Abstract
Introduction:
Dopamine is one of the most frequently used inotropic drugs in neonatal intensive care units (NICUs); however, it does not seem to improve outcomes in premature infants. Given that the ultimate aim of cardiovascular management is to stabilize and maintain organ perfusion, an understanding of dopamine’s effects on organ blood flow will help in judging when to use dopamine and how to titrate the dosage. Such an approach can lead to improved outcomes. This study aimed to evaluate the effects of dopamine on peripheral perfusion in very-low-birth-weight (VLBW) infants within 72 h of birth.
Methods:
This prospective observational study identified and sampled 44 instances of initiation of dopamine treatment or increase in dopamine dose in 29 VLBW infants. Blood pressure, heart rate, and skin and subcutaneous blood flow were measured and compared before and after each instance.
Results:
Blood pressure and skin and subcutaneous blood flow in the lower limbs increased after initiation of dopamine treatment or after dose increase.
Discussion:
Dopamine increases blood pressure as well as skin and subcutaneous blood flow in VLBW infants despite its supposed vasoconstrictive action, indicating that it increases both perfusion pressure and blood flow and is devoid of overwhelming peripheral vasoconstrictive effects.
Similar content being viewed by others
Main
Most very-low-birth-weight (VLBW) infants develop cardiovascular compromise of a complex and varied etiology during the extrauterine transitional period. Cardiovascular compromise can result in a poor prognosis in premature infants. Therefore, VLBW infants often receive cardiac support in neonatal intensive care units (NICUs), although the criteria and protocols for inotropic support vary among institutions (1).
Of late, in most NICUs, the cardiovascular management of VLBW infants has focused on elevating blood pressure (2). Although dopamine is one of the most frequently used vasoactive drugs for elevating blood pressure, there have been no published reports demonstrating improved outcomes after the use of dopamine (3).
The reasons for the failure of vasoactive drugs to improve outcomes are not clear. Because the aim of cardiovascular management is to stabilize and maintain adequate perfusion in each organ, the perfusion of organs should be evaluated. However, few cardiovascular parameters directly represent organ perfusion. Recently, the importance of evaluating the perfusion of each organ has been recognized. Several studies have reported the effects of dopamine on perfusion in various organs, such as the brain (4,5), kidney (6), and intestine (7). In contrast, there is a paucity of clinical data regarding skin perfusion, although skin perfusion is thought to represent peripheral perfusion.
In our previous study, we reported the changes in skin and subcutaneous blood flow in VLBW infants during the extrauterine transitional period (8). Our current study aimed to elucidate the action of dopamine on peripheral perfusion, as measured by skin and subcutaneous blood flow, in VLBW infants. The infants were administered dopamine within 72 h of birth.
Results
A total of 44 instances of dopamine initiation or dose increase were evaluated in 29 infants. In 15 instances, the dosage of dopamine was increased to >10 μg·kg−1·min−1 (defined as “high-dose instances”). The background clinical parameters of all the infants are shown in Table 1 .
Overall, the mean (range) dopamine dose was increased from 4.8 ± 4.3 (0–7) μg·kg−1·min−1 to 8.8 ± 4.9 (2.6–9.7) μg·kg−1·min−1. In high-dose instances alone, the dopamine dose was increased from 9.1 ± 4.2 (3.2–17) μg·kg−1·min−1 to 14.3 ± 3.6 (10.4–20) μg·kg−1·min−1.
