Abstract
Background:
Chronic exposure to supplemental oxygen (O2) induces lung damage and mortality in a sex-dependent manner. The effect of short-term hyperoxia on the newborn pulmonary vasculature is unknown but is, however, of clinical significance in the neonatal resuscitation context. We hypothesize that short-term hyperoxia has a sex-dependent effect on the pulmonary vasculature.
Methods:
Following 1-h 100% O2 exposure, the pulmonary arteries and lung tissues of newborn rats were evaluated.
Results:
Superoxide dismutase 3 (SOD3) expression in female pups’ lungs was increased as compared with that in the lungs of male pups. As compared with air-treated pups, the response of male pups to thromboxane was increased by O2, whereas the opposite effect was documented in the vessels of female pups. The enhanced force of hyperoxia-exposed arteries of the male pups was suppressed with superoxide or peroxynitrite scavengers, and increased lung SOD activity and hydrogen peroxide content were seen in female, but not in male, rats. Hyperoxia induced an increase in lung tissue oxidative products and Rho-kinase (ROCK) activity in male, but not in female, pups.
Conclusion:
A lower lung SOD content and failure to upregulate SOD activity facilitates peroxynitrite generation and ROCK activation in hyperoxia-exposed males, predisposing them to pulmonary vasoconstriction. These observations, if relevant to humans, may explain the increased mortality and higher incidence of pulmonary hypertension in male neonates.
Similar content being viewed by others
Main
Pulmonary vascular resistance is high during fetal life and rapidly decreases after birth to allow for adequate pulmonary blood flow and alveolar gas exchange. Oxygen (O2) is a known pulmonary vasodilator and an increase in PaO2 levels after birth is considered one of the major factors responsible for the rapid decrease in pulmonary vascular resistance during the transition from fetal to postnatal life (1). The clinical use of supplemental O2 for newborn resuscitation was originally predicated on expediting the decrease in pulmonary vascular resistance, thus optimizing arterial oxygenation at birth. Yet exposure to supplemental O2 at birth triggers the production of reactive O2 species (ROS) and promotes oxidative stress–induced tissue damage.
The immediate neonatal period is associated with significant oxidative challenges. Comparative studies of plasma isoprostane levels, a by-product of oxidative stress, show higher levels in the newborn as compared with adult subjects, and this difference is even greater for premature, as opposed to term, neonates (2). Of greater concern is the observation that supplemental O2-induced oxidative stress at birth may result in altered plasma antioxidant levels for up to 28 d after birth (3). The therapeutic use of supplemental O2 during newborn resuscitation of full-term infants is controversial because of its adverse effects. As such, recent guidelines recommend that room air be used in the resuscitation of healthy neonates (4).
Data from randomized clinical studies in neonates demonstrated that, as compared with air, resuscitation with 100% O2 causes oxidative stress and alterations of the glutathione redox cycle enzymes (5,6). Myocardial and renal tissue oxidative insult (7) and increased mortality (8) have also been reported in newborn infants resuscitated with supplemental O2. Little is known, however, about the effect of short-duration exposure to supplemental O2 on the lung during the neonatal period.
Chronic exposure to hyperoxia induces alveolar growth arrest, pulmonary vascular, and airway remodeling changes similar to what is observed clinically in infants with bronchopulmonary dysplasia (9). As compared with adults, however, newborn rodents are more resistant to chronic O2 exposure and are much more likely to survive while breathing O2 (10). Whether short-duration O2 exposure is also less harmful to the rodent lungs during early, as compared with later stages of life, has not been adequately investigated.
Significant sex-related differences in the rodent survival rates were observed following chronic exposure to hyperoxia. Female rodents are more tolerant as compared with males. Castration of young male rats increases their tolerance to chronic hyperoxia exposure for as yet unknown reasons (11).
Female preterm infants exhibit greater survival and lesser morbidity as compared with male neonates (12). In humans, sex differences in the tolerance to oxidative stress also appear to be present because following exposure to antenatal steroids, the antioxidant capacity is greater in female, as compared with male, preterm infants (13).
Supplemental O2-induced ROS formation has a negative impact on the bioavailability of nitric oxide and generates molecules such as peroxynitrite that are powerful pulmonary vasoconstrictors in newborn rodents (14). Superoxide has also been shown to induce pulmonary vasoconstriction in rats via generation of peroxynitrite (the reaction product of nitric oxide and superoxide) and activation of the Ras homolog gene family member A (RhoA)/Rho-kinase (ROCK) pathway (15).
Little is known about the effect of short-term exposure to O2 on the newborn pulmonary vasculature and possible generation of peroxynitrite, as well as ROCK activation in the process. Therefore, the purpose of this study was to evaluate newborn male and female rats exposed for 1 h to 100% O2. We hypothesized that a short-term O2 exposure would alter the pulmonary vascular tone in a sex- and age-dependent manner. In addition, we speculated that a hyperoxia-induced increase in pulmonary arterial muscle contraction potential would involve peroxynitrite generation and ROCK activation.
Results
Oxygen Exposure and Lung Oxidative Stress
Superoxide, a key ROS molecule, once generated is rapidly dismutated into hydrogen peroxide (H2O2) by tissue superoxide dismutases (SODs). As such, we measured lung tissue H2O2 as a marker of ROS generation. Exposure to hyperoxia significantly increased the lung H2O2 content in female, but not in male, pups ( Figure 1 ). Hypothesizing that the sex-related differences in lung H2O2 were related to a distinct hyperoxia effect on SOD activity, we proceeded to comparatively evaluate this parameter. Following hyperoxia, a significant increase in lung tissue total SOD activity, but not mitochondrial SOD activity, was observed in female, but not in male, newborn pups ( Figure 1 ). To further elucidate this sex-dependent SOD activity increase, we comparatively evaluated the SOD1 and SOD3 (SOD2 is the mitochondrial isoform for which no activity changes were observed) lung expression in male and female pups. As compared with males, expression of SOD3 was found to be significantly higher in the female rat lung, whereas no sex difference in SOD1 expression was observed ( Figure 1 ).
