Abstract
We evaluated the potential therapeutic use of exogenous human bone marrow-derived mesenchymal stem cells (hBM-MSCs) in an experimental rat model of necrotizing enterocolitis (NEC). Thirty-six newborn Sprague-Dawley rats were randomly divided into three groups: NEC, NEC + hBM-MSC, and a control (control and control + hBM-MSC). NEC was induced by enteral formula feeding, exposure to hypoxia-hyperoxia, and cold stress. After NEC was induced, iron-labeled hBM-MSCs were administered by intraperitoneal injection. All pups were killed on the fourth day following injection, and the terminal ileum was excised for a histopathological and immunohistochemical evaluation. The pups in the NEC + hBM-MSC group showed significant weight gains and improvements in their clinical sickness scores (p < 0.01). Bowel damage severity observed in the histopathological evaluation was significantly lower in the NEC + hBM-MSC group than that in the NEC group (p = 0.012). The number of MSCs homing to the bowel was significantly higher in the NEC + hBM-MSC group than that in the control + hBM-MSC group. In conclusion, this is the first study that has evaluated the effectiveness of hBM-MSCs in a neonatal rat NEC model. MSCs reduced histopathological damage significantly.
Similar content being viewed by others
Main
Necrotizing enterocolitis (NEC) is the most common gastrointestinal emergency and a leading cause of mortality and morbidity in newborn infants. The etiology and pathophysiology of NEC remains unclear. Although prematurity is the most consistent risk factor, hypoxic-ischemic injury, formula feeding, abnormal bacterial colonization, antenatal and postnatal risk components, and genetic aspects comprise other potential risk factors (1–3). Medical management of severe cases is often inadequate, and surgical intervention may be warranted. Thus, ∼20–40% of neonates eventually require a surgical procedure (1,4,5). In addition, morbid sequelae among survivors include impaired growth, short bowel syndrome, prolonged neonatal hospitalization, and poor long-term neurodevelopment (1–5). Therefore, the introduction of new strategies for the prevention and/or therapy for this devastating disease is essential to increase the survival rate and to reduce significant complications.
Mesenchymal stem cells (MSCs) are multipotent cells capable of differentiating into multiple cell types. In addition, MSCs secrete a wide variety of cytokines and chemokines that have beneficial paracrine actions during tissue repair (6–11). Because of these unique properties, MSCs could be a future option for treating various diseases. Studies on the potential use of stem cells in pediatric diseases have recently aroused interest among clinicians (12–15), although stem cell therapy has not yet been studied as a treatment modality for neonatal intestinal disorders such as NEC. In this study, the therapeutic potential of exogenous human bone marrow-derived (hBM)-MSC therapy was evaluated in an experimental neonatal rat model of NEC.
MATERIALS AND METHODS
Animal model.
Fatih University Institutional Review Board (Ankara, Turkey) approved the animal experiments. Four time-mated Sprague-Dawley pregnant rats delivered spontaneously, and 36 newborn pups were allocated equally into three groups: NEC (subjected to NEC procedure), NEC + MSCs (subjected to NEC procedure and treated with MSCs), and control + MSCs (breast fed with their mother's milk and treated with MSCs). To avoid the preventive effect of breast milk in the study groups, newborn pups were immediately separated from their mothers and kept at 37°C in a humidified incubator. Rat pups were hand fed with 0.2 mL of special rodent formula (15 g Similac 60/40; Ross Pediatrics, Columbus, OH) prepared with 75 mL of canine-puppy milk (Beaphar-Bogena, BV Sedel, The Netherlands). Feeding was started at 0.2 mL every 3 h and was advanced slowly by 0.1 mL increments daily, as tolerated. Rat pups were subjected to 100% CO2 inhalation for 10 min, +4°C cold exposure for 5 min, and 97% O2 for 5 min twice daily for 3 d to induce NEC (16). The pups were weighed daily to the nearest 0.01 g. The behavior of pups was compared by a blinded observer using a modified neonatal rat clinical sickness score that included appearance, response to touch, natural activity, and color each day (17). The number of deaths, approximate time, and cause of death (NEC or iatrogenic cause during NEC procedure) was recorded daily.
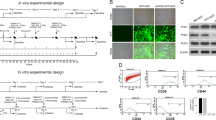
Isolation and culture of hBM-MSCs.