Changes in Parameters of Systemic Circulation
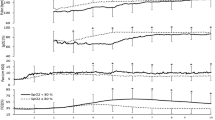
Systolic, diastolic, and mean arterial blood pressures were significantly increased after treatment with dopamine. Mean arterial blood pressure increased in most instances (37/44), and these increases were statistically significant ( Figure 1 ). Systolic blood pressure (mean ± SD) increased from 39.2 ± 5.7 mm Hg to 41.2 ± 6.1 mm Hg (% increase ± SE, 5.6 ± 1.8%; P = 0.006), diastolic blood pressure increased from 22.5 ± 4.4 mm Hg to 25.0 ± 5.1 mm Hg (% increase ± SE, 11.6 ± 2.1%; P < 0.001), and mean arterial blood pressure increased from 29.0 ± 4.0 to 31.8 ± 4.6 mm Hg (% increase ± SE, 10.1 ± 1.6%; P < 0.001) ( Figure 2 ). Invasive measurement of blood pressure was performed in 35/44 (79.5%) instances. A separate analysis of data from these infants also showed significant changes in blood pressure: systolic pressure increased from 38.4 ± 4.9 to 40.8 ± 5.9 mm Hg (P = 0.02), diastolic pressure increased from 22.4 ± 2.9 to 25.1 ± 4.2 mm Hg (P = 0.001), and mean arterial pressure increased from 28.8 ± 3.3 to 31.8 ± 4.1 mm Hg (P < 0.001). In high-dose instances, diastolic and mean blood pressures significantly increased from 22.8 ± 5.2 to 24.9 ± 4.6 mm Hg (% increase ± SE, 11.1 ± 1.7%; P = 0.002) and from 29.0 ± 4.4 to 31.4 ± 3.4 mm Hg (% increase ± SE, 11.1 ± 1.7%; P = 0.003), respectively. However, changes in systolic blood pressure were not significant, i.e., from 38.7 ± 6.0 to 39.3 ± 4.0 mm Hg (% increase ± SE, 3.4 ± 2.0%; P = 0.62).
Individual changes in mean arterial blood pressure, lower-limb blood flow (LBF), and forehead blood flow (FBF) before and after dopamine administration. Mean arterial blood pressure and LBF increased significantly in most instances (37/44 and 31/44, respectively) in response to dopamine administration. LBF seemed to have more variations than mean arterial blood pressure in response to dopamine. There were no consistent trends in FBF. MAP, mean arterial pressure.
Changes in parameters of systemic circulation. Systolic, diastolic, and mean arterial blood pressures increased significantly (systolic: % increase ± SE, 5.6 ± 1.8%, P = 0.006; diastolic: % increase ± SE, 11.6 ± 2.1%, P < 0.001; mean arterial: % increase ± SE, 10.1 ± 1.6%, P < 0.001), whereas heart rate did not change (% increase ± SE, 0.6 ± 0.9%; P = 0.65).
There was a significant increase in heart rates in high-dose instances (161 ± 14 to 165 ± 15 beats/min; % increase ± SE, 2.2 ± 0.4%; P = 0.006). However, when data from all instances were compiled, heart rates increased from 157 ± 14 to 158 ± 15 beats/min, an increase that was not statistically significant (% increase ± SE, 0.6 ± 0.9%; P = 0.65; Figure 2 ).
Changes in Skin and Subcutaneous Blood Flow
Lower-limb blood flow (LBF) increased significantly from 16.8 ± 4.6 ml·100 g−1 tissue weight·min−1 to 17.5 ± 4.6 ml·100 g−1 tissue weight·min−1 (% increase ± SE, 5.6 ± 1.8%; P = 0.012; Figure 3 ); there seemed to be more variations in LBF change than in mean arterial blood pressure change in response to dopamine ( Figure 1 ). There was no significant change in forehead blood flow (FBF) (14.9 ± 5.2 ml·100 g−1 tissue weight·min−1 to 14.7 ± 4.9 ml·100 g−1 tissue weight·min−1; % increase ± SE, 1.0 ± 2.6%; P = 0.59; Figure 2 ).
Changes in skin blood flow and subcutaneous blood flow. After increase in dopamine dosage, the lower-limb blood flow (LBF) increased significantly (% increase ± SE, 5.6 ± 1.8%; P = 0.012), whereas there were no significant changes in forehead blood flow (FBF) (% increase ± SE, 1.0 ± 2.6%; P = 0.59).
In high-dose instances, LBF increased significantly from 17.4 ± 4.9 ml·100 g−1 tissue weight·min−1 to 18.3 ± 4.7 ml·100 g−1 tissue weight·min−1 (% increase ± SE, 7.3 ± 1.6%; P = 0.011), whereas FBF did not undergo any significant change (14.9 ± 4.7 ml·100 g−1 tissue weight·min−1 to 14.8 ± 4.1 ml·100 g−1 tissue weight·min−1; % increase ± SE, 1.2 ± 1.5%; P = 0.94).
Relationships Between Dopamine Dose and Blood Flow
Changes in LBF values were positively correlated with changes in dopamine dose (r = 0.38, P = 0.01) ( Figure 4 ), whereas changes in FBF values were not correlated with changes in dopamine dose (r = 0.18, P = 0.23).