Lung tissue hydrogen peroxide (H2O2) content and superoxide dismutase (SOD) content and activity. Lung (a) H2O2 content and (b) total as well as (c) mitochondrial SOD activity of male and female 1–5-d-old rats exposed to either air or 100% O2 for 1 h. (d) Representative blots. Male and female control newborn rat lung (e) SOD1 and (f) SOD3 normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) content. *P < 0.05 vs. male by Student’s unpaired t-test. **P < 0.01 vs. female air-treated by Student’s unpaired t-test. N = 4 for H2O2; n = 3 per group for SOD activity; and n = 4 per group for SOD content. Open bars, air-exposed; solid bars, oxygen-exposed.
Glutathione is the most important antioxidant and, among other functions, it detoxifies H2O2 via the glutathione peroxidase-facilitated conversion of reduced glutathione (GSH) to oxidized glutathione (GSSG). As glutathione peroxidase activity is upregulated in the presence of excess H2O2, we measured the lung tissue GSH and GSSG in air- and O2-treated animals. Under normoxic conditions, the GSH/GSSG of female pups’ lungs was significantly lower than those of their male counterparts, suggesting increased H2O2 availability ( Figure 2 ). Exposure to O2 increased the lung GSSG content in both sexes but to a greater extent in the female, resulting in a GSH/GSSG ratio that was significantly lower as compared with that of male pups ( Figure 2 ).
Lung glutathione content. (a) Lung reduced (GSH) and (b) oxidized (GSSG) glutathione content and their (c) ratio for male and female 1–5-d-old rats exposed to either air or 100% O2 for 1 h. *P< 0.01 vs. air-treated values and ††P < 0.01 vs. male respective values by two-way ANOVA. n = 11 per group. Open bars, air-exposed; solid bars, oxygen-exposed.
Hyperoxia and Pulmonary Vascular Tone
Hypothesizing that the sex-dependent changes induced by hyperoxia in the lung were associated with alterations in the potential for pulmonary vasoconstriction, we proceeded to comparatively evaluate the response of pulmonary arteries to two distinct contractile agonists: thromboxane A2 analog and KCl.
The dose–response of pulmonary arterial muscle of the newborn (1–5 d old) rat to the thromboxane A2 analog (U46619) in male and female animals studied under normoxic conditions is shown in Figure 3 . A comparable U46619-induced force was observed in male and female pulmonary arteries of air-exposed animals. Exposure to O2 significantly enhanced the U46619-induced force in male arteries, whereas the opposite effect was noted in vessels derived from female pups. A similar increase in pulmonary arterial force was observed in O2-exposed male pups stimulated with 128 mmol/l KCl, whereas no difference was noted in the vessels of female pups as compared with air-treated pups ( Figure 3 ). The pulmonary arterial responses to endothelium-dependent (acetylcholine) and -independent (sodium nitroprusside) vasorelaxant agonists were evaluated, but no significant hyperoxia-induced changes were observed for either sex (data not shown).
Pulmonary arterial muscle contraction in 1–5-d-old rats. Intralobar pulmonary arteries mounted on a wire myograph derived from (a) male and (b) female pups exposed to either air or 100% O2 for 1 h in vivo. Dose–response to the thromboxane A2 analog U46619 (a, b) and (c) 128 mmol/l KCl is shown. **P < 0.01 vs. air-treated by two-way ANOVA (a, b) or Student’s t-test (c). n = 18–20 for males and n = 16–23 for females per group. Open bars or circles, air-exposed; solid bars or circles, oxygen-exposed.
To ascertain whether these changes were also observed following ex vivo exposure to hyperoxia, we studied male and female pulmonary arteries incubated for 1 h with Krebs–Henseleit solution bubbled with 94% O2/6% CO2 for 1 h. Under these conditions, both male and female pulmonary arterial dose–response to U46619 and 128 mmol/l KCl were significantly enhanced ( Figure 4 ).
Pulmonary arterial muscle contraction following ex vivo O2 exposure. Intralobar pulmonary arteries mounted on a wire myograph derived from (a) male and (b) female pups exposed ex vivo to either air/6% CO2 or 94% O2/6% CO2 for 1 h in the muscle bath. Dose–response to the thromboxane A2 analog U46619 (a and b) and 128 mmol/l KCl (c) are shown. **P < 0.01 vs. air-treated by two-way ANOVA (a, b) or Student’s t-test (c). n = 8 for male and female arteries per distinct gas-exposure group. Open bars or circles, air/6% CO2–exposed; solid bars or circles, 94% O2/6% CO2–exposed.
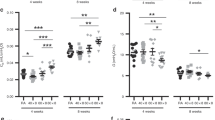
We evaluated whether the O2-induced sex difference in force generation was unique to the immediate neonatal period. Toward this goal, we assessed pulmonary arteries obtained from 2-wk-old animals equally exposed to 1 h of 100% O2 or air in vivo. As shown in Figure 5 , whereas the potentiation of the force dose–response was no longer present in the male vessels, the female pulmonary arteries of hyperoxia-exposed animals exhibited an even greater reduction in the U46619-induced response.
Pulmonary arterial muscle contraction in 2-wk-old rats. Dose–response to the thromboxane A2 analog U46619 of intralobar pulmonary arteries mounted on a wire myograph derived from (a) males and (b) females exposed to either air or 100% O2 for 1 h. *P < 0.05, **P < 0.01 vs. air-treated by two-way ANOVA. n = 4–6 for males and n = 14–16 for females per group. Open circles, air-exposed; solid circles, oxygen-exposed.