Hacettepe University Institutional Review Board (Ankara, Turkey) approved isolation, characterization of human MSCs from healthy BM transplant donors, and labeling for use in in vivo models. hBM-MSCs were isolated and grown in culture at the PEDI-STEM Stem Cell laboratory of Hacettepe University using frozen marrow samples obtained from a healthy, 12-y-old bone marrow transplant donor. An informed consent was obtained from the parents before the application. Two milliliters of marrow sample was used. After dilution in an equal volume of PBS, mononuclear cells were isolated by density centrifugation using Ficoll Hypaque (1077 g/L; Biochrom, Berlin, Germany). Cells were cultured in complete medium (CM) consisting of low-glucose-DMEM; Invitrogen, Carlsbad, CA), 10% fetal bovine serum (FBS; Invitrogen), and 1% penicillin/streptomycin (Biochrom; Fig. 1A). After 24 h, nonadherent cells were discarded, and the CM was changed every 3–4 d, and then incubated at 37°C in 5% CO2. Analyses were performed with P3 cells. hBM-MSCs were confirmed to be negative for hematopoietic markers by flow cytometry and capable of differentiating into osteocytes and adipocytes in vitro (18).
(A) Mesenchymal stem cells (MSCs) in culture. Cells revealed an adherent fibroblastic morphology in culture. A representative photomicrograph of BM-MSCs from a healthy control. (B) Differentiation of MSCs into the adipogenic lineage, stained with Oil Red O. (C) Differentiation of MSCs into the osteogenic lineage, stained with Alizarin Red S. Phase-contrast micrographs in A-C, ×500.
Labeling of hBM-MSCs with iron oxide particles.
hBM-MSCs were allowed to grow until 80–90% confluence, and the medium was exchanged. Cells were magnetically labeled with ferrum oxide (Endorem; Guerbet, Villepinte, France) and complexed to poly-l-lysine (Sigma Chemical Co.-Aldrich, St. Louis, MO), as previously described. The cells were incubated with a labeling medium containing 50 μg/mL iron and 0.375 μg/mL poly-l-lysine for 48 h at 37°C in a 5% CO2 atmosphere. After labeling, viability was tested with trypan blue staining. Cell numbers were determined using a hemocytometer (19).
Differentiation assays.
Adipogenic and osteogenic differentiation tests were performed to confirm that the cells used in the experiments were MSC/stromal cells, as suggested by Dominici et al., (20) and that they represented ISCT recommendations.
Control and labeled (50 μg/mL iron and 0.375 g/mL poly-l-lysine for 48 h) MSCs were grown to confluency (90–100%) on six-well plates, then, exposed to adipogenic medium consisting of LG-DMEM supplemented with 10% FBS, 1 μM dexamethasone, 60 μM indomethacin, 500 μM isobutylmethylxanthine, and 5 μg/mL insulin (Sigma Chemical Co.-Aldrich) for 21 d and stained with Oil Red O (Sigma Chemical Co.-Aldrich; Fig. 1B) (18).
Control and labeled (50 μg/mL iron and 0.375 g/mL poly-l-lysine for 48 h) MSCs were cultured for 5–7 d to 50–60% confluency and exposed to osteogenic induction medium consisting of LG-DMEM supplemented with 10% FBS, 100 nM dexamethasone, 10 mM ß-glycerophosphate, and 0.2 mM ascorbic acid (Sigma Chemical Co.-Aldrich) for 21 d and stained with Alizarin Red S (Sigma Chemical Co.-Aldrich). Extracellular matrix calcification was evident based on the appearance of calcium deposits in the culture (Fig. 1C) (18). All images were captured with a CKX41 microscope (Olympus, Tokyo, Japan).
Immunophenotype of control MSCs and labeled MSCs.
Culture-expanded control and labeled (50 μg/mL iron and 0.375 g/mL poly-l-lysine for 48 h) MSCs were immunophenotypically characterized by flow cytometry (Becton Dickinson FACS Aria; BD Biosciences, Sparks, MD). Detached cells were washed in PBS. Optimal concentrations of directly conjugated MAb were added to a total volume of 100 μL and incubated for 20 min at room temperature. The antibody panel included CD29-FITC, CD44-PE, CD105-PE, CD166-PE, CD73-PE, CD49e-PE, CD90-PE, HLA-ABC-PE (BD Biosciences) as mesenchymal markers, and HLA-DR-FITC (Chemicon, Temecula, CA); CD45-FITC, CD3-FITC, and CD34-FITC (BD Biosciences) as hematopoietic markers, which were used to exclude cells of hematopoietic origin (18).
