Abstract
The developmentally regulated hemodynamic effects of vasoactive medications have not been well characterized. We used traditional and near-infrared spectroscopy monitoring technologies and investigated the changes in heart rate, blood pressure, common carotid artery (CCA) blood flow (BF), cerebral, renal, intestinal, and muscle regional tissue O2 saturation, and acid-base and electrolyte status in response to escalating doses of vasoactive medications in normotensive anesthetized neonatal piglets. We used regional tissue O2 saturation and CCA BF as surrogates of organ and systemic BF, respectively, and controlled minute ventilation and oxygenation. Low to medium doses of dopamine, epinephrine, dobutamine, and norepinephrine increased blood pressure and systemic and regional BF in a drug-specific manner, whereas milrinone exerted minimal effects. At higher doses, dopamine, epinephrine, and norepinephrine but not dobutamine decreased systemic, renal, intestinal, and muscle BF, while cerebral BF remained unchanged. Epinephrine induced significant increases in muscle BF and serum glucose and lactate concentrations. The findings reveal novel drug- and dose-specific differences in the hemodynamic response to escalating doses of vasoactive medications in the neonatal cardiovascular system and provide information for future clinical studies investigating the use of vasoactive medications for the treatment of neonatal cardiovascular compromise.
Similar content being viewed by others
Main
Cardiovascular compromise is a frequently encountered condition in the critically ill preterm and term infant (1) resulting in inadequate tissue O2 delivery, impaired cerebral blood flow (CBF) autoregulation, and, potentially, end-organ injury and death (1–3). Our limited ability to accurately monitor changes in neonatal hemodynamics curtails timely recognition, and thus treatment, of neonatal shock.
Although vasopressor-inotropes, inotropes, and lusitropes have been used to manage neonatal cardiovascular compromise with an attempt to tailor the treatment to the suspected primary etiology (1), there is only limited information available on the safety and effectiveness of these medications (1,4) and little is known about their developmentally regulated dose-dependent hemodynamic actions (4,5). Recent advances in bedside hemodynamic monitoring techniques using, among others, near-infrared spectroscopy (NIRS) have made continuous, noninvasive monitoring of tissue O2 delivery (6) possible. Accordingly, data on tissue O2 delivery and utilization (7) and regional O2 saturation in critically ill adults, children, and, more recently, neonates have become available (2,3,8–10).
To gain insight into the specific, drug-related changes in neonatal hemodynamics, we used traditional and NIRS hemodynamic monitoring technologies and investigated the changes in heart rate, blood pressure (BP), common carotid artery (CCA) blood flow (BF), cerebral regional tissue O2 saturation (CrSO2), renal (kidney) regional tissue O2 saturation (KrSO2), intestinal (gut) regional tissue O2 saturation (GrSO2), muscle regional tissue O2 saturation (MrSO2), and acid-base and electrolyte status in response to escalating doses of vasopressor-inotropes, inotropes, and lusitropes in normotensive anesthetized neonatal piglets.
MATERIALS AND METHODS
Surgical preparation and laboratory measurements.
Neonatal Yorkshire Duroc piglets of 10 ± 3 d of age weighing 2.4 ± 0.6 kg were preanesthetized with ketamine (33 mg/kg) and atropine (0.05 mg/kg), intubated, and anesthetized using 1.5-3.0% isofluorane while mechanically ventilated (Draeger Medical Apollo Anesthesia Machine; Draeger, Germany). Core body temperature was maintained at 38 ± 0.5°C and BP, heart rate, arterial O2 saturation (SpO2; Datex-Ohmeda, GE Healthcare, Milwaukee, WI), end-tidal CO2, fraction of inspired O2, and pulmonary compliance (Medical Apollo Anesthesia Machine; Draeger, Germany) were continuously monitored. Heart rates beyond 250 bpm were captured by the PC-Vet wireless ECG system (Vmed Technology, Mill Creek, WA) or, rarely, by direct counting. Following skin preparation, NIRS sensors were placed on the head, the dorsal abdomen over the right kidney, the abdomen lateral to the umbilicus, and the right leg over the gluteus muscle, and regional tissue O2 saturation (rSO2) of the brain, kidney, gut, and muscle was monitored continuously by an INVOS regional tissue oxygenation monitor (INVOS 5100C, Somanetics, Troy, MI).
