists between levels of HbF at baseline and on HU (14,33,51).
Taken together, these findings lead to the prediction that genetic regulation of HbF expression at baseline overlaps with the control of HU-induced HbF The three major loci related to HbF expression in normal and SCD populations are the following: a SNP upstream of the γ-globin gene within the globin locus on chromosome 11, previously identified by restriction enzyme analysis as the XmnI site (37,60,61); BCL11A, the gene encoding a transcription factor now recognized as a major silencer of HbF expression (51,58,59,61,63); and the intergenic interval between HBS1L and MYB (56,58,61). Additional loci have been identified and await replication (59,64,65), in addition to evaluation of known and probable epigenetic effects ( Figure 3 ) (66).
Phenotypic variability in hydroxyurea (HU) response. A diagram synthesizes the varying clinical and genetic effects of HU in sickle cell disease. The β-globin locus is shown below. ARG1 and 2, arginase 1 and 2; Hb, hemoglobin; HbF, fetal Hb; LCR, locus control region; MYB, myeloblastosis oncogene.
Only a few published studies report on the genetics of HbF response to HU in SCD (33,51,54,64). Compared with genomic studies of more common disorders, sample sizes of studies on HU effects in SCD are inevitably modest. Using the retrospective cohort from the Multicenter Study of Hydroxyurea adult trial and assessing more than two dozen candidate genes, Ma et al. (54) reported significant associations between SNPs and HbF response to HU in loci of genes involved in the metabolism of arginine to NO and in a transcription factor that induces DNA bending. This report predated the identification of BCL11A as a central regulator of HbF expression. Most of the Multicenter Study of Hydroxyurea patients exhibited a small HbF response to HU (54), with less than 5% change in HbF from baseline. This blunted response is not universal in US adults with SCD and may be influenced by both patient characteristics and adherence to HU regimen.
Whether HbF induction by HU occurs through the direct influence of BCL11A is a concept awaiting direct testing. Effects of BCL11A on HbF are probably mediated through its protein partners, upstream or downstream effectors, chromatin structure, and/or telomerase function (recently reviewed in ref. (67)). Other reports include associations between HU response in SCD and polymorphisms in the guanosine triphosphate–binding protein gene sar1a (64), underscoring the complexity of the genetic pathways regulating the HbF response to HU ( Figure 3 ).
Two pediatric pharmacogenetic analyses using candidate SNP markers suggested that just a few genes are associated with baseline HbF, including several SNPs within BCL11A ( Table 2 ). SNP associations with induced HbF are generally not independent of baseline HbF levels (33,51). In contrast with the induced HbF level, the treatment-associated increment appears to be a less relevant marker. Both of these observations probably reflect the association between baseline and induced levels.
In our own smaller multisite analysis, baseline levels of the candidate genes were significantly associated with SNPs within the BCL11A and the β- and ε- globin loci (HBB and HBE, respectively), with an additive attributable variance from these loci of 23% ( Table 2 ) (51). Consistent with studies by Ware et al.(14,33), we reported that baseline HbF levels explained 33% of the variance in induced levels. The variant in HBE accounted for an additional 13% of the variance in induced levels, whereas variants in the HBB and BCL11A loci did not contribute beyond baseline levels. Thus, our data suggest that the combined effects of baseline HbF and one SNP marker contributed an estimated 46% of the variance in HbF (51).
By trend analysis, children with an allele associated with higher HbF (“favorable” allele) in one of the BCL11A and/or either globin marker had significantly higher average values of baseline HbF than those who lacked a favorable allele (51). Effects on baseline HbF from a SNP in each these two genes were additive and were associated with two-fold higher HbF for patients with favorable alleles in both loci. Similarly, having at least one favorable allele in either globin locus and in BCL11A was associated with a higher level of induced HbF. Statistical significance did not withstand adjustment for baseline HbF, probably reflecting the interrelatedness of HbF regulation under both physiological conditions. Genetic studies examining larger pediatric populations on HU, unusual responders, and the influence of specific sequence variants are needed to evaluate the contribution of these and other genetic loci responsible for HbF response.