Hypothesizing that the sex-dependent hyperoxia-induced pulmonary vascular tone changes were related to whether tissue-generated superoxide resulted in peroxynitrite formation or its dismutation to H2O2, we evaluated the newborn pulmonary arteries of both sexes in the absence or presence of a SOD mimetic (Tiron) to scavenge superoxide, 5,10,15,20-Tetrakis(4-sulfonatophenyl) porphyrinate iron (III) chloride, a peroxynitrite decomposition catalyst, or polyethylene glycol (PEG)-catalase, a H2O2 scavenger.
In the presence of 5,10,15,20-Tetrakis(4-sulfonatophenyl) porphyrinate iron (III) chloride, the pulmonary arteries of air- and hyperoxia-exposed pups showed similar agonist-induced force, in support of the likely role of peroxynitrite in the hyperoxia-induced enhancement of the U46619 dose–response in male pups ( Figure 6 ). In the female pulmonary arteries, incubation with either Tiron or PEG-catalase abrogated the reduction in U46619-induced force following hyperoxia exposure ( Figure 6 ).
Response to 5,10,15,20-Tetrakis(4-sulfonatophenyl) porphyrinate iron (III) chloride (FeTPPS), PEG-catalase, and Tiron in pulmonary arteries of 1–5-d-old rats. Intralobar pulmonary arteries mounted on a wire myograph derived from male and female pups exposed to either air or 100% O2 for 1 h. (a–c) Male and (d–f) female dose–response to the thromboxane A2 analog U46619 in the presence of the superoxide dismutase (SOD) mimetic Tiron (a, d), H2O2 catalyst PEG-catalase (b, e), and the peroxynitrite decomposition catalyst FeTPPS (c, f). n = 4–6 for males and n = 4–8 for females per group. Open circles, air-exposed; solid circles, oxygen-exposed.
Finally, we evaluated the newborn rat pulmonary arterial response to H2O2 following smooth muscle precontraction with U46619 ( Figure 7 ). A nonsignificant increase in force was observed with incremental concentrations of H2O2 up to 10–6 mol/l, beyond which significantly increased vasorelaxation was observed, reaching almost 50% of maximum contraction, at 10–4 mol/l. This finding is in keeping with the lower force-response to U46619 observed in the female hyperoxia-exposed pups and thought to be related to the increased SOD activity and H2O2 generation.
Hyperoxia Exposure and RhoA/Rho Kinase Pathway
The lung tissue ROCK I and II, as well as the regulatory myosin light-chain phosphatase regulatory unit (MYPT) phosphorylation, as a measure of ROCK activation, were evaluated in pups exposed to air or hyperoxia ( Figure 8 ). Whereas no significant change in lung ROCK content was observed, its activity was increased in male and decreased in female lung tissue (P < 0.001), in keeping with functional ROCK activation leading to pulmonary vasoconstriction in males exposed to short-term hyperoxia.
Changes in lung Rho-kinase (ROCK) expression and activity following exposure to air and hyperoxia. Western blot analyses of (a) ROCK I and (b) ROCK II content, as well as phosphorylated threonine 850 (P-MYPT)/pan myosin phosphatase target (MYPT) ratio (c), as a marker of ROCK activity. Representative western blots from (d) male and (e) female lungs are shown. **P < 0.01 as compared with air values by ANOVA. n = 4 samples per group. Open bars, air-exposed; solid bars, oxygen-exposed.
We obtained further ROCK activation measurements in third- to fourth-generation intrapulmonary arteries of air- and oxygen-treated pups of both sexes. As shown in Supplementary Figure S1 online, no statistically significant differences were noted between the sexes.
Lung DHE and 3-Nitrotyrosine Immunostaining
Lung dihydroethidium (DHE) immunofluorescence was obtained in air- and oxygen-treated newborn animals of both sexes. As noted in Figure 9 , a significant increase in SOD-dependent DHE fluorescence was observed in the lungs of both male and female pups. There was a trend toward the male oxygen-treated lung having a higher fluorescence, as compared with those of the females, which was close to statistical significance (P = 0.06). Lung peroxynitrite formation was qualitatively assessed in the lung tissues of male and female animals. As compared with female lungs, a greater 3-nitrotyrosine immunostaining of pulmonary arterial tissue was apparent in the large and small vessels of male pups ( Figure 9 ).
Lung dihydroethidium (DHE) immunofluorescence and 3-nitrotyrosine immunostaining. Superoxide dismutase (SOD)-dependent fluorescence intensity of DHE was evaluated as described in the Methods section. Representative pictures from each group are shown in the insert and the relative data in (a). Bar = 50 μm. (b, c) 3-Nitrotyrosine immunostaining of large and small vessels shown in low (×10, bar = 100 μm) and high magnification (×40, bar = 20 μm), respectively. PA, pulmonary artery. *P < 0.05 vs. male air-treated by Student’s unpaired t-test. Male pulmonary arteries, n = 7–12; female pulmonary arteries, n = 11–12. Open bars, air-exposed; solid bars, oxygen-exposed.
Discussion
We observed that short-term exposure to hyperoxia induces significant changes in the newborn rat pulmonary arterial vascular contractile potential that are highly dependent on sex and postnatal age. Stimulation with the thromboxane A2 analog U46619 resulted in enhanced force in the hyperoxia-exposed male, as compared with the opposite effect in the pulmonary arteries of female pups. This sex-dependent effect appears to be regulated by a greater expression of SOD3 and hyperoxia-induced SOD activation in the female lung. These changes likely account for the higher lung H2O2 content and reduced pulmonary arterial force generation in female pups, whereas the opposite was true for males. Addition of a peroxynitrite decomposition catalyst to the incubation media abolished the enhanced dose–response of the male newborn pulmonary arteries to U46619, suggesting that this molecule plays a role in the hyperoxia-induced changes in the newborn vascular tone. Lung SOD-dependent DHE fluorescence and the qualitative assessment of nitrotyrosine immunostaining further support a greater generation of peroxynitrite in male and H2O2 in female pups.