MSC transplantation procedure.
After inducing NEC, hBM-MSC transplantation was performed by injecting 6 × 105 labeled cells in 50 μL PBS into the peritoneal cavity of each rat in the NEC + hBM-MSC group (n = 12) and in control animals (non-NEC + hBM-MSC; n = 6) on the third day of the study. Animals for the control and study groups were randomly selected. After the transplantation procedure, pups were followed daily to document weights and to assess the NEC neonatal rat clinical sickness score (17).
Histopathological evaluation.
All pups were killed on the fourth day of transplantation under deep anesthesia with ketamine (100 mg/kg intraperitoneally). The abdominal cavity was opened, and the terminal ileum was excised and fixed in 10% neutral-buffered formalin. Tissues were paraffin embedded, and paraffin blocks were sliced into 4–5-μm portions and stained with hematoxylin and eosin. Histological findings were graded by two pathologists in a blinded fashion as follows: grade 0, normal; grade 1, focal mild injury confined to villous tips; grade 2, partial or total loss of villi; grade 3, necrosis extending to the submucosa; grade 4, transmural necrosis (Fig. 2) (21).
Histological changes in the ileum of rat pups with NEC, showing representative sections of each grading score (hematoxylin and eosin staining; original magnification, ×400). (A) grade 0, normal; (B) grade 1, focal mild injury confined to villous tips; (C) grade 2, partial or total loss of villi; (D) grade 3, necrosis extending to the submucosa; and (E) grade 4, transmural necrosis.
Detection of iron-labeled hBM-MSCs in the tissues.
Iron-labeled hBM-MSCs were counterstained with the Van Gieson picrofuchsin stain package (Bio-Optica, Milan, Italy) to distinguish injected human cells from host intestinal cells. After all procedures were completed, the stained sections were examined under a light microscope. Ferric reactive iron in the MSCs was visualized as blue (Fig. 3).
Photographs of homing hBM-MSCs with Van Gieson picrofuchsin staining; original magnification, ×400. Ferric reactive iron in hBM-MSCs is visualized as blue. (A) Normal intestinal tissue; (B) intestinal tissue with NEC; (C) control + hBM-MSCs showing a low level of hBM-MSCs homing; and (D) NEC + hBM-MSCs demonstrating prominent ferric reactive iron staining (blue) in the lamina propria and intestinal villi.
Immunohistochemical examination of hBM-MSCs in tissues.
Sections of 5 μm thickness were processed with polylysin microscope slides. For the immunohistochemical examination, endogenous peroxidase activity was blocked in 3% hydrogen peroxide (cat #TA-125-HP, lot #AHP40114; LabVision, Fremont, CA). Epitopes were stabilized with a serum blocking solution (cat #85-9043, lot #724944A; Invitrogen, Carlsbad, CA). Sections were incubated with β-2-microglobulin [MAb (2213), sc-80668] diluted in PBS (cat #00-3000; Zymed Laboratories, Inc., San Francisco, CA) overnight at 4°C. β-2 microglobulin was used to detect cells of human origin. Biotinylated secondary antibody (cat #85-9043, lot #724944A; Invitrogen) and horseradish peroxidase (cat #85-9043, lot #724944A; Invitrogen) were applied to the slides. DAB (3,3′-diaminobenzidine; cat #00-2020, lot #720221A; Invitrogen) was used as the chromogen. The slides were then counterstained with Mayer's hematoxylin and examined under a DM 4000 photolight microscope (Leica, Wetzlar, Germany). β-2-microglobulin positive hBM-MSCs were surrounded by brown-stained cell membranes (Fig. 4).