Other Physiological Effects of HU
Cellular Biology
The effects of HU largely depend on its effects on nucleic acid synthesis in dividing RBC progenitors. HU affects the S-phase by inhibiting ribonucleotide reductase, an enzyme important for DNA synthesis. Depletion of DNA precursors by HU causes arrest of the replication fork, leading to cell death. A cell-based ex vivo assay for HbF induction, burst-forming unit erythroid colonies grown in methylcellulose from blood of children with SCD, demonstrated that HU decreases the number of burst-forming unit erythroid colonies. HU and other ribonucleotide reductase inhibitors increase HbF production in that system. Interestingly, other cytotoxic agents that are not of that drug category, such as cytarabine and alkylating agents, decreased burst-forming unit erythroid counts but did not induce HbF (41,68).
HU’s lethal effect of on ribonucleotide reductase and cell survival are also seen in laboratory bacteria such as Escherichia coli (69). Whether the bactericidal effects influence investigation using animal models or even in patients has not previously been studied. Direct bacterial effects on the HU-dampened expression of adhesion molecules should be addressed in a murine model of bacterial infection.
HbF response to temporary marrow toxicity is probably attributable to transcriptional and epigenetic effects on the progenitor developmental program (66,70). HU signaling appears to involve cGMP (cyclic guanine monophosphate), cAMP (cyclic adenosine monophosphate), p38MAPK (mitogen-activated protein kinase), and other pathways. Activation of cGMP may induce HbF via enhancing production of NO (47,68,71,72). NO may also support HbF production (47). HU induces a small guanosine triphosphate–binding protein, the secretion-associated and Ras-related protein (SAR) (73). SAR may be involved in the activation of transcription factors and signal transduction pathways in erythroleukemia K562 cells and in human bone marrow–derived progenitor cells. HU may also function through kinase and signal transduction pathways, such as globin transcription factor-1, to enhance γ- and β-globin synthesis in erythroid cells (47).
Gene Expression
Comparing whole mRNA at the pre- and post-initiation stages of therapy revealed that HU affects expression of a number of genes involved in transcription, translation, ribosome assembly, and chromosomal organization (66,70). Results may vary with age, dosing, or other clinical conditions. Variation in cell source, whether from bone marrow or purified early reticulocytes, would be expected to affect detection of expressed genes. HU may also affect expression of genes that link HU and HbF to BCL11A (66,70). Epigenetic analysis of the γ-globin promoter did not reveal much impact from HU (74). Interestingly, HU appears to upregulate specific microRNAs (74). These results require further investigation but underscore the view that HU is involved with complex pathways of gene regulation.
Testing for Oncogenicity
The primary effect is damaging DNA replication by inhibiting ribonucleotide reductase. This effect raises concerns about an oncogenic potential, especially after prolonged use. These fears have been amplified by its original use as chemotherapeutic agent for chronic myeloid leukemia, the latent phase of acute leukemia. Although links with acute leukemia outside of chronic myeloid leukemia have been disproven (75), concerns for the safety of long-term use in children persist. Several studies have tested DNA and cellular toxicity from pediatric HU users. No genotoxicity was detected using several different assays in vitro, including karyotype, illegitimate VDJ recombination of the variable regions of rearranged T-cell receptor genes, and chromatid breaks (9). Increased reticulocyte micronuclei were observed, but this effect was highly variable among patients and did not increase with time (76). In all, oncogenicity of HU is probably quite low or nonexistent. A few cases of acute leukemia were reported in patients after many years of HU treatment, but these do not appear to be more frequent than in the untreated population (75).
Potential Pharmacological Alternatives to HU
Other HbF inducers have been assessed during the past few decades, including nucleoside analogs such as 5-azacytidine and decitabine. However, they are often poorly tolerated, potentially oncogenic, and lack proof of effectiveness comparable with HU (recently reviewed in ref. (41)). Additional HbF-inducing drugs are histone deacetylase inhibitors, erythropoietin (already high in SCD and shown not to induce HbF in SCD), valproate, thalidomide derivatives (e.g., pomalidimide), and kit ligand. In all, a variety of cellular stresses and stimuli can promote coordinated stress responses, including activation of the γ-globin gene (66,70). Based on results from SCD mouse models, inhibitors of phosphodiesterase 9 (71) or hypoxia-inducible factor-1α (HIF-1α) (77), alone or in combination with HU, may be clinically useful to stimulate cGMP and NO for HbF production and/or to enhance its antisickling impact (71).