Until recently, neonates were commonly exposed to supplemental O2 during the resuscitation process. Such a practice was viewed as beneficial toward the successful transition from fetal to postnatal life by ensuring a rapid decrease in pulmonary vascular resistance and adequate tissue oxygenation. Mounting evidence of short- and long-term harmful effects of supplemental O2–induced oxidative stress challenged this practice. Accordingly, the North American and European pediatric associations, among others, now recommend air resuscitation for full-term infants (4).
Exposure to supplemental O2 during the neonatal period, however, is not just confined to resuscitation at birth. Infants with respiratory distress are therapeutically managed with supplemental O2 to maintain adequate arterial O2 saturation. Although ensuring normoxemia (adequate blood oxygenation), this approach exposes proximal and distal airways and the pulmonary arterial bed to high O2 concentrations. The impact of exposure to supplemental O2 has been mostly studied from the clinical perspective of biotrauma and the development of chronic lung disease in neonates. Bronchopulmonary dysplasia, the disease most commonly associated with exposure to supplemental O2, is histologically characterized by different degrees of pulmonary vascular remodeling (16). Yet little is known about the potential harmful effects to the pulmonary vasculature of short-term O2 exposure.
Acutely, hyperoxia is a pulmonary vasodilator, whereas hypoxia induces pulmonary vasoconstriction (17). Exposure to an inspired O2 concentration different from air for even short periods of time can alter the pulmonary vascular tone (1,18). The mechanism accounting for these changes is poorly understood and only limited data are available on newborn animals.
In this study, we showed that a relatively short exposure to supplemental O2 induces significant changes in the pulmonary vascular response to contractile agonists that persist after hyperoxia is terminated. The mechanism accounting for this lasting response is unclear but is likely related to supplemental O2–induced ROS generation by endothelial cells (19) or peroxynitrite/isoprostanes at the level of the alveoli that reach the vasculature via diffusion (20). Mitochondrial matrix oxidant stress has also been documented in pulmonary arterial smooth muscle cells of newborn lambs exposed to 100% O2 (21).
In designing this study, we reasoned that 100% O2 exposure would result in superoxide generation at the level of the pulmonary arteries. Once formed, superoxide has a very short half-life and is either dismutated to H2O2 by SOD or this reaction is outcompeted by nitric oxide to produce peroxynitrite, a powerful pulmonary vasoconstrictor in newborn rats (14). Superoxide may enhance pulmonary vascular tone in rats via its direct stimulation of ROCK, thus promoting inhibition of myosin light-chain phosphatase activity and calcium sensitization (15).
We have previously shown that H2O2 is a pulmonary vasodilator in mice (22) and have documented the same response in the pulmonary arteries of newborn rats in this study. Therefore, we speculate that lung SOD content and activity may modulate the fate of hyperoxia-induced superoxide formation thereby favoring a reduction or an increase in pulmonary arterial response to thromboxane.
Pulmonary SOD activity is known to significantly increase in humans following birth and with advancing postnatal age (23). In the newborn rat lung, O2 exposure induces an increase in SOD activity (24). Yet this response is mostly seen during the neonatal period and may account for the increased tolerance to hyperoxia early in life (25). Of note, Frank et al. (26) reported more than 30 y ago that SOD activity of sick neonates with hyaline membrane disease fails to increase following exposure to O2, possibly contributing to superoxide-induced pulmonary hypertension.
Survival following chronic exposure to O2 in rats depends on sex. Female juvenile rats are more tolerant of O2 toxicity, and survival of chronic exposure to O2 is increased following castration in male animals (11). However, sex-related differences were not previously evaluated in the studies addressing the hyperoxia effect on lung tissue SOD activity.
In this study, we showed that 100% O2 for 1 h induces an increase in lung SOD activity of female, but not male, newborn rats. In addition, we documented that lung SOD3 content was significantly greater in female, as compared with male, newborn rats. Therefore, the higher lung SOD content and SOD activity following exposure to hyperoxia appear to be the key factors accounting for the sex-related differences in the newborn pulmonary vascular tone changes associated with exposure to hyperoxia.
A reduced pulmonary arterial dose–response to U46619 was documented in this study in female pups, whereas the opposite effect occurred in males. This finding, in conjunction with the hyperoxia-induced increase in lung H2O2 observed solely in females, strongly suggests that the reduced thromboxane-mediated force development in the pulmonary arteries reflects dismutation of the excess superoxide by SOD. Despite the published evidence that H2O2 can inactivate SOD3 (27), our data on hyperoxia-exposed female pups suggest otherwise. This apparent discrepancy might be related to the fact that although the lung H2O2 content was increased, the levels measured in the present experiments are significantly lower than those required for SOD inactivation, as previously reported (27).
It is unlikely that endothelial nitric oxide synthase uncoupling accounted for the enhanced force observed in the hyperoxia-exposed pulmonary arteries of male, but not female, pups. Such a conclusion is based on the lack of significant change in endothelium-dependent pulmonary vasorelaxation following hyperoxia exposure. Nitric oxide synthase uncoupling results in the formation of superoxide instead of nitric oxide. Had this happened to the hyperoxia-treated pulmonary arteries of male pups, one would expect marked reduction or suppression of the endothelial-dependent vasorelaxation.
There is evidence that following oxidative stress ROCK activation occurs either as a result of peroxynitrite generation or via superoxide-induced RhoA signaling (28,29). In this study, we documented a significant increase in MYPT phosphorylation. Such phosphorylation has been shown to inhibit the enzyme ability to dephosphorylate myosin, thus promoting further contraction independent of intracellular calcium changes in rat pulmonary arteries (15). We have recently shown that chronic exposure to hypoxia promotes pulmonary hypertension in newborn rats via the Rho kinases pathway (30).
In our study, we observed significant ROCK activation in hyperoxia-treated male pup lungs but not their third-generation pulmonary arteries. Such apparent discrepancy likely relates to the fact that the whole lung protein extraction has a greater representation of smaller resistance vessels where ROCK activation is more likely to occur, as compared with the near resistance arteries assayed individually. The possible contribution of airway and pulmonary vascular venous tissue to the enhanced ROCK activation demonstrated in whole lung extracts cannot be ruled out and requires further investigation.