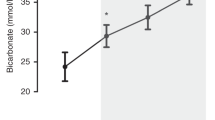
Serum electrolytes, glucose, lactate and Hb concentration, and arterial blood gasses were followed by iSTAT (Abbott Point of Care, Inc., Princeton, NJ) as per the experimental protocol (Fig. 1). Animals received i.v. heparin (200 units/kg/h), physiologic saline (10 mL/kg/h), and 10 g/dL of dextrose-water to adjust serum glucose levels using Genie Plus syringe pumps validated for stability of infusion rate (Kent Scientific, Torrington, CT). Serum electrolytes, pH (7.20–7.45), base excess (−6 to + 6), partial arterial O2 pressure (Pao2; 80–120 mm Hg) and partial arterial CO2 pressure (Paco2; 35–45 mm Hg), and SpO2 (90–100%) were maintained in the target ranges using sodium bicarbonate, potassium chloride, lactated Ringer's solution, and ventilatory changes, respectively.
Experimental protocol. *, CCA BF was not monitored in all animals; **, ABGs were obtained every 15 or 30 min to ensure that pH, Paco2, and base excess were within the target ranges. ABG, arterial blood gas; HR, heart rate; ETCO2, end-tidal CO2; FiO2, fraction of inspired oxygen; MABP, mean arterial BP; MAC, mean alveolar concentration (of anesthetics). See text for details.
Both femoral veins and left femoral artery were cannulated for fluid and drug administration and arterial BP measurements and blood sampling, respectively. In three of the seven animals in the studies with dopamine, epinephrine, dobutamine, and milrinone, and in all four animals in the norepinephrine experiments, the CCA was instrumented for continuous BF measurements using an ultrasonic flow-meter (T400; Transonic Systems, Inc., Ithaca, NY). Urine output was not measured because of technical difficulties associated with catheterization of newborn piglets. Data were collected real time at sampling rates between 9 and 240 samples/min and stored on a computer for offline analysis (The RugLoop Program, Dmed, Temse, Belgium).
Experimental protocols.
The study was approved by the Institutional Animal Care and Use Committee at Providence Hospital, Detroit, MI.
Stability studies.
Four piglets without CCA flow measurements were monitored for 4 h to document cardiovascular stability of the preparation.
Dose-escalation studies.
Changes in the hemodynamic parameters and serum electrolyte, lactate, and glucose and Hb concentration were followed during graded doses of selected vasoactive medications. After a 15-min postsurgical stabilization period, baseline measurements were recorded, followed by dose escalation and a 15-min washout period after discontinuation of the medication (Fig. 1). Except for milrinone, each drug dose was given for 15 min.
-
Group 1 (seven animals): dopamine was administered in concentrations of 5, 10, 15, 20, 25, and 30 μg/kg/min.
-
Group 2 (seven animals): epinephrine was administered in concentrations of 0.25, 0.5, 0.75, 1.0, 1.5, and 2.0 μg/kg/min.
-
Group 3 (four animals): norpinephrine was administered in concentrations of 0.25, 0.5, 0.75, 1.0, 1.25, and 1.5 μg/kg/min.
-
Group 4 (seven animals): dobutamine was administered in concentrations of 5, 10, 15, 20, 25, and 30 μg/kg/min.
-
Group 5 (six animals): milrinone was administered as a bolus of 50 μg/kg over 15 min followed by continuous infusions of 0.375 μg/kg/min and 0.75 μg/kg/min, each given for 30 min.
Statistical analysis
Hemodynamic parameters.
A computer code in MatLab (MathWorks, Natick, MA) was written for loading, processing, and statistical analysis of the data from the RugLoop program.
The mean values of the hemodynamic parameters were first calculated for every 1-min block followed by calculation of the mean values of the last 5 min of each dose block. We assumed that the last 5 min of each dose block represented the equilibrium of the effects at each dose most accurately. ANOVA was used to determine statistical significance among the mean values of the last 5-min blocks. If these findings were statistically significant, a pairwise comparison using Tukey-multiple comparison was performed.