Barriers to Use of HU
Outside of clinical trials with HU, ample documentation exists of incomplete clinical effectiveness of HU. Uneven drug adherence has been well documented (78,79). Provider non- and underutilization is well documented (75,80). Our recent multisite survey of parents of children with SCD revealed several family barriers to use of HU, such as lack of FDA approval, near-universal safety concerns, and highly varied knowledge about its benefits, including many for whom its basic property of decreasing episodes of pain was unknown (81). Use of HU was positively correlated with fundamental knowledge of parents regarding the basic positive effects of HU on disease, independent of parental demographics such as education level, language spoken, or ethnicity. Barriers in effective communication between providers and families may be exacerbated by issues arising from medical delivery systems.
The mixed uptake of HU by families may also reflect family perspectives on the long-term effects of SCD. A single-site survey of parents revealed that the majority believed that the disease effects were going to diminish over time and would not affect life goals or life span (82). These poignant perceptions will need to be addressed if families are to embrace the long-term benefits of HU against its inconveniences and largely theoretical risks.
Conclusion
HU is a remarkably effective drug for a large proportion of children with SCD. Efforts to achieve expanded understanding of the scientific underpinnings of its effects on SCD, predict individual response, and perfect the clinical applications for modifying disease effects are ongoing. Clinical trials will continue to test the uncertain benefits of HU ( Table 1 ), such as primary prevention of brain infarcts (clinicaltrials.gov/show/NCT01389024). Murine models will facilitate insight into the benefits provided by induced HbF, altered expression of adhesion molecules, reduced BCL11A levels, and other mechanisms. Genetic epidemiology will be used to identify specific variants in regulatory genes and gene pathways.
The accumulating science of HU is anticipated to lead to three direct effects for children with HbSS: (i) use at earlier ages; (ii) wider clinical indications; and (iii) delineation of children who are less likely to enjoy substantive benefit from HU. For this last group, more aggressive consideration of chronic transfusion, hematopoietic stem cell transplantation, or trial of emerging alternative agents may be warranted. To date, early clinical trials of other experimental HbF-inducing drugs have demonstrated considerable short- and long-term toxicity compared with HU. Therefore, HU is predicted to remain the mainstay of pharmacological therapy for SCD in the foreseeable future.
Progress toward the Healthy People 2020 goals will occur through increased use of HU. Nonetheless, the entire SCD population may not benefit from HU alone. Dampened impact on clinical complications and HbF induction occurs for those with certain genotypes, many patients with HbSC (compound heterozygous for HbS and HbC), some adult patients, and those with renal compromise. New, effective, and safe therapies, alone or in combination with HU, are still needed to maximize pharmacological benefit for everyone living with SCD. Constructive engagement must be made to assist families in undertaking long-term HU use to help them to balance the optimism of HU treatment and its potential toxicities with the risk of accumulating disease consequences. Although several important crisis modulators are currently under investigation, disease modifiers that prevent crises and other morbidities are arguably the primary therapeutic targets.
Statement of Financial Support
The authors acknowledge support from the Clinical Translational Science Award at Columbia University, 5UL1RR024156 (H.N. Ginsberg, principal investigator).
Disclosure
No commercial or other conflict of interest is reported by the authors.
References
Healthy People 2020. (http://healthypeople.gov/2020/topicsobjectives2020/overview.aspx?topicid=4.) Accessed 6 September 2012.
Hassell KL . Population estimates of sickle cell disease in the U.S. Am J Prev Med 2010;38:Suppl:S512–21.
Hamideh D, Alvarez O . Sickle cell disease related mortality in the United States (1999-2009). Pediatr Blood Cancer 2013;60:1482–6.
Quinn CT, Rogers ZR, McCavit TL, Buchanan GR . Improved survival of children and adolescents with sickle cell disease. Blood 2010;115:3447–52.
Yanni E, Grosse SD, Yang Q, Olney RS . Trends in pediatric sickle cell disease-related mortality in the United States, 1983-2002. J Pediatr 2009;154:541–5.
Lanzkron S, Carroll CP, Haywood C Jr . Mortality rates and age at death from sickle cell disease: U.S., 1979-2005. Public Health Rep 2013;128:110–6.
Charache S, Terrin ML, Moore RD, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. N Engl J Med 1995;332:1317–22.
