Abstract
Introduction:
This study examined the temporal expression of angiotensin (Ang)-converting enzyme 2 (ACE2) during renal, heart, lung, and brain organogenesis in the mouse.
Results:
We demonstrate that kidney ACE2 mRNA levels are low on embryonic day (E) 12.5, increase fourfold during development, and decline in adulthood. In extrarenal tissues, ACE2 mRNA levels are also low during early gestation, increase in perinatal period, and peak in adulthood. The lung shows the highest age-related increase in ACE2 mRNA levels followed by the brain, kidney, and heart. ACE2 protein levels and enzymatic activity are high in all organs studied during gestation and decline postnatally. Ang II decreases ACE2 mRNA levels and enzymatic activity in kidneys grown ex vivo. These effects of Ang II are blocked by the specific Ang II AT1 receptor (AT1R) antagonist candesartan, but not by the AT2 receptor (AT2R) antagonist PD123319.
Discussion:
We conclude that ACE2 gene and protein expression and enzymatic activity are developmentally regulated in a tissue-specific manner. Ang II, acting through AT1R, exerts a negative feedback on ACE2 during kidney development. We postulate that relatively high ACE2 protein levels and enzymatic activity observed during gestation may play a role in kidney, lung, brain, and heart organogenesis.
Similar content being viewed by others
Main
The renin–angiotensin system (RAS) plays a fundamental role in the regulation of arterial blood pressure, fluid, and electrolyte homeostasis (1). In the RAS, renin cleaves angiotensinogen to generate angiotensin (Ang) I, Ang-(1–10). Ang I is converted by angiotensin-converting enzyme (ACE) to Ang II, Ang-(1–8), the most powerful effector peptide hormone of the RAS (1). Ang II acts through two major G protein–coupled receptors: AT1 receptor (AT1R) and AT2 receptor (AT2R) (1). Most of the hypertensinogenic and sodium-retaining actions of Ang II are attributed to AT1R (1,2). In contrast to AT1R, AT2R elicits vasodilation, promotes renal sodium excretion, and inhibits proliferation in mesangial cells (3,4).
The ACE2 gene has been mapped to the X chromosome (Xp22). The human ACE2 (EC 3.4.15.1) is a zinc metallopeptidase containing 805 amino acids and is 42% homologous with ACE (5). During adult life, ACE2 is expressed in the kidney, heart, vasculature, brain, and other organs (5,6,7). ACE2 is a carboxypeptidase that hydrolyzes a single amino acid from Ang II to generate Ang-(1–7), which acts through the G protein–coupled receptor Mas, encoded by the Mas protooncogene, to oppose hypertensinogenic effects of the ACE–Ang II–AT1R axis (8).
Convincing evidence indicates that the RAS is critical for proper kidney development. Use of ACE inhibitors or AT1R antagonists during fetal life, as well as mutations in the genes encoding angiotensinogen, renin, ACE, and AT1R in humans, are associated with renal tubular dysgenesis (9). Genetic inactivation of angiotensinogen, renin, ACE, or AT1R in mice causes abnormal glomerular and renal vascular development, pelvic dilation (hydronephrosis), and hypoplastic papilla (10). Accumulating evidence indicates that ACE2 plays an important role in the regulation of cardiac structure and function (11), age-related glomerulosclerosis (12), central regulation of blood pressure (13), and acute lung injury (14). To our knowledge, the expression and the role of ACE2 during kidney, lung, heart, and brain development is largely unknown. Given the recognized relationship between disturbed intrauterine organogenesis and health outcome during postnatal life, we examined the spatiotemporal ontogeny of ACE2 in the kidney, lung, heart, and brain of mice and determined whether Ang II regulates ACE2 in the developing kidney.
Results
Developmental Changes in ACE2 Gene Expression
We chose a mouse model to test the hypothesis that ACE2 gene expression is developmentally regulated in a tissue-specific manner because ACE2-mutant mice have been generated and represent a valuable tool to study further the role of ACE2 during development. Although all ACE2-mutant lines were generated with randomly mixed genetic backgrounds derived from C57BL/6 and 129 mice (11,15,16), the differences in cardiac structure and function, the dominant phenotype reported in ACE2-null adult mice (11), do not appear to be due to variability in genetic background (17). Because we have previously reported an important role for Ang II and its AT1R in ureteric bud (UB) morphogenesis during embryonic kidney development in CD1 mice (18), we chose to examine ACE2 ontogeny in CD1 mice.