To determine the rate of change of the hemodynamic parameters, the data obtained were subdivided into “baseline,” “low dose” (first drug dose), “medium dose” (second and third drug doses), “high dose” (fourth-sixth drug doses), and “postmedication” blocks. The slopes of changes in each block were calculated from the best linear fit to the data (MatLab-Regression Diagnostics, Natick, MA), and the R2 value for the goodness of fit was determined. The slopes between BP and CrSO2, BP and CCA BF, and CCA BF and CrSO2 were tested for statistical difference using F-statistics (MatLab-Linear Hypothesis Test; Natick, MA). The p < 0.05 was considered significant.
Laboratory measurements.
Data are given as mean ± SD unless indicated otherwise. ANOVA for repeated measures (ANOVA-RM. SPSS, Chicago, IL, release 18.0.0) was used where more than two datasets were available for the given parameter. In case of statistical significance, a pairwise comparison with Bonferroni adjustment was performed. For laboratory tests with two measurements across the dose-escalation experiments, paired t test was used. The p < 0.05 was considered significant.
RESULTS
Stability studies.
In the four animals studied to assess the stability of the preparation over time, all hemodynamic parameters monitored (data not shown) and, except for the initial serum glucose concentration, all laboratory parameters remained stable and within the target range for 4 h (Table 1; serum electrolytes and Hb values not shown). Based on these findings, the initial glucose delivery rate was adjusted in the dose-escalation experiments.
Dose-escalation studies
Group 1.
Dopamine (5–30 μg/kg/min) caused dose-dependent increases in heart rate, BP, CrSO2, KrSO2, GrSO2, and CCA flow (Fig. 2A). However, the pattern of changes was different among the hemodynamic parameters. The increase in BP and CrSO2 paralleled at the low- and medium-dose blocks (p = 0.7 and 0.19, respectively). In the high-dose block (20–30 μg/kg/min), the slopes of BP and CrSO2 differed as BP tended to increase while CrSO2 remained unchanged (p < 0.001). At medium doses, CCA BF increased out of proportion of the increase in BP (p < 0.001) and slightly decreased in the high-dose block, whereas BP continued to rise (p < 0.001). At the lowest dose, KrSO2 and GrSO2 increased significantly and out of proportion of the change in BP. Among all drugs tested, dopamine increased GrSO2 the most (Fig. 3C). Higher doses of dopamine tended to attenuate the increases in KrSO2 more than in GrSO2. No apparent changes in MrSO2 were noted. On discontinuation of dopamine, all values returned toward or below baseline. The same pattern was seen in the washout phase with the other medications. Among the laboratory parameters, only serum glucose (Table 1) and Hb increased.
Hemodynamic effects of escalating doses of dopamine (A), epinephrine (B), norepinephrine (C), dobutamine (D), and milrinone (E). Changes in heart rate (HR; 1/min; shown as actual rate divided by 10), mean BP (MBP; mm Hg), and CCA BF (mL/min) on the right y axis and CrSO2 (%), KrSO2 (%), GrSO2 (%), and MrSO2 (%) on the left y axis are shown before, during, and after the experiments with dopamine (A; n = 7; CCA-flow = 3), epinephrine (B; n = 7; CCA-flow = 3), norepinephrine (C; n = 4; CCA-flow = 4), dobutamine (D; n = 7; CCA-flow = 3), and milrinone (E). Except for milrinone, each dose was given for 15 min, and the escalating doses (mcg/kg/min) are shown in each panel above the x axis in colored boxes. See text for details. CCA-BF (black); CrSO2 (blue); GrSO2 (gold); heart rate (HR) × 10 (gray); KrSO2, (green); mean BP (MBP) (white); MrSO2 (red).
Relative organ-specific changes in rSO2 in response to infusion of escalating doses of vasoactive medications. Relative changes compared with baseline in CrSO2 (A), KrSO2 (B), GrSO2 (C), and MrSO2 (D) (y axis) in response to advancing doses of dopamine (brown), epinephrine (green), norepinephrine (yellow), dobutamine (blue), and milrinone (gold) are shown (time in hours on the x axis). The relative protection of CBF from vasoconstriction is suggested by the lack of a decrease in CrSO2 at high doses of vasopressor-inotropes (A). See text for details.