Ferster A, Vermylen C, Cornu G, et al. Hydroxyurea for treatment of severe sickle cell anemia: a pediatric clinical trial. Blood 1996;88:1960–4.
McGann PT, Ware RE . Hydroxyurea for sickle cell anemia: what have we learned and what questions still remain? Curr Opin Hematol 2011;18:158–65.
Strouse JJ, Heeney MM . Hydroxyurea for the treatment of sickle cell disease: efficacy, barriers, toxicity, and management in children. Pediatr Blood Cancer 2012;59:365–71.
Strouse JJ, Lanzkron S, Beach MC, et al. Hydroxyurea for sickle cell disease: a systematic review for efficacy and toxicity in children. Pediatrics 2008;122:1332–42.
Kinney TR, Helms RW, O’Branski EE, et al. Safety of hydroxyurea in children with sickle cell anemia: results of the HUG-KIDS study, a phase I/II trial. Pediatric Hydroxyurea Group. Blood 1999;94:1550–4.
Wang WC, Helms RW, Lynn HS, et al. Effect of hydroxyurea on growth in children with sickle cell anemia: results of the HUG-KIDS Study. J Pediatr 2002;140:225–9.
Ware RE, Eggleston B, Redding-Lallinger R, et al. Predictors of fetal hemoglobin response in children with sickle cell anemia receiving hydroxyurea therapy. Blood 2002;99:10–4.
Hankins JS, Ware RE, Rogers ZR, et al. Long-term hydroxyurea therapy for infants with sickle cell anemia: the HUSOFT extension study. Blood 2005;106:2269–75.
Thornburg CD, Dixon N, Burgett S, et al. A pilot study of hydroxyurea to prevent chronic organ damage in young children with sickle cell anemia. Pediatr Blood Cancer 2009;52:609–15.
Thornburg CD, Files BA, Luo Z, et al.; BABY HUG Investigators. Impact of hydroxyurea on clinical events in the BABY HUG trial. Blood 2012;120:4304–10; quiz 4448.
Wang WC, Ware RE, Miller ST, et al.; BABY HUG investigators. Hydroxycarbamide in very young children with sickle-cell anaemia: a multicentre, randomised, controlled trial (BABY HUG). Lancet 2011;377:1663–72.
Bernaudin F, Verlhac S, Arnaud C, et al. Impact of early transcranial Doppler screening and intensive therapy on cerebral vasculopathy outcome in a newborn sickle cell anemia cohort. Blood 2011;117:1130–40; quiz 1436.
de Montalembert M, Brousse V, Elie C, Bernaudin F, Shi J, Landais P ; French Study Group on Sickle Cell Disease. Long-term hydroxyurea treatment in children with sickle cell disease: tolerance and clinical outcomes. Haematologica 2006;91:125–8.
Thornburg CD, Calatroni A, Panepinto JA . Differences in health-related quality of life in children with sickle cell disease receiving hydroxyurea. J Pediatr Hematol Oncol 2011;33:251–4.
Dampier C, Lieff S, LeBeau P, et al.; Comprehensive Sickle Cell Centers (CSCC) Clinical Trial Consortium (CTC). Health-related quality of life in children with sickle cell disease: a report from the Comprehensive Sickle Cell Centers Clinical Trial Consortium. Pediatr Blood Cancer 2010;55:485–94.
Zimmerman SA, Schultz WH, Davis JS, et al. Sustained long-term hematologic efficacy of hydroxyurea at maximum tolerated dose in children with sickle cell disease. Blood 2004;103:2039–45.
Aygun B, Mortier NA, Smeltzer MP, Shulkin BL, Hankins JS, Ware RE . Hydroxyurea treatment decreases glomerular hyperfiltration in children with sickle cell anemia. Am J Hematol 2013;88:116–9.
Alvarez O, Miller ST, Wang WC, et al.; BABY HUG Investigators. Effect of hydroxyurea treatment on renal function parameters: results from the multi-center placebo-controlled BABY HUG clinical trial for infants with sickle cell anemia. Pediatr Blood Cancer 2012;59:668–74.
Kratovil T, Bulas D, Driscoll MC, Speller-Brown B, McCarter R, Minniti CP . Hydroxyurea therapy lowers TCD velocities in children with sickle cell disease. Pediatr Blood Cancer 2006;47:894–900.