The quantitative levels of ACE2 mRNA, as determined by real-time reverse transcriptase PCR (RT-PCR), are shown in Figure 1 . In each tissue, the relative levels of ACE2 mRNA on embryonic day 12.5 (E12.5) were assigned a value of 1. During early embryonic development (E12.5–E14.5), ACE2 mRNA expression was low in all the tissues studied and increased significantly thereafter. However, the temporal changes in ACE2 mRNA abundance differed with each organ. In the lung and brain, ACE2 mRNA levels increased progressively with maturation and peaked in adulthood. In contrast, renal and cardiac ACE2 gene expression peaked during the perinatal period and declined in adult life. At all stages of development except adulthood, ACE2 mRNA is expressed in the following order of abundance: kidney > lung > brain > heart ( Figure 1e ).
Bar graphs showing developmental expression of angiotensin-converting enzyme 2 (ACE2) mRNA factored per expression of glyceraldehyde 3-phosphate dehydrogenase mRNA in the mouse (a) kidney, (b) lung, (c) heart, and (d) brain by quantitative reverse-transcription polymerase chain reaction (RT-PCR). (e) Relative tissue-specific developmental expression of ACE2 mRNA (white columns: kidney, black: lung, gray: heart, hatched: brain). (a) *P < 0.05 vs. E14.5, §P < 0.05 vs. E16.5, **P < 0.05 vs. P10. (b) *P < 0.05 vs. E16.5, §P < 0.05 vs. P10. (c) *P < 0.05 vs. other groups, §P < 0.05 vs. E12.5–E18.5. (d) *P < 0.05 vs. E12.5–E16.5, §P < 0.05 vs. other groups, **P < 0.05 vs. other groups. E, day of embryonic life; P, day of postnatal life.
Developmental Changes in ACE2 Protein Levels
Western blot analysis revealed the presence of a single ACE2 band with the molecular weight of 110 kDa ( Figure 2a ). ACE2 protein is present in developing organs studied as early as on E12.5. In contrast to the temporal profile of ACE2 mRNA expression, ACE2 protein contents are high throughout gestation in all organs studied, decline gradually during postnatal development, and reach lowest levels in adulthood ( Figure 2b–e ).
Developmental expression of angiotensin-converting enzyme 2 (ACE2) protein in the kidney, lung, brain, and heart. (a) Western blots (30 µg total protein per lane) showing ACE2 and β-actin protein expression. (b–e) Bar graphs show the ontogeny of tissue-specific ACE2 protein expression factored per β-actin protein expression. (b) Kidney: *P < 0.05 vs. other groups. (c) Lung: *P < 0.05 vs. other groups; §P < 0.05 vs. E18.5, P1, P10, adult; †P < 0.05 vs. P1, P10, adult; ‡P < 0.05 vs. other groups. (d) Heart: *P < 0.05 vs. P1, P10, and adult; §P < 0.05 vs. E12.5–E18.5, P10, adult; **P < 0.05 vs. other groups. (e) Brain: *P < 0.05 vs. P1, P10, adult; **P < 0.05 vs. E12.5–E18.5, adult; §P < 0.05 vs. other groups. E, day of embryonic life; P, day of postnatal life.