In keeping with the sex-dependent fate of hyperoxia-induced superoxide, we showed in this study that lung SOD-dependent DHE fluorescence is increased following oxygen exposure in both sexes but with a tendency for higher values in male pups. 3-Nitrotyrosine immunostaining of the lung confirmed peroxynitrite generation that was qualitatively increased in the pulmonary vessels of male, as compared with female, pups.
In humans, neonatal survival is clearly sex dependent, being significantly greater for females, even in the postsurfactant era (12). Given that most of the neonatal mortality relates to premature birth and these neonates usually have surfactant deficiency requiring supplemental O2, it is tempting to relate sex differences in the handling of oxidative stress to survival and morbidity. Age appears to also play an important role in the sex-dependent hyperoxia-induced changes in pulmonary vascular tone. Whereas a significant increase in the thromboxane-induced force dose–response was seen in the first days of life in male rats, animals exposed to hyperoxia during their second week of life did not show this effect.
Prenatal exposure to corticosteroids has been shown to accelerate maturation of the lung antioxidant enzymes in rodents (31) and humans (32). Glutathione and other pulmonary antioxidant enzymes markedly increase during fetal gestation, likely as a result of the mitochondrial and microsomal-generated ROS generation prenatally (33). In our study, we showed that although glutathione oxidation in response to hyperoxia exposure was present in both sexes, the magnitude of change was significantly greater in the lungs of female, as compared with male, pups. This, we believe, relates to the increased H2O2 content in female newborn pup lungs exposed to 100% O2 because in its presence glutathione peroxidase activity increases (34).
Finally, the discrepant results obtained when inducing hyperoxia ex vivo vs. in vivo merit discussion. In contrast to the in vivo studies in which the pups were allowed to breathe 100% O2, the ex vivo experiments involved testing the pulmonary arterial response following a 1-h exposure in the muscle bath to 94% O2. In contrast with the in vivo experiments, no sex-dependent differences were noted for the ex vivo hyperoxia-exposed arteries. This apparent discrepancy can be explained as follows. First, we use air when evaluating the mechanical properties of pulmonary arterial smooth muscle in the muscle bath. Bubbling air generates a higher than physiologic O2 tension in the bath (PO2 = 125 mm Hg), yet this is necessary to ensure adequate oxygenation of the tissue core as the vasa vasorum circulation of these arteries is not active ex vivo. Bubbling 94% O2 in the bath generates a PO2 of 500 mm Hg, which would certainly trigger a number of nonphysiologic events in the pulmonary arteries. An ex vivo increase in PO2 has been shown to promote vascular smooth muscle contraction via L-type calcium channels (35), and this phenomenon is operative in resistance pulmonary arteries (36). Yet acute hyperoxia is known to induce pulmonary vasodilation in several newborn animal species (37). Thus, the pulmonary vascular changes induced by high oxygen concentration breathing are distinct from the nonphysiologic exposure to hyperoxia ex vivo. Such important differences likely explain the discrepancy with regard to the sex-dependent data obtained under the two conditions and emphasize the importance of evaluating the pulmonary vascular tissue following the physiologic in vivo, as compared with ex vivo, conditions.
In summary, this study documents significant sex- and age-related differences in response to short-term hyperoxia. Enhanced pulmonary vascular tone following 100% exposure to O2 is seen in male and not in female neonates as a result of differences in both lung SOD content and its hyperoxia-induced upregulation. These findings may be of clinical relevance to the increased incidence of persistent pulmonary hypertension syndrome in male newborns as compared with female newborns.
Methods
Chemicals and Reagents
All chemicals and reagents were obtained from Sigma Aldrich (Oakville, Ontario, Canada), unless otherwise indicated.
Animals
All procedures were conducted according to the criteria established by the Canadian Council on Animal Care and were approved by the animal care committees of The Hospital for Sick Children Research Institutes.
Sprague-Dawley rats of both sexes were studied between 1 and 14 d of life. Immediately after exposure to either air or hyperoxia, the animals were killed with an overdose of pentobarbital sodium (50 mg/kg i.p.; BHD, Toronto, Ontario, Canada) and the lungs quickly removed. For the organ bath measurements, third-generation intrapulmonary arteries were obtained while the lungs were maintained in ice-cold bubbled Krebs–Henseleit solution (NaCl, 115 mmol/l; NaHCO3, 25 mmol/l; NaHPO4, 1.38 mmol/l; KCl, 2.51 mmol/l; MgSO4·7H2O, 2.46 mmol/l; CaCl2, 1.91 mmol/l; and dextrose, 5.56 mmol/l). The lung tissue to be used for biochemical measurements was flushed clear of blood with cold phosphate-buffered saline and snap-frozen in liquid nitrogen.
Hyperoxia Exposure
Animals were retrieved from their mothers and immediately placed in a temperature-controlled environment maintained at 37 °C to prevent hypothermia. The pups were exposed to 100% O2 for 1 h, whereas a similarly treated control group was maintained under air conditions. All pups tolerated the exposure to hyperoxia well and no deaths were observed.
Organ Bath Studies
The functional in vitro evaluation of newborn pulmonary arteries has been previously described (38). Briefly, third-generation lung intralobar pulmonary artery ring segments (average diameter 80–100 μm and length 2 mm) were dissected free and mounted in a wire myograph (Danish Myo Technology A/S, Aarhus, Denmark). Isometric changes were digitized and recorded online (Myodaq, Danish Myo Technology A/S). Tissues were bathed in Krebs–Henseleit buffer bubbled with air/6% CO2 and maintained at 37 °C. After 1 h of equilibration, the optimal tissue resting tension was determined by repeated stimulation with 128 mmol/l KCl until maximum active tension was reached. All subsequent force measurements were obtained at optimal resting tension.