Zimmerman SA, Schultz WH, Burgett S, Mortier NA, Ware RE . Hydroxyurea therapy lowers transcranial Doppler flow velocities in children with sickle cell anemia. Blood 2007;110:1043–7.
Ware RE, Helms RW ; SWiTCH Investigators. Stroke With Transfusions Changing to Hydroxyurea (SWiTCH). Blood 2012;119:3925–32.
Alvarez O, Yovetich NA, Scott JP, et al.; Investigators of the Stroke With Transfusions Changing to Hydroxyurea Clinical Trial (SWiTCH). Pain and other non-neurological adverse events in children with sickle cell anemia and previous stroke who received hydroxyurea and phlebotomy or chronic transfusions and chelation: results from the SWiTCH clinical trial. Am J Hematol 2013;88:932–8.
Steinberg MH, McCarthy WF, Castro O, et al.; Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia and MSH Patients’ Follow-Up. The risks and benefits of long-term use of hydroxyurea in sickle cell anemia: a 17.5 year follow-up. Am J Hematol 2010;85:403–8.
Voskaridou E, Christoulas D, Bilalis A, et al. The effect of prolonged administration of hydroxyurea on morbidity and mortality in adult patients with sickle cell syndromes: results of a 17-year, single-center trial (LaSHS). Blood 2010;115:2354–63.
Lobo CL, Pinto JF, Nascimento EM, Moura PG, Cardoso GP, Hankins JS . The effect of hydroxcarbamide therapy on survival of children with sickle cell disease. Br J Haematol 2013;161:852–60.
Ware RE, Despotovic JM, Mortier NA, et al. Pharmacokinetics, pharmacodynamics, and pharmacogenetics of hydroxyurea treatment for children with sickle cell anemia. Blood 2011;118:4985–91.
Platt OS . Hydroxyurea for the treatment of sickle cell anemia. N Engl J Med 2008;358:1362–9.
Bunn HF . Subunit assembly of hemoglobin: an important determinant of hematologic phenotype. Blood 1987;69:1–6.
Powars DR, Weiss JN, Chan LS, Schroeder WA . Is there a threshold level of fetal hemoglobin that ameliorates morbidity in sickle cell anemia? Blood 1984;63:921–6.
Green NS, Fabry ME, Kaptue-Noche L, Nagel RL . Senegal haplotype is associated with higher HbF than Benin and Cameroon haplotypes in African children with sickle cell anemia. Am J Hematol 1993;44:145–6.
Makani J, Menzel S, Nkya S, et al. Genetics of fetal hemoglobin in Tanzanian and British patients with sickle cell anemia. Blood 2011;117:1390–2.
Nagel RL, Erlingsson S, Fabry ME, et al. The Senegal DNA haplotype is associated with the amelioration of anemia in African-American sickle cell anemia patients. Blood 1991;77:1371–5.
Solovieff N, Hartley SW, Baldwin CT, et al. Ancestry of African Americans with sickle cell disease. Blood Cells Mol Dis 2011;47:41–5.
Fathallah H, Atweh GF . Induction of fetal hemoglobin in the treatment of sickle cell disease. Hematology Am Soc Hematol Educ Program 2006;1:58–62.
Lebensburger JD, Pestina TI, Ware RE, Boyd KL, Persons DA . Hydroxyurea therapy requires HbF induction for clinical benefit in a sickle cell mouse model. Haematologica 2010;95:1599–603.
Gambero S, Canalli AA, Traina F, et al. Therapy with hydroxyurea is associated with reduced adhesion molecule gene and protein expression in sickle red cells with a concomitant reduction in adhesive properties. Eur J Haematol 2007;78:144–51.
Tsai M, Kita A, Leach J, et al. In vitro modeling of the microvascular occlusion and thrombosis that occur in hematologic diseases using microfluidic technology. J Clin Invest 2012;122:408–18.
Nébor D, Romana M, Santiago R, et al. Fetal hemoglobin and hydroxycarbamide moduate both plasma concentration and cellular origin of circulating microparticles in sickle cell anemia children. Haematologica 2013;98:862–7.
Lebensburger JD, Howard T, Hu Y, et al. Hydroxyurea therapy of a murine model of sickle cell anemia inhibits the progression of pneumococcal disease by down-modulating E-selectin. Blood 2012;119:1915–21.