Developmental Changes in ACE2 Enzymatic Activity
We previously reported that ACE enzymatic activity is developmentally regulated in the rat kidney, heart, and lung (19). Given that ACE/Ang II and ACE2/Ang-(1–7) have opposing effects on blood pressure (7), we tested whether developmental changes in ACE2 activity are temporally linked with ACE activity during ontogeny. The specific ACE2 inhibitor DX600 inhibited ACE2 activity (percent inhibition) in the kidney: postnatal day 10 (P10)—99%, adult—88%; in the brain: P10—99.5%, adult—87%; and in plasma: P10—96%, adult—90%, demonstrating that the observed increase in fluorescence was attributable specifically to ACE2 activity. In the embryonic kidney, ACE2 activity is highest on E12.5, declines significantly on E14.5 (16 ± 2.2 vs. 170 ± 44 units, P < 0.05), and increases during later gestation to decline on P1 ( Figure 3a ). ACE2 activity increases on P10 and declines in the adult kidney. In the lung, ACE2 activity is low on E12.5, increases on E14.5–E16.5, declines perinatally, and peaks on P10 to decline again in the adult ( Figure 3b ). Cardiac ACE2 activity increases progressively with gestation, declines after birth, and remains relatively low. Similar to the kidney, brain ACE2 activity is highest on E12.5, declines on E14.5 (5.9 ± 0.6 vs. 40 ± 10 units, P < 0.05), and increases during later gestation to decrease and remain low after birth. In the adult male (P60), ACE2 activity is highest in the kidney (22 ± 2.6 units), followed by the lung (13 ± 1.2 units), heart (7.5 ± 1.6 units), and brain (4.4 ± 0.9 units). These data are consistent with previous reports in adult C57Bl/6 mice, which manifest highest ACE2 activity in the kidney, followed by the lung and heart (6). Circulating ACE2 activity remains unchanged during early postnatal development (4.1 ± 1.3 units on P1, 5.2 ± 0.7 units on P10) and declines in adulthood (2.7 ± 0.7 vs. 5.2 ± 0.7 units on P10, P < 0.05; Figure 3e ). Thus, tissue and circulating ACE2 activities are coordinately regulated during postnatal maturation.
Bar graphs showing developmental expression of angiotensin-converting enzyme 2ACE2 enzymatic activity (pmol Mca/µg protein/min) in the mouse (a) kidney, (b) lung, (c) heart, (d) brain, and (e) plasma. (a) *P < 0.05 vs. other groups; **P < 0.05 vs. E14.5, P1, adult; §P < 0.05 vs. E14.5, P1, adult. (b) *P < 0.05 vs. other groups; **P < 0.05 vs. E18.5, P1, adult; §P < 0.05 vs. adult. (c) *P < 0.05 vs. other groups; **P < 0.05 vs. E12.5, E14.5, P1, P10, adult. (d) *P < 0.05 vs. other groups; **P < 0.05 vs. E14.5, P1, P10, adult. (e) *P < 0.05 vs. other groups. E, day of embryonic life; P, day of postnatal life.
Immunohistochemistry for ACE2
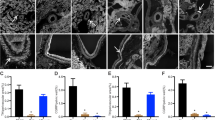
To determine the identity of ACE2-expressing cells in the developing kidney, lung, heart, and brain, we mapped the cellular expression of ACE2 protein during mouse organogenesis by immunohistochemistry. On E12.5, ACE2 immunoreactivity is widespread throughout the brain ( Figure 4 ). On E16.5 and E18.5, ACE2 is most prominent in the ependymal lining of the ventricle and cerebellum. In the E12.5 fetal kidney, ACE2 is expressed in a diffuse stippled fashion on the apical aspects of UBs, in maturing glomeruli, and in the mesenchyme ( Figure 5 ). On E14.5, ACE2 is most prominent in the inner tubules, which resemble morphologically proximal tubules, with weaker expression in the UBs. On E16.5, ACE2 is expressed predominantly in the tubules, which resemble morphologically proximal tubules. A similar staining pattern is observed on E18.5 (not shown). In the fetal heart, ACE2 is expressed diffusely in the myocardium ( Figure 6 ). In the E16.5 and E18.5 lung, ACE2 is expressed at highest levels in the epithelium of the bronchial tree.
Immunolocalization of angiotensin-converting enzyme 2 (ACE2) protein in the fetal mouse brain on embryonic days (E) 12.4, 16.5, and 18.5. (a) On E12.5, ACE2 immunoreactivity (brown staining) is present in a diffuse stippled fashion throughout the brain (×4). (b) Control section, where the addition of the primary antibody was omitted, demonstrates no staining (×40). (c) On E16.5, ACE2 is expressed most prominently in the ependymal lining of the ventricle (V) (×20). (d) On E18.5, ACE2 is most prominent in the cerebellum (C) (×10).