Photographs of hBM-MSCs homing to intestines in the immunohistochemical (β-2-microglobulin MAb) examination; Panel 1 (A and B) normal intestinal tissue, Panel 2 (A and B): control + hBM-MSCs indicated that β-2 microglobulin immunoreactivity was identified weakly in the epithelium of villi at the membranous level and in intestinal glands. Panel 3 (A and B): intestinal tissue with necrotizing enterocolitis (NEC). Panel 4 (A and B): intestinal wall indicated better histological outcomes in the NEC + hBM-MSC group than in the NEC group. Strong β-2-microglobulin immunoreactivity was observed in the apical cytoplasm of epithelial cells, the lamina propria, and the muscularis compared with that in the control group (immunoperoxidase and hematoxylin, original magnification A, ×100; B, ×400). E, epithelial cells; V, villi; (§), intestinal glands; Lp, lamina propria; Tm, muscularis layer; Lc, lacteals.
Statistical analysis.
The SPSS statistical package (v15.0; SPSS, Inc., Chicago, IL) was used for the statistical analysis. The distribution of the values was examined graphically with the Shapiro-Wilk test. The median interquartile range (IQR) and the mean ± SD were used to display descriptive statistics. An analysis of variance with Bonferroni adjustment was used to analyze the independent data with a normal distribution and for intergroup analyses of parametric variables, whereas the Mann-Whitney U test was used to compare data that were not normally distributed and for intergroup comparisons of nonparametric independent variables. Friedman's test and Wilcoxon's test with Bonferroni adjustment were applied for intergroup comparisons of nonparametric dependent variables. A p < 0.05 was considered statistically significant. A power analysis was conducted; if 0.60 of the effect size and an alpha of 0.05 were used to obtain 80% power, eight animals were adequate for each group. Four animals were added to each group to compensate for unpredictable losses.
RESULTS
Characterization of hBM-MSCs.
In vitro culture-expanded hBM-MSCs showed plastic adherent and fibroblastic morphology. The cells differentiated into adipogenic and osteoblastic lineages under defined culture conditions (Fig. 1). The immunophenotyping analysis of the MSCs revealed a positive response for CD105, CD29, CD44, CD73, CD166, HLA-ABC, CD49e, and CD90 surface antigens but negative staining for hematopoietic markers, including CD45, CD34, and CD3. These characteristics confirmed the stromal nature of the cells by immunophenotype and multipotentiality.
Animal study.
Five pups died in the NEC group and one died in the NEC + hBM-MSC group, but no significant differences were found for survival between two groups (p = 0.155). Thirty pups were alive at the end of the study. On the third day of the study, weight loss in the NEC and the NEC + hBM-MSC groups was more remarkable than that in the control group (p < 0.001) (Table 1). After hBM-MSCs transplantation, the pups in the NEC + hBM-MSC group started to gain weight. Thus, at the end of the study, the mean body weight of the pups in the NEC + hBM-MSC group was higher than that in the NEC group (p < 0.001; Table 1, Fig. 5).
Rat behavior, assessed with the clinical sickness score (0 = best, 12 = worst) (17), demonstrated no significant difference among the groups including the NEC [6.0 (2.0)] and NEC + hBM-MSC [5.5 (2.3)] on the third day (p = 0.72). However, after the hBM-MSCs transplantation, the pups in the NEC + hBM-MSC group started to improve in terms of clinical sickness score until the end of the study [7.0 (2.8) versus 3.0 (1.0); p < 0.001; Table 1, Fig. 6].
The severity of bowel damage was determined as grades 0–4 (Fig. 2). No histological changes were observed in the control group. However, pups in the NEC + hBM-MSCs group had less bowel damage compared with that in the NEC group (p = 0.012; Table 1). MSC homing was shown by the detection of iron-labeled hBM-MSCs (Fig. 3) and an immunohistochemical examination of human cells in the intestinal tissues (Fig. 4). The iron-labeled hBM-MSCs count was 42.5 ± 6.4 per 50 high power fields in the control + hBM-MSCs group and 2544.3 ± 635.7 per 50 high power fields (Nikon, ×40 objective, 0.152 mm2) in the NEC + hBM-MSC group (p < 0.005). MSC homing was significantly lower in the control + hBM-MSC group than in the NEC + hBM-MSC group.
DISCUSSION
We investigated the potential therapeutic use of hBM-MSCs in a rat model of NEC. Our data indicated that hBM-MSCs homed to injured sites in the bowel and reduced pathological damage. Thus, hBM-MSCs had beneficial effects on the clinical sickness score and the body weight of rat pups when compared with those not exposed to MSCs. This is the first report indicating that human MSCs improved pathological changes in the small intestine of a neonatal NEC rat model.