Group 2.
Epinephrine (0.25-2 μg/kg/min) increased heart rate in a dose-dependent manner. At lower doses, BP, CCA flow, CrSO2, KrSO2, GrSO2, and MrSO2 increased significantly (Fig. 2B), and the increase in KrSO2 was similar to that seen with low to medium doses of dopamine (Fig. 3B). As with dopamine, the increase in BP and CrSO2 paralleled in the low- and medium-dose blocks (p = 0.108 and 0.995, respectively). At higher doses of epinephrine, CCA flow, KrSO2, GrSO2, and MrSO2 gradually decreased; CrSO2 remained unchanged; and BP continued to rise (BP versus CrSO2 slope; p < 0.001). Among the medications tested, low, medium, and high doses of epinephrine caused the most significant increase in MrSO2, CCA flow, and BP, respectively (Fig. 3D). BP and CCA flow showed a low-frequency high-amplitude oscillatory pattern. KrSO2, GrSO2, and MrSO2 followed the oscillations but CrSO2 did not. Serum glucose and lactate and Hb increased, and pH and base excess decreased (Table 1). Epinephrine caused the most significant increase in serum glucose and lactate (Table 1).
Group 3.
At 0.25 μg/kg/min, norepinephrine did not affect the hemodynamic parameters (Fig. 2C). At 0.5 μg/kg/min the drug abruptly increased heart rate, BP, CCA BF, CrSO2, and MrSO2 and to a lesser extent GrSO2 and KrSO2. Among the medications studied, norepinephrine caused the most abrupt and single-dose-related increase in BP, CCA BF, and CrSO2 (Fig. 2C). In the medium- and high-dose blocks, the slopes of CrSO2 and BP did not differ (p = 0.36 and 0.31, respectively). At higher doses, CCA flow, KrSO2, GrSO2, and MrSO2 were significantly attenuated. In the high-dose block, the BP tracing revealed a low-frequency oscillatory pattern with variable amplitude and without changes in the baseline (slope = 0.06 ± 0.01). As with epinephrine, CCA flow, GrSO2, and KrSO2, but not CrSO2, mirrored the oscillations on BP. Serum chloride and glucose and Hb increased, while serum lactate trended to increase (Table 1).
Group 4.
Dobutamine (5–30 μg/kg/min) increased heart rate in a dose-dependent manner (Fig. 2D). At doses of 5 and 10 μg/kg/min, CrSO2, KrSO2, GrSO2, and BP increased in a dose-dependent manner. The slopes of BP and CrSO2 and BP and CCA flow were different in the medium- and high-dose blocks (p < 0.001 for both comparisons). BP and CCA flow increased abruptly at 10 μg/kg/min followed by small increases in CCA flow but not in BP. Although the magnitude of the BP increase was similar to that seen with dopamine, the dobutamine-induced increases in CCA flow, GrSO2, and KrSO2 were smaller (Figs. 3A–C). No significant changes in MrSO2 occurred while serum glucose and Hb slightly increased (Table 1).
Group 5.
The 50 μg/kg bolus of milrinone caused a slight increase in heart rate, CCA BF, KrSO2 GrSO2, and MrSO2 (Fig. 2E). During the dose-escalation phase, BP, heart rate, and CCA flow decreased toward or slightly below baseline, CrSO2, KrSO2, and MrSO2 remained unchanged and MrSO2 continued to slightly increase (Fig. 2E). As BP tended to decrease during the dose-escalation phase, a significant difference between the slopes of BP and CrSO2 was noted in the high-dose block (p < 0.001). All laboratory parameters remained stable (Table 1).
DISCUSSION
To our knowledge, this is the first study investigating the developmentally regulated dose-dependent effects of dopamine, epinephrine, norepinephrine, dobutamine and milrinone on heart rate, BP, systemic and organ BF, O2 delivery, and acid-base and electrolyte status. Our findings reveal significant drug- and dose-specific differences in the hemodynamic and metabolic response among these vasoactive medications in normotensive anesthetized neonatal piglets.