Immunolocalization of angiotensin-converting enzyme 2 (ACE2) protein in the fetal mouse kidney on embryonic days (E) 12.4, 14.5, and 16.5. (a) On E12.5, ACE2 is expressed in a diffuse stippled fashion on the apical aspects of ureteric buds (UBs), in maturing glomeruli (G), and in the mesenchyme (×40). (b) On E14.5, ACE2 is most prominent in the tubules (T), followed by UBs and nascent glomeruli (G) (×10). (c) On E16.5, ACE2 is most prominent in the tubules (×20). (d) Control section, where the addition of the primary antibody was omitted, demonstrates no staining (×20).
Immunolocalization of angiotensin-converting enzyme 2 (ACE2) protein in the fetal mouse heart and lung on embryonic days (E) 16.5 and 18.5. (a,b) In the heart, ACE2 immunoreactivity is present diffusely in the myocardium (M) (a ×40, b ×20). (c,d) In the lung, ACE2 is expressed at highest levels in the epithelium of the bronchial tree (arrows) (c ×20, d ×40).
Regulation of ACE2 by Ang II in the Newborn Kidney
To determine whether Ang II regulates ACE2 in the developing kidney, we examined the effect of Ang II on ACE2 mRNA expression and enzymatic activity in the fetal, newborn, and adult metanephroi. Ang II (10−6 M) decreased ACE2 mRNA levels and activity in the E18.5, P1, and P10, but not in the E12.5 or E14.5, kidney compared to control (media; Figure 7a,b ). These effects of Ang II were abrogated by pretreatment with the specific Ang II AT1R antagonist candesartan, but not by the AT2R antagonist PD123319. In the adult kidney, ACE2 expression was increased by candesartan (mRNA: 1.5 ± 0.14 vs. 1.0 ± 0.1, P < 0.05; activity: 42.3 ± 4.5 vs. 30.6 ± 4.0, P < 0.05) but not by PD123319 (mRNA: 0.87 ± 0.03 vs. 1.0 ± 0.0, P = NS; activity: 29.3 ± 3.1 vs. 30.6 ± 4.0, P = NS). Thus, Ang II, acting through AT1R, exerts a negative feedback on kidney ACE2 during later gestation and postnatal life. It is conceivable that the lack of effect of Ang II on renal ACE2 on E12.5 and E14.5 is due to low expression levels of AT1R during early metanephric development (20).
Regulation of angiotensin-converting enzyme 2 (ACE2) in the developing kidney. (a,b) Effect of media (control, white columns), Ang II (10−6 M, black columns), Ang II combined with candesartan (10−6 M, gray columns), or PD123319 (10−6 M, hatched columns) on ACE2 mRNA levels (a) and enzymatic activity (b). A: Media (C) values in each group are normalized to 1 and data are presented as relative fold difference. *P < 0.05 vs. media, **P < 0.05 vs. media, §P < 0.05 vs. Ang II or PD123319.
Discussion
The present study demonstrates that ACE2 is expressed in the developing kidney, lung, heart, and brain during embryonic development in the mouse. Moreover, ACE2 mRNA, protein, and enzymatic activity are developmentally regulated in a tissue-specific manner. In addition, Ang II, acting through AT1R, exerts a negative feedback on ACE2 in the developing kidney.
The developing metanephric kidney expresses all the components of the RAS (11,21,22,23). The activity of the renal RAS is high during fetal and neonatal life and declines during postnatal maturation (22). In the rat kidney, ACE mRNA levels and enzymatic activity are low in the newborn, peak on P15, and decline in adulthood (19). The temporal profile of renal ACE2 expression during postnatal period seen in this study recapitulates changes observed in the rat. These findings imply that Ang II generation and degradation are coordinately regulated during postnatal kidney development. In the fetal kidney, ACE2 activity is highest on E12.5 and declines later in gestation. Spatial distribution of ACE2 immunoreactivity during gestation shifts from diffuse staining in the UBs, nascent glomeruli, and the mesenchyme on E12.5 to a more localized expression in the tubular epithelia that resemble morphologically proximal tubules during E14.5–E18.5. We have recently reported that Ang II, when applied directly to whole intact E11.5 mouse metanephroi cultured in vitro, stimulates UB branching (18). The presence of ACE2 in the UB branches suggests a novel role for ACE2 in the regulation of UB morphogenesis. The enrichment of ACE2 expression in the tubules during E14.5–E18.5 suggests that ACE2 may play a role in tubulogenesis. Since Ang-(1–7) inhibits growth and proliferation of vascular smooth muscle cells (24), ACE2 may also be important for renal vascular development.