NEC is a complex multifactorial disease, and many factors contribute to its pathogenesis. Hypoxic-ischemic injury to the gastrointestinal tract is believed to be a major contributing and potentially inciting factor in NEC (1–4). A recent study indicated that local transplantation of MSCs was effective for reducing pathological damage and preserving intestinal mechanical barrier function after an ischemia-reperfusion injury (22). Crisostomo et al. (23) demonstrated that human MSCs produce vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), IGF-1, and IL-6 (IL-6) after noxious stimulation. Weil et al. (24) also reported that MSCs might increase the viability and proliferative capacity of fetal intestinal epithelial cells after hypoxic injury, such as that occurring with NEC, by releasing paracrine mediators such as VEGF, hepatocyte growth factor (HGF), and IL-6 from MSCs. Several different cytokines, including IL-6, HGF, FGF, and VEGF, play key roles in gut mucosal barrier function and gastrointestinal healing. Therefore, increased concentrations of these factors may promote healing and gut reconstitution by increasing the angiogenic potential, lowering apoptosis during acute inflammation and ischemia, and enhancing stem cell survival during transplantation via a paracrine mechanism (25–30). In premature infants, the immature intestine has multiple potential developmental defects that increase susceptibility to intestinal epithelial injury and apoptosis (1–3). A recent study reported that MSCs reduce apoptosis in fetal intestinal epithelial cells by down-regulating proapoptotic signaling after hypoxic injury (24). Furthermore, hBM-MSCs inhibit inflammatory and immune responses (31,32). The immature intestine is thought to respond to injury with excess inflammation, which is likely the final common pathway in the pathogenesis of NEC (1–3). One may speculate that the anti-inflammatory effect of hBM-MSCs might have played a role in the favorable response achieved in the present study (31–33). The attenuation of the histopathological damage and protection of the intestine from severe damage was attributable to the regenerative effects of the MSCs.
Another important contributing factor to NEC is abnormal bacterial colonization that predisposes the intestine to injury and bacterial translocation. However, MSC administration reduces bacterial translocation (22). Thus, MSCs may lessen the harmful effects of bacteria during the course of NEC. In NEC, the gastrointestinal system shows edema, epithelial cell necrosis, disruption of mucosal integrity, bacterial translocation, a systemic inflammatory response (SIR), multiple organ dysfunction (MOD), and eventually, death (1–5). However, MSC administration significantly attenuates histological damage, villous injuries, and accelerates the recovery of intestinal barrier function (22). Therefore, decreased intestinal damage and recovery of intestinal tissues may increase the intestinal absorptive surface. Moreover, reduced inflammation and bacterial translocation may reduce the risk of SIR and MOD. In the present study, the beneficial effects of hBM-MCs therapy were demonstrated by improvements in the rat clinical sickness score and body weight after hBM-MSCs transplantation.
MSCs have the ability to migrate and home to tissues and organs. Some experimental studies evaluating the biodistribution and potential therapeutic effect of human MSCs have indicated that MSCs can home to a wide variety of tissues with a high engraftment level, particularly in the presence of injury or inflammation; the mechanism by which MSCs home to tissues and migrate across the endothelium, however, is not yet fully understood (34–36). The possibility exists that injured tissues express specific receptors or ligands to facilitate trafficking, adhesion, and infiltration of MSCs to the site of injury (36). Inflammation is an important contributing factor in the pathogenesis of NEC. The immature intestine responds to injury by activating molecular pathways that increase the release of inflammatory mediators, tissue chemokines, and chemokine receptors, which may attract stem cells to damaged tissues, where they may home and differentiate in NEC (1–3). In the present study, hBM-MSCs were transplanted to pups in the control group to evaluate homing of hBM-MSCs in normal tissue. MSC homing was observed much less in the control + hBM-MSC group than in the NEC + hBM-MSC group. Our data might support the opinion that MSCs home to injured intestinal tissues with high engraftment levels during the course of NEC. Another important feature of MSCs is their multipotentiality, with the capacity to differentiate into multiple cell types, including intestinal epithelia, endothelia, and connective tissue cells, as these tissues are lost in the intestine in NEC (8,9,37). Therefore, MSCs may contribute to repair by supporting the regeneration of damaged cells or by substituting for injured cells.