For the ex vivo hyperoxia exposure experiments, third-generation pulmonary arteries of control animals were maintained in Krebs–Henseleit buffer bubbled with 94% O2/6% CO2 in the muscle bath for 1 h. Following this, the arteries were switched to Krebs–Henseleit buffer bubbled with air/6% CO2 for the measurement of their agonist-induced force generation. Control arteries were incubated for a similar period of time on Krebs–Henseleit buffer bubbled with air/6% CO2.
The pulmonary vascular muscle contraction was induced with the thromboxane A2-mimetic U46619 (Cayman Chemical, Ann Arbor, MI) or high potassium concentration (128 mmol/l). Following precontraction with U46619 at concentrations equivalent to the effective concentration to induce 75% of maximal contraction (EC75), the endothelium-dependent and -independent relaxation dose–responses were obtained with acetylcholine and sodium nitroprusside, respectively, as well as H2O2. The former was evaluated in the presence and absence of the respective scavengers for superoxide (Tiron: 100 µmol/l; PEG-catalase: 200 µ/ml) and peroxynitrite (5,10,15,20-Tetrakis(4-sulfonatophenyl) porphyrinate iron (III) chloride; 100 µmol/l; Millipore, Billerica, MA). Contractile responses were normalized to the tissue cross-sectional area as follows: (width × diameter) × 2 and expressed as mN/mm2.
H2O2 Microsensor
Lung homogenate supernatants were tested using a calibrated H2O2-specific sensor (World Precision Instruments, Saratosa, FL) attached to an Apollo 4000 Free Radical Analyzer (World Precision Instruments). Results were measured as µmole H2O2/g protein for lung and expressed as a percentage of the respective age wild-type values.
Glutathione Determination
Reduced (GSH) and oxidized (GSSG) glutathione were determined by ultraperformance liquid chromatography coupled to tandem mass spectrometry (UPLC-MS/MS) following the procedure of Harwood et al. (39) with some modifications based on Nishiyama and Kuninori’s method of determination of thiols (40). Briefly, lung tissue was homogenized in the presence of N-ethylmaleimide 10 mmol/l dissolved in phosphate-buffered saline at a 4:1 (vol/wt). Samples were deproteinized with 4% vol/vol perchloric acid and centrifuged at 11000 rpm for 15 min at 4 °C. Five microliters of phenylalanine-d5 (3.5 µmol/l) was added to 100 μl supernatant and subjected to UPLC-MS/MS analysis using an Acquity UPLC coupled to a Xevo TQ-S MS (Waters, Manchester, UK). Chromatographic separations were carried out at 50 °C using a C18 Kinetex column (Phenomenex, Torrance, CA) and an injection volume of 2 µl. A 6-min gradient elution was performed at a flow rate of 400 µl min–1. The following solvents were used as the mobile and stationary phases: water + formic acid 0.1% vol/vol and acetonitrile + formic acid 0.1% vol/vol, respectively. These were kept for 1 min, followed by a linear gradient up to 85% of basal for 1.5 min, then isocratic condition was held for 2 min. Finally, a 0.25-min linear gradient was used to return to the initial conditions. Positive ion electrospray MS/MS was recorded using the following conditions: capillary voltage 3 kV; source temperature 350 °C; and cone and nebulization gases set at 750 and 180 l/h, respectively. The limits of detection and quantitation for both GSH and GSSG were in the 5–10 nmol/l range.
SOD Activity
Total and mitochondrial lung SOD activity was measured in the tissue homogenate supernatant using a commercially available assay kit (Cayman Chemical).
SOD Western Blot Analysis
Lung tissue was homogenized in 10 mmol/l Tris–HCl pH 7.4 lysis buffer-containing 1% Triton X-100 and protease/phosphatase inhibitors (Roche Diagnostics Canada, Laval, Quebec, Canada) and centrifuged at 13000g for 30 min. Equivalent amounts of lysate proteins in Laemmli buffer were fractionated on SDS/PAGE and immunoblotted. The following primary antibodies were used: SOD1, 1:4,000; SOD3/EC-SOD, 1:4,000 (goat immunoglobulin G; R&D Systems, Minneapolis, MN). Appropriate immunoglobulin G conjugated with horseradish peroxidase (1:20,000 dilution) was used as secondary antibody. The enhanced chemiluminescence (Perkin Elmer, Shelton, CT) reagent was used for detection. Band intensities were quantified and expressed relative to glyceraldehyde 3-phosphate dehydrogenase.
In Situ Detection of ROS
Freshly excised lungs distended at a constant pressure of 20 cm H2O were cryopreserved in optimal cutting temperature compound (OCT; Sakura Finetek USA, Torrance, CA), snap-frozen in liquid N2, and stored at –80 °C; parallel sections (15–20 µm) were cut and slides stored at –80 °C. ROS scavenging was achieved by a 30-min preincubation with PEG-SOD (100 U/ml) to analyze SOD-dependent ROS. Sections were then incubated with 10 μmol/l DHE at room temperature in the dark for 30 min and imaged with an epifluorescent microscope (Axioskop; Carl Zeiss, Oberkochen, Germany) with appropriate filters (excitation 540 nm and emission 605 nm). DHE fluorescence level in each field of the microscope was quantified using Image-Pro Plus (version 7.0.1; Media Cybernetics, Bethesda, MD). SOD-dependent ROS was evaluated as the difference of fluorescence level in PEG-SOD-treated and untreated lung sections. From each sex and exposure group (air vs. hyperoxia), three animals were evaluated, and the fluorescence of the pulmonary arteries was scored by a group assignment–blinded investigator.