We have previously shown that endogenous Ang II, acting through AT1R, exerts a negative feedback on renal ACE during postnatal development in the rat (25). Our present findings demonstrate that Ang II, acting through AT1R, downregulates ACE2 mRNA expression and activity in the developing kidney. These results are consistent with the findings that Ang II decreases ACE2 gene expression and activity in neonatal cardiomyocytes and in human tubular epithelial (HK-2) cells in vitro through AT1R (26,27). Coordinate regulation of ACE and ACE2 by Ang II during postnatal life may represent a dual mechanism by which Ang II prevents its ACE-dependent generation from Ang I as well as inhibits its ACE2-mediated degradation to Ang-(1–7).
Genetic studies in mice demonstrated that ACE2 plays an important role in the pathogenesis of cardiac hypertrophy due to aortic constriction or chronic Ang II infusion (19,21). Given that ACE2 mediates antihypertrophic effects in cardiomyocytes (15), it is conceivable that progressively increasing cardiac ACE2 activity during gestation may regulate growth of the myocardium. Central overexpression of ACE2 downregulates AT1R expression in the brain, attenuates hypertension, and improves arterial baroreflex induced by Ang II infusion or in spontaneously hypertensive rats (13). Collectively, these findings indicate a critical role for ACE2 in the central regulation of blood pressure and the development of hypertension. High ACE2 protein levels and activity observed in the fetal brain suggest a role for ACE2 in brain development and regulation of local RAS activity. This possibility is supported by the finding that the fetal brain expresses all the components of the classic RAS that are subject to epigenetic programming by maternal protein restriction during gestation (28). The enrichment of ACE2 in pulmonary bronchioles supports a novel function for ACE2 in the regulation of epithelial branching morphogenesis.
An interesting finding of this study is that the temporal profile of ACE2 mRNA expression differs among the various tissues. Whereas pulmonary and brain ACE2 mRNA levels peak in adulthood, renal and cardiac ACE2 levels are lower in the adult compared with developing animal. The factors regulating tissue-specific developmental expression of ACE2 remain to be determined. One potential mechanism may involve transcriptional repression of ACE2 by hypoxia-inducible factor 1α or activation by hepatocyte nuclear factor 1β (29,30). In addition, ACE2 expression can be induced by retinoic acid (vitamin A). Functionally, induction of ACE2 expression by retinoic acid is associated with reduction in blood pressure in spontaneously hypertensive rats (31).
We observed an apparent divergence between ACE2 mRNA and protein levels in several organs. Whereas ACE2 mRNA levels are relatively low during E12.5–E16.5 in all organs studied, ACE2 proteins are easily detected. In contrast, ACE2 mRNA/protein ratios increase in all organs after E18.5. The relatively low ACE2 mRNA/protein ratios observed during early gestation may be due to increased stability of nonabundant ACE2 mRNA or due to resistance of ACE2 protein to degradation. One specific mechanism may involve microRNAs (miRNAs), which function to downregulate protein expression without degrading the mRNA. In this regard, reduced expression of the miRNAs that putatively increase translation of ACE2 is observed in fetal lung (32). Thus, posttranscriptional epigenetic programming to increase protein translation may account for discrepancies in the pulmonary mRNA and protein levels observed in the present study. Another mechanism may involve inhibition of ACE2 gene transcription by hypoxia-inducible factor 1α (30). Relatively lower ACE2 protein contents during postnatal life may also be due to shedding, as demonstrated for ACE (33). Enhanced shedding may lead to increased secretion of ACE2 and lowering of its protein content. In addition, ACE2 protein abundance may be differentially regulated during ontogeny by the variation in the activity of peptidases, as shown for neutral peptidase activity in the rat kidney (35).
In summary, the present study demonstrates that ACE2 is expressed in the developing kidney, heart, lung, and brain as early as on E12.5 in the mouse. Moreover, ACE2 gene and protein expression and enzymatic activity are developmentally regulated in a tissue-specific manner. Ang II, acting though AT1R, exerts a negative feedback on ACE2 during kidney development. We postulate that relatively high ACE2 protein levels and enzymatic activity observed during gestation may play a role in kidney, lung, brain, and heart organogenesis.