Our results indicated high hBM-MSC engraftment levels in the injured intestine, which resulted in an improvement in the intestinal damage after intraperitoneal administration. MSCs may have played a role in inducing re-epithelization of blunted villi and prevented fibrosis possibly by providing secretory support. Moreover, MSCs home to injury sites and play a significant role hastening organ/tissue repair by providing growth factors, angiogenic support, and avoiding excess inflammation and fibrosis rather than trans-differentiating into cells not of connective tissue origin (33). Human MSCs were used based on literature data confirming their immunosuppressive nature and escape from immune rejection, even in xenogenic models (38). Previous experimental studies have shown that hB-MSCs can survive and be engrafted in an immunocompetent environment (38–40). Considering the neonatal status and thus the immunologically naive and highly regenerative state of the recipient animals in our study, human MSCs could escape from immunological rejection, home to injured sites, and contribute to intestinal repair. Another reason that human cells were used was to demonstrate the repair potential of human cells, which may be the candidate cell type for further clinical application.
In this study, carrier control experiments were not performed because carrier was not expected to have an effect on improving NEC. The primary focus of this study was to determine the homing and repair potential of hBM-MSCs to injured intestine and to identify a potential treatment to human subjects whether hBM-MSCs infiltrate primarily injured intestines with intraperitoneal applications of hBM-MSCs. After this preliminary study, histologic examination of other major organs (brain, kidney, heart, lungs, liver, and spleen) would inform us about the risk for potential side effects of this treatment for the future.
In conclusion, the results suggest that MSCs might represent a novel treatment modality for the repair and regeneration of injured intestinal tissues in NEC because of their beneficial effects on reducing inflammation and enhancing tissue regeneration. However, further studies are warranted to understand the mechanism of MSC action in NEC and to present them as a new treatment option for NEC.
Abbreviations
- BM:
-
bone marrow
- hBM-MSC:
-
human bone marrow-mesenchymal stem cell
- IQR:
-
median interquartile range
- MSC:
-
mesenchymal stem cell
- NEC:
-
necrotizing enterocolitis
References
Lin PW, Nasr TR, Stoll BJ 2008 Necrotizing enterocolitis: recent scientific advances in pathophysiology and prevention. Semin Perinatol 32: 70–82
Bradshaw WT 2009 Necrotizing enterocolitis: etiology, presentation, management, and outcomes. J Perinat Neonatal Nurs 23: 87–94
Thompson AM, Bizzarro MJ 2008 Necrotizing enterocolitis in newborns: pathogenesis, prevention and management. Drugs 68: 1227–1238
Petrosyan M, Guner YS, Williams M, Grishin A, Ford HR 2009 Current concepts regarding the pathogenesis of necrotizing enterocolitis. Pediatr Surg Int 25: 309–318
Carter BM 2007 Treatment outcomes of necrotizing enterocolitis for preterm infants. J Obstet Gynecol Neonatal Nurs 36: 377–384
Gnecchi M, Melo LG 2009 Bone marrow-derived mesenchymal stem cells: isolation, expansion, characterization, viral transduction, and production of conditioned medium. Methods Mol Biol 482: 281–294
Caplan AI, Bruder SP 2001 Mesenchymal stem cells: building blocks for molecular medicine in the 21st century. Trends Mol Med 7: 259–264
Markel TA, Crisostomo PR, Lahm T, Novotny NM, Rescorla FJ, Tector J, Meldrum DR 2008 Stem cells as a potential future treatment of pediatric intestinal disorders. J Pediatr Surg 43: 1953–1963
Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM 2002 Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 418: 41–49