3-Nitrotyrosine Immunohistochemistry
Lungs were quickly excised and the pulmonary circulation was flushed with 1 ml phosphate-buffered saline containing 1 U/ml heparin to clear the blood while inflated with air at a constant pressure of 20 cm H2O. The lungs were then perfusion-fixed 4% (wt/vol) with paraformaldehyde in phosphate-buffered saline, excised en bloc, dehydrated, cleared in xylene, and embedded in paraffin. Sections (5 μm) were incubated with anti-nitrotyrosine rabbit polyclonal antiserum overnight at 4 °C at a dilution of 1/200 (Millipore) followed by an anti-rabbit biotin-conjugated secondary antibody at a dilution of 1/200 (Santa Cruz Biotechnology, Santa Cruz, CA) for 2 h at room temperature, and stained for a precise and consistent duration using an avidin–biotin–peroxidase method. The lung sections were counterstained with hematoxylin for cell-type-specific identification. Microscopic images of small and large pulmonary arteries were obtained (Axioskop; Carl Zeiss) for qualitative assessment of the 3-nitrotyrosine immunostaining.
RhoA/ROCK Pathway
Lung tissue and third-generation intrapulmonary arteries were lysed in radioimmunoprecipitation assay buffer, and protein samples (50 μg/lane) were blotted, as previously described (30). Myosin phosphatase target subunit 1 (MYPT-1) is one of the substrates of ROCK. ROCK activity was quantified by the measurement of phosphothreonine 850 normalized to pan-MYPT-1, as previously described (30). Protein bands were identified using enhanced chemiluminescent substrate (Immobilon; Millipore) and images were digitally captured using a MicroChemi chemiluminescent image analysis system (DNR Bio-imaging Systems, Jerusalem, Israel). Bands were quantified by digital densitometry of nonsaturated images with any background density removed (ImageJ; National Institutes of Health, Bethesda, MD).
Data Analysis
Data were evaluated by one- or two-way ANOVA with multiple comparisons obtained by the Tukey–Kramer test or unpaired Student’s t-test, when appropriate. Statistical significance was accepted at P < 0.05. All statistical analyses were performed with the Number Cruncher Statistical System (NCSS, Kaysville, UT). Data are presented as means ± SEM.
Statement of Financial Support
This work was supported by grants from the Canadian Institutes of Health Research (MOP 93710 to J.B. and MOP 84290 to R.P.J.) and the Spanish Ministry of Science & Innovation (RD08/0072/022 to M.V.). E.C. was the recipient of the 2010 INO Therapeutics Linde research grant. J.E. is supported by a postdoctoral fellowship from Sara Borrell from the CARLOS III Spanish Health Institute. M.E. is supported by Takatsuki General Hospital (Japan).
References
Lakshminrusimha S, Swartz DD, Gugino SF, et al. Oxygen concentration and pulmonary hemodynamics in newborn lambs with pulmonary hypertension. Pediatr Res 2009;66:539–44.
Comporti M, Signorini C, Leoncini S, Buonocore G, Rossi V, Ciccoli L . Plasma F2-isoprostanes are elevated in newborns and inversely correlated to gestational age. Free Radic Biol Med 2004;37:724–32.
Vento M, Asensi M, Sastre J, García-Sala F, Pallardó FV, Viña J . Resuscitation with room air instead of 100% oxygen prevents oxidative stress in moderately asphyxiated term neonates. Pediatrics 2001;107:642–7.
Vento M, Saugstad OD . Oxygen supplementation in the delivery room: updated information. J Pediatr 2011;158:2 Suppl:e5–7.
Vento M, Asensi M, Sastre J, et al. Hyperoxemia caused by resuscitation with pure oxygen may alter intracellular redox status by increasing oxidized glutathione in asphyxiated newly born infants. Semin Perinatol 2002;26:406–10.
Vento M, Asensi M, Sastre J, Lloret A, García-Sala F, Viña J . Oxidative stress in asphyxiated term infants resuscitated with 100% oxygen. J Pediatr 2003;142:240–6.
Vento M, Sastre J, Asensi MA, Viña J . Room-air resuscitation causes less damage to heart and kidney than 100% oxygen. Am J Respir Crit Care Med 2005;172:1393–8.
Saugstad OD, Ramji S, Soll RF, Vento M . Resuscitation of newborn infants with 21% or 100% oxygen: an updated systematic review and meta-analysis. Neonatology 2008;94:176–82.
Lau M, Masood A, Yi M, Belcastro R, Li J, Tanswell AK . Long-term failure of alveologenesis after an early short-term exposure to a PDGF-receptor antagonist. Am J Physiol Lung Cell Mol Physiol 2011;300:L534–47.
Bhandari V . Hyperoxia-derived lung damage in preterm infants. Semin Fetal Neonatal Med 2010;15:223–9.
Neriishi K, Frank L . Castration prolongs tolerance of young male rats to pulmonary O2 toxicity. Am J Physiol 1984;247(3 Pt 2):R475–81.
Binet ME, Bujold E, Lefebvre F, Tremblay Y, Piedboeuf B; Canadian Neonatal Network™. Role of gender in morbidity and mortality of extremely premature neonates. Am J Perinatol 2012;29:159–66.
Vento M, Aguar M, Escobar J, et al. Antenatal steroids and antioxidant enzyme activity in preterm infants: influence of gender and timing. Antioxid Redox Signal 2009;11:2945–55.
Belik J, Jankov RP, Pan J, Tanswell AK . Peroxynitrite inhibits relaxation and induces pulmonary artery muscle contraction in the newborn rat. Free Radic Biol Med 2004;37:1384–92.
Knock GA, Snetkov VA, Shaifta Y, et al. Superoxide constricts rat pulmonary arteries via Rho-kinase-mediated Ca(2+) sensitization. Free Radic Biol Med 2009;46:633–42.
Bhandari A, Bhandari V . Pitfalls, problems, and progress in bronchopulmonary dysplasia. Pediatrics 2009;123:1562–73.
Sommer N, Dietrich A, Schermuly RT, et al. Regulation of hypoxic pulmonary vasoconstriction: basic mechanisms. Eur Respir J 2008;32:1639–51.