Methods
Animals
Tissues were dissected from CD1 mice (Charles River Laboratories, Wilmington, MA) on embryonic days E12.5–E18.5 and postnatal days P1, P10, and P60 (adult male). All experiments involving mice were approved by Tulane Institutional Animal Care and Use Committee.
Quantitative Real-Time RT-PCR
Quantitative real-time RT-PCR was utilized to determine the expression of ACE2 mRNA in the whole metanephroi, heart, lung, and brain. SYBR Green quantitative real-time RT-PCR with ACE2 primers (SA Biosciences, Frederick, MD) was conducted as described previously (18). The quantity of ACE2 mRNA expression was normalized by that of glyceraldehyde 3-phosphate dehydrogenase mRNA expression. For E12.5–E18.5, organs were pooled from one litter. For P1–P60, n = 4 animals per age group. RNA samples per age group were analyzed in triplicates in each run. PCR was performed three times.
Western Blot Analysis
Organs were homogenized in cold lysis buffer containing a cocktail of enzyme inhibitors (35). The samples were centrifuged and the supernatants containing proteins (40 µg/lane) were resolved on 10% sodium dodecyl sulfate-polyacrylamide gels and transferred to nitrocellulose membranes. The membranes were incubated with the ACE2 antibody (1:200, Open Biosystems, Huntsville, AL) and β-actin monoclonal antibody (1:5,000; Sigma, St Louis, MO). Immunoreactive bands were visualized using the enhanced chemiluminescence detection system (Amersham, Piscataway, NJ) as previously described (35).
ACE2 Enzymatic Activity
Organs were dissected free of connective tissue, blotted dry, and immediately homogenized in ice-cold Tris-HCl buffer (50 mM, pH 7.4) containing 2 μM phenylmethanesulfonyl fluoride. ACE2 activity was measured by fluorometric assay of the enzymatic cleavage of 7-methoxycoumarin-acetyl (Mca) from the fluorogenic substrate Mca-APK-dinitrophenyl (Dnp) Mca-Ala-Pro-Lys(Dnp) (Enzo Life Sciences, Plymouth Meeting, PA). All reactions were carried out in duplicates in 96-well, clear, flat-bottom polystyrene microplates (Corning, Lowell, MA) at a final volume of 100 μl. Reactions were initiated by the addition of 10 μl protein extract to MES buffer (50 mM final concentration, pH 6.5, Sigma) containing NaCl (300 mM, Sigma), ZnCl2 (10 μM, Sigma), and Mca-APK(Dnp) (40 μM). Fluorescence of the reaction product was measured following 1 h incubation at 37 °C using a Spectramax M5E microplate reader (Molecular Devices, Sunnyvale, CA) (excitation = 328 μm, emission = 393 μm). Specificity was determined by preincubating plasma or tissue extracts with the specific ACE2 inhibitor DX600 (10−6 M, AnaSpec, Fremont, CA). ACE2 activity was expressed as picomoles Mca formation per microgram protein per minute.
Immunohistochemistry for ACE2
Immunolocalization of ACE2 protein was performed in CD1 mouse embryos from E12.5 to E18.5 (n = 3 per age group). Immunostaining was performed by the immunoperoxidase technique using the Vectastain Elite kit (Vector Laboratories, Burlingame, CA). A polyclonal goat ACE2 antibody (R&D Systems, Minneapolis, MN) was utilized at concentrations of 1/50 to 1/100. Specificity of immunostaining was documented by the elimination of immunoreactivity after omission of the primary antibody.