Discussion
In this study, we found that dopamine significantly increased skin and subcutaneous blood flow in a dose-dependent manner, even at doses >10 μg·kg−1·min−1, in VLBW infants within 72 h of birth. This finding has some clinical significance. Dopamine is generally considered to have peripheral vasoconstrictive action, especially at high doses (exceeding 10 μg·kg−1·min−1) (9). Inappropriately high doses of dopamine may result in vasoconstriction with signs of peripheral hypoperfusion, such as pale skin color, coldness of the skin, and prolonged capillary refill time. However, the findings of our study indicated that dopamine, even at doses >10 μg·kg−1·min−1, may not be the cause of peripheral hypoperfusion in VLBW infants. Dopamine exerts vasoconstrictive action on vessels through α-adrenergic receptors. In preterm infants, under the influence of certain factors such as immature cardiovascular adrenergic receptor expression, receptor downregulation caused by critical illness and exogenous catecholamine administration (10), and dysregulated local vasodilator production caused by conditions such as septic shock, dopamine doses >20 μg·kg−1·min−1 may be required to achieve the same effects as those seen in adults (6). In this study, there tended to be more variations in LBF response to dopamine, whereas the response of arterial blood pressure to the drug was more consistent. This finding suggests that subcutaneous blood flow is regulated by other factors in addition to local adrenergic receptor function. These findings may be clinically important for the management of patients with cardiovascular failure consequent to distributional shock (such as septic shock or vasodilative shock), which is not uncommon in infants with chorioamnionitis (11).
In this study, dopamine had the effect of increasing blood pressure regardless of dose. It is believed that dopamine increases blood pressure by increasing both myocardial contractility and peripheral vascular resistance (12), although several reports have suggested that these actions are impaired in certain conditions, such as critical illness (13), relative steroid deficiency (14), and immaturity (15) in preterm infants. Several authors have used echocardiography to evaluate the myocardial effects of dopamine. Although complicating factors such as the existence of patent ductus arteriosus in preterm infants may make the interpretation of echocardiographical data difficult, most studies have demonstrated positive results of echocardiographical evaluation, such as increased myocardial contractility (16) and increased cardiac output (17,18). In contrast, some studies found that dopamine increased blood pressure without causing an improvement in echocardiographical parameters such as left ventricular output (19) and superior vena cava blood flow (20). Rather, dopamine appears to increase blood pressure by increasing calculated systemic vascular resistance (21). These studies therefore concluded that vasoconstriction may be the primary mechanism underlying blood pressure elevation by dopamine in premature infants. This interpretation is not consistent with our findings or with a number of earlier findings reported in the literature (16,17,18). The similarity in the magnitude of increase in blood pressure and LBF may indicate that peripheral vascular resistance is unchanged by dopamine administration per the equation “vascular resistance = blood pressure/blood flow.” However, one must be cautious about applying this formula to peripheral vascular resistance because it is not clear whether peripheral perfusion pressure changes in parallel with systemic (mean) blood pressure. Furthermore, increases in skin and subcutaneous blood flow cannot be explained by peripheral vasoconstriction alone; they can be explained by an increase in myocardial contractility or by increases in both myocardial contractility and peripheral vasoconstriction. No evidence of any vasoconstrictive effects of dopamine was found in our study, which reported an increase in both mean arterial blood pressure and LBF. It is possible that vasoconstriction, leading to increased peripheral vascular resistance, was induced by dopamine in peripheral organs other than skin and subcutaneous tissue, such as muscles, kidneys, and intestines; however, this seems unlikely because the skin and subcutaneous tissue have neuroendocrine compensatory mechanisms that act through adrenergic receptors to reduce blood flow immediately in the event of cardiovascular failure (22).
Differences in the distributions of adrenergic and dopaminergic receptors between different organs may have contributed to changes in blood flow distribution (9). For a more detailed understanding of the drug response mechanism of dopamine, further studies evaluating the distributional changes in perfusion among peripheral and vital organs are required. These studies could be carried out by simultaneously measuring perfusion with a laser Doppler flowmeter and near-infrared spectroscopy.
In our study, the changes in blood flow were different at the forehead than at the lower limb. As discussed in our previous studies (8,23), this difference may be explained by differences in autonomic innervation between the skin over the forehead and that over the lower limbs. LBF seems to be a more sensitive measure of circulatory changes and a better indicator of skin perfusion and subcutaneous perfusion responses to treatment.