Lakshminrusimha S, Russell JA, Steinhorn RH, et al. Pulmonary hemodynamics in neonatal lambs resuscitated with 21%, 50%, and 100% oxygen. Pediatr Res 2007;62:313–8.
Roy S, Parinandi N, Zeigelstein R, et al. Hyperoxia alters phorbol ester-induced phospholipase D activation in bovine lung microvascular endothelial cells. Antioxid Redox Signal 2003;5:217–28.
Lakshminrusimha S, Russell JA, Steinhorn RH, et al. Pulmonary arterial contractility in neonatal lambs increases with 100% oxygen resuscitation. Pediatr Res 2006;59:137–41.
Farrow KN, Wedgwood S, Lee KJ, et al. Mitochondrial oxidant stress increases PDE5 activity in persistent pulmonary hypertension of the newborn. Respir Physiol Neurobiol 2010;174:272–81.
Belik J, Jerkic M, McIntyre BA, et al. Age-dependent endothelial nitric oxide synthase uncoupling in pulmonary arteries of endoglin heterozygous mice. Am J Physiol Lung Cell Mol Physiol 2009;297:L1170–8.
Autor AP, Frank L, Roberts RJ . Developmental characteristics of pulmonary superoxide dismutase: relationship to idiopathic respiratory distress syndrome. Pediatr Res 1976;10:154–8.
Stevens JB, Autor AP . Induction of superoxide dismutase by oxygen in neonatal rat lung. J Biol Chem 1977;252:3509–14.
Yam J, Frank L, Roberts RJ . Oxygen toxicity: comparison of lung biochemical responses in neonatal and adult rats. Pediatr Res 1978;12:115–9.
Frank L, Autor AP, Roberts RJ . Oxygen therapy and hyaline membrane disease: the effect of hyperoxia on pulmonary superoxide dismutase activity and the mediating role of plasma or serum. J Pediatr 1977;90:105–10.
Hink HU, Santanam N, Dikalov S, et al. Peroxidase properties of extracellular superoxide dismutase: role of uric acid in modulating in vivo activity. Arterioscler Thromb Vasc Biol 2002;22:1402–8.
Chandra S, Romero MJ, Shatanawi A, Alkilany AM, Caldwell RB, Caldwell RW . Oxidative species increase arginase activity in endothelial cells through the RhoA/Rho kinase pathway. Br J Pharmacol 2012;165:506–19.
Touyz RM . Reactive oxygen species as mediators of calcium signaling by angiotensin II: implications in vascular physiology and pathophysiology. Antioxid Redox Signal 2005;7:1302–14.
Peng G, Ivanovska J, Kantores C, et al. Sustained therapeutic hypercapnia attenuates pulmonary arterial Rho-kinase activity and ameliorates chronic hypoxic pulmonary hypertension in juvenile rats. Am J Physiol Heart Circ Physiol 2012;302:H2599–611.
Frank L . Prenatal dexamethasone treatment improves survival of newborn rats during prolonged high O2 exposure. Pediatr Res 1992;32:215–21.
Vento M, Aguar M, Escobar J, et al. Antenatal steroids and antioxidant enzyme activity in preterm infants: influence of gender and timing. Antioxid Redox Signal 2009;11:2945–55.
Frank L, Price LT, Whitney PL . Possible mechanism for late gestational development of the antioxidant enzymes in the fetal rat lung. Biol Neonate 1996;70:116–27.
Bhandari V, Maulik N, Kresch M . Hyperoxia causes an increase in antioxidant enzyme activity in adult and fetal rat type II pneumocytes. Lung 2000;178:53–60.
Welsh DG, Jackson WF, Segal SS . Oxygen induces electromechanical coupling in arteriolar smooth muscle cells: a role for L-type Ca2+ channels. Am J Physiol 1998;274(6 Pt 2):H2018–24.
Franco-Obregón A, López-Barneo J . Differential oxygen sensitivity of calcium channels in rabbit smooth muscle cells of conduit and resistance pulmonary arteries. J Physiol (Lond) 1996;491 (Pt 2):511–8.
Crowley MR . Oxygen-induced pulmonary vasodilation is mediated by adenosine triphosphate in newborn lambs. J Cardiovasc Pharmacol 1997;30:102–9.
Belik J, Jerkic M, McIntyre BA, et al. Age-dependent endothelial nitric oxide synthase uncoupling in pulmonary arteries of endoglin heterozygous mice. Am J Physiol Lung Cell Mol Physiol 2009;297:L1170–78.
Harwood DT, Kettle AJ, Brennan S, Winterbourn CC . Simultaneous determination of reduced glutathione, glutathione disulphide and glutathione sulphonamide in cells and physiological fluids by isotope dilution liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2009;877:3393–9.
Nishiyama J, Kuninori T . Assay of thiols and disulfides based on the reversibility of N-ethylmaleimide alkylation of thiols combined with electrolysis. Anal Biochem 1992;200:230–4.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Figure S1.
(PDF 240 kb)
Rights and permissions
About this article
Cite this article
Enomoto, M., Gosal, K., Cubells, E. et al. Sex-dependent changes in the pulmonary vasoconstriction potential of newborn rats following short-term oxygen exposure. Pediatr Res 72, 468–478 (2012). https://doi.org/10.1038/pr.2012.120
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2012.120
This article is cited by
-
Acetaminophen increases pulmonary and systemic vasomotor tone in the newborn rat
Pediatric Research (2020)
-
Surfactant phospholipid composition of gastric aspirate samples differs between male and female very preterm infants
Pediatric Research (2017)
-
Metabolic adaptation and neuroprotection differ in the retina and choroid in a piglet model of acute postnatal hypoxia
Pediatric Research (2014)
-
Age dependency of vasopressin pulmonary vasodilatory effect in rats
Pediatric Research (2014)
-
Rho-kinase inhibitor Y-27632 attenuates pulmonary hypertension in hyperoxia-exposed newborn rats
Acta Pharmacologica Sinica (2013)