Regulation of ACE2 by Ang II in the Developing Kidney
E12.5, E14.5, E18.5, P1, and P10 CD1 mouse kidneys were grown on air–fluid interface on polycarbonate Transwell filters (Corning Costar, Lowell, MA; 0.5 μm) inserted into six-well plates containing Dulbecco’s modified Eagle’s medium/F12 medium (Gibco, Carlsbad, CA) alone (n = 3) or in the presence of Ang II (10−6 M) alone (n = 3) or combined with the AT1R antagonist candesartan (10−6 M, Sigma) (n = 3) or AT2R antagonist PD123319 (10−6 M, Sigma) for 16 h at 37 °C and 5% CO2, and then processed for RNA or protein extraction. Adult males (P60) were injected intraperitoneally with normal saline (control), candesartan (2 mg/kg/day), or PD123319 (20 mg/kg/day) twice daily for 3 d (n = 3 per group). Kidney ACE2 mRNA levels were examined by quantitative RT-PCR, and ACE2 activity was measured as described above.
Statistical Analysis
Data are presented as mean ± SD. Differences among the age groups in mRNA, protein levels, and enzymatic activity were analyzed by one-way ANOVA followed by Student–Newman–Keuls test. A P value of <0.05 was considered statistically significant.
Statement of Financial Support
This work was supported by NIH grants P20 RR17659 and DK-71699 (I.V.Y.).
References
Kobori H, Ozawa Y, Suzaki Y, et al. Young Scholars Award Lecture: Intratubular angiotensinogen in hypertension and kidney diseases. Am J Hypertens 2006;19: 541–50.
Ito M, Oliverio MI, Mannon PJ, et al. Regulation of blood pressure by the type 1A angiotensin II receptor gene. Proc Natl Acad Sci USA 1995;92: 3521–5.
Siragy HM, Carey RM . The subtype 2 (AT2) angiotensin receptor mediates renal production of nitric oxide in conscious rats. J Clin Invest 1997;100: 264–9.
Tsutsumi Y, Matsubara H, Masaki H,et al. Angiotensin II type 2 receptor overexpression activates the vascular kinin system and causes vasodilation. J Clin Invest 1999;104: 925–35.
Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ . A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem 2000;275: 33238–43.
Gembardt F, Sterner-Kock A, Imboden H, et al. Organ-specific distribution of ACE2 mRNA and correlating peptidase activity in rodents. Peptides 2005;26: 1270–7.
Ferrario CM . ACE2: more of Ang-(1-7) or less Ang II? Curr Opin Nephrol Hypertens 2011;20: 1–6.
Santos RA, Simoes e Silva AC, Maric C, et al. Angiotensin-(1-7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci USA 2003;100: 8258–63.
Lacoste M, Cai Y, Guicharnaud L, et al. Renal tubular dysgenesis, a not uncommon autosomal recessive disorder leading to oligohydramnios: Role of the Renin-Angiotensin system. J Am Soc Nephrol 2006;17: 2253–63.
Yosypiv IV . Renin-angiotensin system-growth factor cross-talk: a novel mechanism for ureteric bud morphogenesis. Pediatr Nephrol 2009;24: 1113–20.
Crackower MA, Sarao R, Oudit GY, et al. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature 2002;417: 822–8.
Oudit GY, Herzenberg AM, Kassiri Z, et al. Loss of angiotensin-converting enzyme-2 leads to the late development of angiotensin II-dependent glomerulosclerosis. Am J Pathol 2006;168: 1808–20.
Feng Y, Xia H, Cai Y, et al. Brain-selective overexpression of human Angiotensin-converting enzyme type 2 attenuates neurogenic hypertension. Circ Res 2010;106: 373–82.
Imai Y, Kuba K, Rao S, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 2005;436: 112–6.
Yamamoto K, Ohishi M, Katsuya T, et al. Deletion of angiotensin-converting enzyme 2 accelerates pressure overload-induced cardiac dysfunction by increasing local angiotensin II. Hypertension 2006;47: 718–26.
Gurley SB, Allred A, Le TH, et al. Altered blood pressure responses and normal cardiac phenotype in ACE2-null mice. J Clin Invest 2006;116: 2218–25.
Gurley SB, Coffman TM . Angiotensin-converting enzyme 2 gene targeting studies in mice: mixed messages. Exp Physiol 2008;93: 538–42.
Yosypiv IV, Boh MK, Spera MA, El-Dahr SS . Downregulation of Spry-1, an inhibitor of GDNF/Ret, causes angiotensin II-induced ureteric bud branching. Kidney Int 2008;74: 1287–93.
Yosipiv IV, Dipp S, el-Dahr SS . Ontogeny of somatic angiotensin-converting enzyme. Hypertension 1994;23: 369–74.