We measured CCA BF directly and assessed BF changes to the brain, kidneys, intestine, and muscle by monitoring rSO2, using NIRS. As long as metabolic rate and oxygenation remain unchanged, changes in rSO2 reflect changes in regional BF (7,11). In our study, SpO2 and Pao2 remained unchanged and, with the exception of epinephrine (12), metabolic rate is unlikely to have changed during the experiments. Therefore, we used rSO2 as a surrogate of regional BF. Finally, because we kept Paco2 and Pao2, the most potent acute regulators of CBF (13) constant, we could assess the direct effects of vasoactive medications on CBF.
Dopamine exerts its complex cardiovascular, renal, and endocrine effects by the dose-dependent stimulation of the dopaminergic (low doses), beta-adrenergic (medium doses), alpha-adrenergic (high doses), and serotoninergic receptors (4). In our normotensive anesthetized neonatal piglet model, the drug caused the expected dose-dependent changes in heart rate, BP, and renal and mesenteric BF (4,14,15). Although selective dopaminergic renal and mesenteric vasodilation has been consistently documented in animals and humans across the spectrum of development (4,14,15), dopamine, between 2 and 32 μg/kg/min, did not affect renal and mesenteric BF in chronically instrumented, awake piglets (16). The reason for this finding is unclear but chronic instrumentation of the renal and superior mesenteric artery might have affected vasoreactivity in these vessels.
Because the changes in CrSO2 and BP paralleled during low and medium doses of dopamine, CBF autoregulation was impaired in the anesthetized piglets. This finding is explained by the anesthesia-induced impairment of this protective vascular mechanism (17). The increase in CCA BF out of proportion of the increase in BP and CrSO2 at low to medium doses suggests an increase in cardiac output and, perhaps, a selective dopaminergic vasodilation in the extracranial vessels of the neck and head (4). The findings that, at the highest doses, BP increased out of proportion to CrSO2, whereas KrSO2, GrSO2, and CCA BF decreased suggest an alpha-adrenoreceptor-mediated peripheral vasoconstriction resulting in a decrease in cardiac output (4), a relative sparing of the cerebral vasculature from vasoconstriction and an impaired yet not completely diminished CBF autoregulation (4,13). The finding that the increase in GrSO2 was greater at low to medium doses and was less attenuated at higher doses than that in KrSO2 indicates that, similar to the immature rat (18) and unlike in the preterm neonate (15), dopamine induces a more pronounced mesenteric than renal vasodilation in the newborn piglet. These findings point to important interspecies differences in the hemodynamic response to dopamine. The small increase in MrSO2 at low to medium doses of dopamine and the moderate increase in serum glucose suggest a limited peripheral β2-adrenoreceptor stimulatory effect of the drug.
Dopamine had no effect on blood gasses and serum lactate and electrolytes. We speculate that the hemoconcentration observed by the end of the experiments was the consequence of increased urine output caused by both the hemodynamic and direct renal effects of dopamine (4,14). The observation that hemoconcentration only occurred with drugs causing both an increase in BP and adrenoreceptor stimulation (dopamine, epinephrine, norepinephrine, and dobutamine but not milrinone), supports this speculation.
Epinephrine exerts its complex cardiovascular, renal, and endocrine effects primarily by the dose-dependent stimulation of beta- and alpha-adrenergic receptors (19). As with dopamine, at low doses of epinephrine, there was evidence of impaired CBF autoregulation and an increase in renal and mesenteric BF and cardiac output (17). However, CBF autoregulation was not completely abolished because CrSO2 did not mirror the fluctuations in BP, whereas CCA BF did. Based on the attenuated fluctuations in KrSO2, GrSO2, and MrSO2, BF autoregulation functioned at the level expected for these organs. The fluctuations in BP might have been due to rapid desensitization of adrenergic receptors (1,20) and a myogenic and peripheral autonomic nervous system response to the abrupt increases in perfusion pressure. The significant increase in muscle BF at low and medium doses and the increase in serum glucose and lactate are due to the high affinity of epinephrine to β2-adrenoreceptors (18) and underscore the physiological role of epinephrine in assisting the “fight-or-flight” response during stress (12). At the highest dose range, the alpha-adrenoreceptor-mediated vasoconstriction resulted in a decrease in BF to all organs but the brain, indicating a preservation of brain BF even at very high doses of epinephrine. Because BP continued to rise while CCA BF declined, cardiac output likely also decreased somewhat at high doses. These findings demonstrate a relative sparing of the cerebral vasculature from vasoconstriction and that epinephrine is a more potent vasopressor than dopamine (4,12,19).