In conclusion, this is the first report, to the best of our knowledge, of dopamine-induced changes in skin and subcutaneous blood flow in VLBW infants. Dopamine is an important drug for the management of blood circulation in premature infants. Simultaneous measurement of skin and subcutaneous blood flow, and comparison of these with blood flow in other organs, will elucidate the mechanism underlying the cardiovascular action of dopamine in VLBW infants.
Methods
Subjects
This prospective observational study enrolled VLBW infants born between 1 September 2008 and 31 May 2011 and admitted to the NICU at Saitama Medical Center, Japan. Infants with congenital heart disease, intracranial complications, chromosomal anomalies, diseases with intrathoracic space–occupying lesions, and severe asphyxia were excluded. We collected data on instances of initiation of dopamine treatment and increase in dopamine dose within 72 h of birth. We excluded instances wherein skin and subcutaneous blood flow were not measured, and those wherein concomitant changes were made in the doses of drugs other than dopamine.
Written informed consent was obtained from the infants’ parents immediately after admission of the infant into the NICU. This study was approved by the ethical committee of Saitama Medical Center, Saitama, Japan.
Dopamine treatment was initiated during the early neonatal period, primarily for hypotension. In a few cases, a small dose of dopamine was administered in an attempt to increase renal and/or intestinal blood flow. Hypotension was defined as mean arterial pressure below the 10th percentile of gestational age-dependent normal values (24). Dopamine was usually initiated at a low to moderate dose (<10 μg·kg−1·min−1) and continuously infused via a peripheral central catheter or an umbilical venous line. If the blood pressure was not sufficiently elevated in response to this treatment, the dose was increased to ~20 μg·kg−1·min−1. Alternatively, the same dose of dobutamine was added with or without a single volume expander (10 ml·kg−1). These treatments were administered according to the instructions of attending physicians. If these measures were also ineffective, epinephrine or corticosteroids were administered.
All neonates were placed in incubators during the study period, and their skin temperature was maintained at 37.0 ± 0.5 °C.
Blood Flow Measurement
A noninvasive continuous monitoring technique was used for measuring skin and subcutaneous blood flow, as described in our previous studies (8,23). Probes were attached to the middle of the forehead and to the dorsum of the right or left foot, avoiding visible vessels. The FBF and LBF were measured simultaneously, and the values were displayed separately. The data were stored in a computer for the 72-h period. Once the data were collected, blood flow values were extracted at 10-s intervals.
Cardiovascular Parameters
Blood pressure was measured in all neonates. Systolic, diastolic, and mean arterial blood pressures were measured continuously using an umbilical or peripheral arterial catheter connected to a pressure transducer or via oscillometric methods, from 1 h before and after dopamine treatment initiation or dopamine dose increase.
Data Analysis and Statistics
We compared the value of each parameter before and after each initiation or change in dose. In addition, we studied instances wherein the dopamine dose was increased to more than 10 μg·kg−1·min−1. This was done because dopamine at doses of >10 μg·kg−1·min−1 was expected to exert peripheral vasoconstrictive action via α-adrenergic receptors (9) if cardiovascular adrenergic receptors were not significantly downregulated (6).
We also evaluated the correlation between the degree of change in dopamine dose and LBF and FBF.
All statistical values are shown as means ± SD unless otherwise indicated, and a two-tailed P value of <0.05 was considered statistically significant. Values before and after each instance were compared using a paired t-test. The extent of change in dopamine dose as compared to those in skin blood flow and subcutaneous blood flow were examined for correlation, using Pearson’s correlation coefficient. Stat Flex (StatFlex; Artec, Osaka, Japan) was used for all statistical analyses.
Statement of Financial Support
This study was supported by the Kawano Masanori Memorial Foundation for Promotion of Pediatrics.
References
Laughon M, Bose C, Allred E, et al. Factors associated with treatment for hypotension in extremely low gestational age newborns during the first postnatal week. Pediatrics 2007;119:273–80.
Dempsey EM, Barrington KJ . Diagnostic criteria and therapeutic interventions for the hypotensive very low birth weight infant. J Perinatol 2006;26:677–81.
Barrington KJ . Hypotension and shock in the preterm infant. Semin Fetal Neonatal Med 2008;13:16–23.