García-Villalba P, Denkers ND, Wittwer CT, Hoff C, Nelson RD, Mauch TJ . Real-time PCR quantification of AT1 and AT2 angiotensin receptor mRNA expression in the developing rat kidney. Nephron Exp Nephrol 2003;94: e154–9.
Gomez RA, Lynch KR, Sturgill BC, et al. Distribution of renin mRNA and its protein in the developing kidney. Am J Physiol 1989;257: F850–8.
Yosipiv IV, el-Dahr SS . Activation of angiotensin-generating systems in the developing rat kidney. Hypertension 1996;27: 281–6.
Jung FF, Bouyounes B, Barrio R, Tang SS, Diamant D, Ingelfinger JR . Angiotensin converting enzyme in renal ontogeny: hypothesis for multiple roles. Pediatr Nephrol 1993;7: 834–40.
Freeman EJ, Chisolm GM, Ferrario CM, Tallant EA . Angiotensin-(1-7) inhibits vascular smooth muscle cell growth. Hypertension 1996;28: 104–8.
Yosipiv IV, el-Dahr SS . Developmental regulation of ACE gene expression by endogenous kinins and angiotensin II. Am J Physiol 1995;269: F172–9.
Koka V, Huang XR, Chung AC, Wang W, Truong LD, Lan HY . Angiotensin II up-regulates angiotensin I-converting enzyme (ACE), but down-regulates ACE2 via the AT1-ERK/p38 MAP kinase pathway. Am J Pathol 2008;172: 1174–83.
Gallagher PE, Ferrario CM, Tallant EA . Regulation of ACE2 in cardiac myocytes and fibroblasts. Am J Physiol Heart Circ Physiol 2008;295: H2373–79.
Goyal R, Goyal D, Leitzke A, Gheorghe CP, Longo LD . Brain renin-angiotensin system: fetal epigenetic programming by maternal protein restriction during pregnancy. Reprod Sci 2010;17: 227–38.
Zhang R, Wu Y, Zhao M, et al. Role of HIF-1alpha in the regulation ACE and ACE2 expression in hypoxic human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 2009;297: L631–40.
Senkel S, Lucas B, Klein-Hitpass L, Ryffel GU . Identification of target genes of the transcription factor HNF1beta and HNF1alpha in a human embryonic kidney cell line. Biochim Biophys Acta 2005;1731: 179–90.
Zhong JC, Huang DY, Yang YM, et al. Upregulation of angiotensin-converting enzyme 2 by all-trans retinoic acid in spontaneously hypertensive rats. Hypertension 2004;44: 907–12.
Goyal R, Leitzke A, Goyal D, Gheorghe CP, Longo LD . Antenatal maternal hypoxic stress: adaptations in fetal lung Renin-Angiotensin system. Reprod Sci 2011;18: 180–9.
Donoghue M, Hsieh F, Baronas E,et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res 2000;87: E1–9.
Edwards RM, Pullen M, Nambi P . Distribution of neutral endopeptidase activity along the rat and rabbit nephron. Pharmacology 1999;59: 45–50.
Yosypiv IV, Schroeder M, El-Dahr SS . Angiotensin II type 1 receptor-EGF receptor cross-talk regulates ureteric bud branching morphogenesis. J Am Soc Nephrol 2006;17: 1005–14.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Song, R., Preston, G. & Yosypiv, I. Ontogeny of angiotensin-converting enzyme 2. Pediatr Res 71, 13–19 (2012). https://doi.org/10.1038/pr.2011.7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2011.7
This article is cited by
-
COVID-19 and the Pediatric Nervous System: Global Collaboration to Meet a Global Need
Neurocritical Care (2021)
-
Clinical Profile and Outcome of Children Infected with SARS-CoV-2
Indian Journal of Pediatrics (2021)
-
Obesity is associated with increased severity of disease in COVID-19 pneumonia: a systematic review and meta-analysis
European Journal of Medical Research (2020)
-
COVID-19 PICU guidelines: for high- and limited-resource settings
Pediatric Research (2020)
-
Pathophysiology of COVID-19: Why Children Fare Better than Adults?
The Indian Journal of Pediatrics (2020)