Lou TF, Singh M, Mackie A, Li W, Pace BS . Hydroxyurea generates nitric oxide in human erythroid cells: mechanisms for gamma-globin gene activation. Exp Biol Med (Maywood) 2009;234:1374–82.
Kato GJ, Hebbel RP, Steinberg MH, Gladwin MT . Vasculopathy in sickle cell disease: biology, pathophysiology, genetics, translational medicine, and new research directions. Am J Hematol 2009;84:618–25.
King SB . Nitric oxide production from hydroxyurea. Free Radic Biol Med 2004;37:737–44.
Bunn HF, Nathan DG, Dover GJ, et al. Pulmonary hypertension and nitric oxide depletion in sickle cell disease. Blood 2010;116:687–92.
Green NS, Ender KL, Pashankar F, et al. Candidate sequence variants and fetal hemoglobin in children with sickle cell disease treated with hydroxyurea. PLoS ONE 2013;8:e55709.
Meier ER, Byrnes C, Weissman M, Noel P, Luban NL, Miller JL . Expression patterns of fetal hemoglobin in sickle cell erythrocytes are both patient- and treatment-specific during childhood. Pediatr Blood Cancer 2011;56:103–9.
Italia K, Jain D, Gattani S, et al. Hydroxyurea in sickle cell disease–a study of clinico-pharmacological efficacy in the Indian haplotype. Blood Cells Mol Dis 2009;42:25–31.
Ma Q, Wyszynski DF, Farrell JJ, et al. Fetal hemoglobin in sickle cell anemia: genetic determinants of response to hydroxyurea. Pharmacogenomics J 2007;7:386–94.
Steinberg MH, Voskaridou E, Kutlar A, et al. Concordant fetal hemoglobin response to hydroxyurea in siblings with sickle cell disease. Am J Hematol 2003;72:121–6.
Thein SL, Menzel S . Discovering the genetics underlying foetal haemoglobin production in adults. Br J Haematol 2009;145:455–67.
Garner C, Tatu T, Reittie JE, et al. Genetic influences on F cells and other hematologic variables: a twin heritability study. Blood 2000;95:342–6.
Bae HT, Baldwin CT, Sebastiani P, et al. Meta-analysis of 2040 sickle cell anemia patients: BCL11A and HBS1L-MYB are the major modifiers of HbF in African Americans. Blood 2012;120:1961–2.
Bhatnagar P, Purvis S, Barron-Casella E, et al. Genome-wide association study identifies genetic variants influencing F-cell levels in sickle-cell patients. J Hum Genet 2011;56:316–23.
Galarneau G, Palmer CD, Sankaran VG, Orkin SH, Hirschhorn JN, Lettre G . Fine-mapping at three loci known to affect fetal hemoglobin levels explains additional genetic variation. Nat Genet 2010;42:1049–51.
Lettre G, Sankaran VG, Bezerra MA, et al. DNA polymorphisms at the BCL11A, HBS1L-MYB, and beta-globin loci associate with fetal hemoglobin levels and pain crises in sickle cell disease. Proc Natl Acad Sci USA 2008;105:11869–74.
Sebastiani P, Solovieff N, Hartley SW, et al. Genetic modifiers of the severity of sickle cell anemia identified through a genome-wide association study. Am J Hematol 2010;85:29–35.
Sedgewick AE, Timofeev N, Sebastiani P, et al. BCL11A is a major HbF quantitative trait locus in three different populations with beta-hemoglobinopathies. Blood Cells Mol Dis 2008;41:255–8.
Kumkhaek C, Taylor JG 6th, Zhu J, Hoppe C, Kato GJ, Rodgers GP . Fetal haemoglobin response to hydroxycarbamide treatment and sar1a promoter polymorphisms in sickle cell anaemia. Br J Haematol 2008;141:254–9.
Solovieff N, Milton JN, Hartley SW, et al. Fetal hemoglobin in sickle cell anemia: genome-wide association studies suggest a regulatory region in the 5’ olfactory receptor gene cluster. Blood 2010;115:1815–22.
Flanagan JM, Steward S, Howard TA, et al. Hydroxycarbamide alters erythroid gene expression in children with sickle cell anaemia. Br J Haematol 2012;157:240–8.