Caplan AI, Dennis JE 2006 Mesenchymal stem cells as trophic mediators. J Cell Biochem 98: 1076–1084
Haynesworth SE, Baber MA, Caplan AI 1996 Cytokine expression by human marrow-derived mesenchymal progenitor cells in vitro: effects of dexamethasone and IL-1 alpha. J Cell Physiol 166: 585–592
Pillekamp F, Reppel M, Brockmeier K, Hescheler J 2006 Stem cells and their potential relevance to pediatric cardiology. Cardiol Young 16: 117–124
Gardner SL 2004 Application of stem cell transplant for brain tumors. Pediatr Transplant 8: 28–32
Santner-Nanan B, Peek MJ, McCullagh P, Nanan R 2005 Therapeutic potential of stem cells in perinatal medicine. Aust N Z J Obstet Gynaecol 45: 102–107
van Haaften T, Byrne R, Bonnet S, Rochefort GY, Akabutu J, Bouchentouf M, Rey-Parra GJ, Galipeau J, Haromy A, Eaton F, Chen M, Hashimoto K, Abley D, Korbutt G, Archer SL, Thébaud B 2009 Airway delivery of mesenchymal stem cells prevents arrested alveolar growth in neonatal lung injury in rats. Am J Respir Crit Care Med 180: 1131–1142
Guven A, Gundogdu G, Vurucu S, Uysal B, Oztas E, Ozturk H, Korkmaz A 2009 Medical ozone therapy reduces oxidative stress and intestinal damage in an experimental model of necrotizing enterocolitis in neonatal rats. J Pediatr Surg 44: 1730–1735
Zani A, Eaton S, Leon FF, Malerba A, Hall NJ, De Coppi P, Smith VV, Pierro A 2008 Captopril reduces the severity of bowel damage in a neonatal rat model of necrotizing enterocolitis. J Pediatr Surg 43: 308–314
Uçkan D, Kilic E, Sharafi P, Kazik M, Kaya F, Erdemli E, Can A, Tezcaner A, Kocaefe C 2009 Adipocyte differentiation defect in mesenchymal stem cells of patients with malignant infantile osteopetrozis. Cytotherapy 11: 392–402
Amsalem Y, Mardor Y, Feinberg MS, Landa N, Miller L, Daniels D, Ocherashvilli A, Holbova R, Yosef O, Barbash IM, Leor J 2007 Iron-oxide labeling and outcome of transplanted mesenchymal stem cells in the infarcted myocardium. Circulation 116: I38–I45
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E 2006 Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8: 315–317
Caplan MS, Hedlund E, Adler L, Hsueh W 1994 Role of asphyxia and feeding in a neonatal rat model of necrotizing enterocolitis. Pediatr Pathol 14: 1017–1028
Jiang H, Qu L, Li Y, Gu L, Shi Y, Zhang J, Zhu W, Li J 2011 Bone marrow mesenchymal stem cells reduce intestinal ischemia/reperfusion injuries in rats. J Surg Res 168: 127–134
Crisostomo PR, Wang Y, Markel TA, Wang M, Lahm T, Meldrum DR 2008 Human mesenchymal stem cells stimulated by TNF-alpha, LPS, or hypoxia produce growth factors by an NF kappaB- but not JNK-dependent mechanism. Am J Physiol Cell Physiol 294: C675–C682
Weil BR, Markel TA, Herrmann JL, Abarbanell AM, Meldrum DR 2009 Mesenchymal stem cells enhance the viability and proliferation of human fetal intestinal epithelial cells following hypoxic injury via paracrine mechanisms. Surgery 146: 190–197
Jeschke MG, Bolder U, Finnerty CC, Przkora R, Muller U, Maihofer R, Thompson JC, Wolf SE, Herndon DN 2005 The effect of hepatocyte growth factor on gut mucosal apoptosis and proliferation, and cellular mediators after severe trauma. Surgery 138: 482–489
Rollwagen FM, Madhavan S, Singh A, Li YY, Wolcott K, Maheshwari R 2006 IL-6 protects enterocytes from hypoxia-induced apoptosis by induction of bcl-2 mRNA and reduction of fas mRNA. Biochem Biophys Res Commun 347: 1094–1098
Wang Y, Ahmad N, Wani MA, Ashraf M 2004 Hepatocyte growth factor prevents ventricular remodeling and dysfunction in mice via Akt pathway and angiogenesis. J Mol Cell Cardiol 37: 1041–1052
von Dobschuetz E, Meyer S, Thorn D, Marme D, Hopt UT, Thomusch O 2006 Targeting vascular endothelial growth factor pathway offers new possibilities to counteract microvascular disturbances during ischemia/reperfusion of the pancreas. Transplantation 82: 543–549