Pellicer A, Valverde E, Elorza MD, et al. Cardiovascular support for low birth weight infants and cerebral hemodynamics: a randomized, blinded, clinical trial. Pediatrics 2005;115:1501–12.
Munro MJ, Walker AM, Barfield CP . Hypotensive extremely low birth weight infants have reduced cerebral blood flow. Pediatrics 2004;114:1591–6.
Seri I . Circulatory support of the sick preterm infant. Semin Neonatol 2001;6:85–95.
Seri I, Abbasi S, Wood DC, Gerdes JS . Regional hemodynamic effects of dopamine in the sick preterm neonate. J Pediatr 1998;133:728–34.
Ishiguro A, Sekine T, Suzuki K, et al. Changes in skin and subcutaneous perfusion in very-low-birth-weight infants during the transitional period. Neonatology 2011;100:162–8.
Seri I . Cardiovascular, renal, and endocrine actions of dopamine in neonates and children. J Pediatr 1995;126:333–44.
Noori S, Friedlich P, Seri I . Developmentally regulated cardiovascular, renal, and neuroen-docrine effects of dopamine. Neoreviews 2003;4:E283–8.
Yanowitz TD, Jordan JA, Gilmour CH, et al. Hemodynamic disturbances in premature infants born after chorioamnionitis: association with cord blood cytokine concentrations. Pediatr Res 2002;51:310–6.
Seri I . Systemic and pulmonary effects of vasopressors and inotropes in the neonate. Biol Neonate 2006;89:340–2.
Hausdorff WP, Caron MG, Lefkowitz RJ . Turning off the signal: desensitization of beta-adrenergic receptor function. FASEB J 1990;4:2881–9.
Watterberg KL, Gerdes JS, Gifford KL, Lin HM . Prophylaxis against early adrenal insufficiency to prevent chronic lung disease in premature infants. Pediatrics 1999;104:1258–63.
Huysman MW, Hokken-Koelega AC, De Ridder MA, Sauer PJ . Adrenal function in sick very preterm infants. Pediatr Res 2000;48:629–33.
Clark SJ, Yoxall CW, Subhedar NV . Right ventricular performance in hypotensive preterm neonates treated with dopamine. Pediatr Cardiol 2002;23:167–70.
Lundstrøm K, Pryds O, Greisen G . The haemodynamic effects of dopamine and volume expansion in sick preterm infants. Early Hum Dev 2000;57:157–63.
Padbury JF, Agata Y, Baylen BG, et al. Dopamine pharmacokinetics in critically ill newborn infants. J Pediatr 1987;110:293–8.
Rozé JC, Tohier C, Maingueneau C, Lefèvre M, Mouzard A . Response to dobutamine and dopamine in the hypotensive very preterm infant. Arch Dis Child 1993;69:1 Spec No:59–63.
Osborn D, Evans N, Kluckow M . Randomized trial of dobutamine versus dopamine in preterm infants with low systemic blood flow. J Pediatr 2002;140:183–91.
Zhang J, Penny DJ, Kim NS, Yu VY, Smolich JJ . Mechanisms of blood pressure increase induced by dopamine in hypotensive preterm neonates. Arch Dis Child Fetal Neonatal Ed 1999;81:F99–104.
Noori S, Friedlich PS, Seri I . Pathophysiology of shock in the fetus and neonate. In: Polin RA, Fox WW, Abman SH, eds. Fetal and Neonatal Physiology, 3rd edn. Philadelphia: Saunders, 2004:772–81.
Ishiguro A, Sekine T, Kakiuchi S, et al. Skin and subcutaneous blood flows of very low birth weight infants during the first 3 postnatal days. J Matern Fetal Neonatal Med 2010;23:522–8.
Lee J, Rajadurai VS, Tan KW . Blood pressure standards for very low birthweight infants during the first day of life. Arch Dis Child Fetal Neonatal Ed 1999;81:F168–70.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ishiguro, A., Suzuki, K., Sekine, T. et al. Effect of dopamine on peripheral perfusion in very-low-birth-weight infants during the transitional period. Pediatr Res 72, 86–89 (2012). https://doi.org/10.1038/pr.2012.37
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2012.37
This article is cited by
-
Influence of sympathetic activity in the control of peripheral microvascular tone in preterm infants
Pediatric Research (2016)
-
Skin blood flow as a predictor of intraventricular hemorrhage in very-low-birth-weight infants
Pediatric Research (2014)