Bauer DE, Orkin SH . Update on fetal hemoglobin gene regulation in hemoglobinopathies. Curr Opin Pediatr 2011;23:1–8.
Yang YM, Pace B . Pharmacologic induction of fetal hemoglobin synthesis: cellular and molecular mechanisms. Pediatr Pathol Mol Med 2001;20:87–106.
Bollenbach T, Quan S, Chait R, Kishony R . Nonoptimal microbial response to antibiotics underlies suppressive drug interactions. Cell 2009;139:707–18.
Costa FC, da Cunha AF, Fattori A, et al. Gene expression profiles of erythroid precursors characterise several mechanisms of the action of hydroxycarbamide in sickle cell anaemia. Br J Haematol 2007;136:333–42.
Almeida CB, Scheiermann C, Jang JE, et al. Hydroxyurea and a cGMP-amplifying agent have immediate benefits on acute vaso-occlusive events in sickle cell disease mice. Blood 2012;120:2879–88.
Cokic VP, Andric SA, Stojilkovic SS, Noguchi CT, Schechter AN . Hydroxyurea nitrosylates and activates soluble guanylyl cyclase in human erythroid cells. Blood 2008;111:1117–23.
Tang DC, Zhu J, Liu W, et al. The hydroxyurea-induced small GTP-binding protein SAR modulates gamma-globin gene expression in human erythroid cells. Blood 2005;106:3256–63.
Walker AL, Steward S, Howard TA, et al. Epigenetic and molecular profiles of erythroid cells after hydroxyurea treatment in sickle cell anemia. Blood 2011;118:5664–70.
Lanzkron S, Strouse JJ, Wilson R, et al. Systematic review: hydroxyurea for the treatment of adults with sickle cell disease. Ann Intern Med 2008;148:939–55.
Flanagan JM, Howard TA, Mortier N, et al. Assessment of genotoxicity associated with hydroxyurea therapy in children with sickle cell anemia. Mutat Res 2010;698:38–42.
Kaul DK, Fabry ME, Suzuka SM, Zhang X . Antisickling fetal hemoglobin reduces hypoxia-inducible factor-1a expression in normoxic sickle mice: microvascular implications. Am J Physiol Heart Circ Physiol 2013;304:H42–50.
Candrilli SD, O’Brien SH, Ware RE, Nahata MC, Seiber EE, Balkrishnan R . Hydroxyurea adherence and associated outcomes among Medicaid enrollees with sickle cell disease. Am J Hematol 2011;86:273–7.
Thornburg CD, Rogers ZR, Jeng MR, et al.; BABY HUG Investigators. Adherence to study medication and visits: data from the BABY HUG trial. Pediatr Blood Cancer 2010;54:260–4.
Haywood C Jr, Beach MC, Lanzkron S, et al. A systematic review of barriers and interventions to improve appropriate use of therapies for sickle cell disease. J Natl Med Assoc 2009;101:1022–33.
Oyeku SO, Driscoll MC, Cohen HW, et al. Parental and other factors associated with hydroxyurea use for pediatric sickle cell disease. Pediatr Blood Cancer 2013;60:653–8.
Roth M, Krystal J, Manwani D, Driscoll C, Ricafort R . Stem cell transplant for children with sickle cell anemia: parent and patient interest. Biol Blood Marrow Transplant 2012;18:1709–15.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Green, N., Barral, S. Emerging science of hydroxyurea therapy for pediatric sickle cell disease. Pediatr Res 75, 196–204 (2014). https://doi.org/10.1038/pr.2013.227
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2013.227
This article is cited by
-
Sickle cell disease and acute leukemia: one case report and an extensive review
Annals of Hematology (2023)
-
Genome-based therapeutic interventions for β-type hemoglobinopathies
Human Genomics (2021)
-
High fetal hemoglobin level is associated with increased risk of cerebral vasculopathy in children with sickle cell disease in Mayotte
BMC Pediatrics (2020)
-
Functional polymorphisms of BCL11A and HBS1L-MYB genes affect both fetal hemoglobin level and clinical outcomes in a cohort of children with sickle cell anemia
Annals of Hematology (2020)
-
Population Pharmacokinetic and Exposure–Response Analyses of Prasugrel in Pediatric Patients with Sickle Cell Anemia
Clinical Pharmacokinetics (2018)