Wang Y, Haider HK, Ahmad N, Xu M, Ge R, Ashraf M 2006 Combining pharmacological mobilization with intramyocardial delivery of bone marrow cells over-expressing VEGF is more effective for cardiac repair. J Mol Cell Cardiol 40: 736–745
Gerber HP, Malik AK, Solar GP, Sherman D, Liang XH, Meng G 2002 VEGF regulates haematopoietic stem cell survival by an internal autocrine loop mechanism. Nature 417: 954–958
Ortiz LA, Dutreil M, Fattman C, Pandey AC, Torres G, Go K, Phinney DG 2007 Interleukin 1 receptor antagonist mediates the anti-inflammatory and anti-fibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci U S A 104: 11002–11007
Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, Giunti D, Ceravolo A, Cazzanti F, Frassoni F, Mancardi G, Uccelli A 2005 Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood 106: 1755–1761
Manieri NA, Stappenbeck TS 2011 Mesenchymal stem cell therapy of intestinal disease: are their effects systemic or localized?. Curr Opin Gastroenterol 27: 119–124
Allers C, Sierralta WD, Neubauer S, Rivera F, Minguell JJ, Conget PA 2004 Dynamic of distribution of human bone marrow-derived mesenchymal stem cells after transplantation into adult unconditioned mice. Transplantation 78: 503–508
François S, Bensidhoum M, Mouiseddine M, Mazurier C, Allenet B, Semont A, Frick J, Saché A, Bouchet S, Thierry D, Gourmelon P, Gorin NC, Chapel A 2006 Local irradiation not only induces homing of human mesenchymal stem cells at exposed sites but promotes their widespread engraftment to multiple organs: a study of their quantitative distribution after irradiation damage. Stem Cells 24: 1020–1029
Sordi V 2009 Mesenchymal stem cell homing capacity. Transplantation 87: S42–S45
Matsumoto T, Okamoto R, Yajima T, Mori T, Okamoto S, Ikeda Y, Mukai M, Yamazaki M, Oshima S, Tsuchiya K, Nakamura T, Kanai T, Okano H, Inazawa J, Hibi T, Watanabe M 2005 Increase of bone marrow-derived secretory lineage epithelial cells during regeneration in the human intestine. Gastroenterology 128: 1851–1867
Lee JA, Kim BI, Jo CH, Choi CW, Kim EK, Kim HS, Yoon KS, Choi JH 2010 Mesenchymal stem-cell transplantation for hypoxic-ischemic brain injury in neonatal rat model. Pediatr Res 67: 42–46
Ryan JM, Barry FP, Murphy JM, Mahon BP 2005 Mesenchymal stem cells avoid allogeneic rejection. J Inflamm (Lond) 2: 8
Niemeyer P, Vohrer J, Schmal H, Kasten P, Fellenberg J, Suedkamp NP, Mehlhorn AT 2008 Survival of human mesenchymal stromal cells from bone marrow and adipose tissue after xenogenic transplantation in immunocompetent mice. Cytotherapy 10: 784–795
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by a Grant of Fatih University Scientific Research Commity and Hacettepe University PEDI-STEM Center for Stem Cell Research and Development with a Project number DPT 2006K 120 640-06-01.
The authors report no conflicts of interest.
Rights and permissions
About this article
Cite this article
Tayman, C., Uckan, D., Kilic, E. et al. Mesenchymal Stem Cell Therapy in Necrotizing Enterocolitis: A Rat Study. Pediatr Res 70, 489–494 (2011). https://doi.org/10.1203/PDR.0b013e31822d7ef2
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/PDR.0b013e31822d7ef2
This article is cited by
-
Dedifferentiated fat cells administration ameliorates abnormal expressions of fatty acids metabolism-related protein expressions and intestinal tissue damage in experimental necrotizing enterocolitis
Scientific Reports (2023)
-
Necrotizing enterocolitis: recent advances in treatment with translational potential
Pediatric Surgery International (2023)
-
Stem cell therapy as a promising strategy in necrotizing enterocolitis
Molecular Medicine (2022)
-
Amniotic fluid stem cell administration can prevent epithelial injury from necrotizing enterocolitis
Pediatric Research (2022)
-
Stem cells and exosomes: promising candidates for necrotizing enterocolitis therapy
Stem Cell Research & Therapy (2021)








 , NEC + MSCs.
, NEC + MSCs.