The increase in serum glucose and lactate and the associated acidosis were because of the enhanced gluconeogenesis caused by the drug-induced β2-adrenoreceptor stimulation (21). The lactic acidosis during low- to medium-dose epinephrine administration is not caused by excessive vasoconstriction (22) and has been described in neonatal piglets (23) and preterm neonates (24).
Norepinephrine exerts its complex cardiovascular and endocrine effects by the dose-dependent stimulation of beta- and alpha-adrenergic receptors (19). Compared with epinephrine, norepinephrine has more enhanced alpha- and minimal peripheral vascular beta adrenoreceptor stimulatory effects (19). Accordingly, the major differences in the hemodynamic response between norepinephrine and epinephrine are explained by the overwhelming peripheral vasoconstrictive actions of norepinephrine at medium-to high doses as BF to all organs but the brain decreased and CCA flow paralleled the decline in BP. Interestingly though, at a narrow dose range at low doses, norepinephrine increased BP and CCA and regional organ BFs. This finding maybe of clinical importance and suggests that low-dose norepinephrine increases cardiac output more than systemic vascular resistance even in the immature cardiovascular system. Because of its relatively decreased affinity to β2-adrenoreceptors, norepinephrine increased serum glucose levels less than epinephrine (19). The cause of the small but statistically significant increase in serum chloride concentration is unclear.
Dobutamine has relatively selective cardiac beta- and alpha-adrenoreceptor stimulatory effects (25). Accordingly, dobutamine administration results in direct inotropic and chronotropic actions and a variable decrease in systemic vascular resistance (19,25). Low and medium doses of dobutamine caused small increases in BP and relatively larger increases in CCA flow indicating an increase in cardiac output. Perhaps of clinical relevance is the finding that renal and mesenteric BF increased as much as by low-dose epinephrine. The modest increase in serum glucose and muscle BF suggests a weak affinity of dobutamine to β2-adrenoreceptors.
Relatively selective phosphodiesterase type-III inhibitors such as milrinone exert their cardiovascular effects (positive inotropy, improved diastolic function, and vasodilation) primarily by increasing intracellular cAMP concentrations (26). Their positive inotropic effect has been documented in mature but not newborn (27) animals and children and adults (26,28). Milrinone exerted minimal hemodynamic and no metabolic effects in the normotensive piglets. The unchanged CBF in face of the slight decrease in BP suggests that cardiac output was maintained and CBF autoregulation was effective within the range of the BP changes. Interestingly, a recent study found that milrinone is as effective as epinephrine or dobutamine in improving cardiac output and systemic BP in asphyxiated neonatal piglets (29).
Although we controlled for the most important acute regulators of organ, especially brain BF, such as pH, Paco2, and Pao2, our study has several limitations. First, we used rSO2 as a surrogate of organ BF. We did this because oxygenation was maintained constant during the experiments and, except for epinephrine, which may have increased basic metabolic rate by 7-15% (12), metabolic rate is unlikely to have changed with the use of the other medications. Furthermore, because we documented a varying pattern of changes in rSO2 among the different drugs, it is unlikely that these rapid organ-specific changes in rSO2 were caused by changes in metabolic activity rather than in BF. Second, because cardiac output was not directly measured, changes in CCA BF were used as a surrogate to changes in cardiac output. As regional hemodynamic effects such as changes in CBF also affect CCA BF, in the discussion we always refer to changes in systemic BF in context of the changes in CBF. However, these interpretations must be viewed with caution. Third, direct measurement of CCA BF was not performed in all animals except for the experiments with norepinephrine. However, as the drug-specific hemodynamic response was similar among the animals in each group, it is unlikely that the CCA BF findings would have been affected by enrolling more animals with direct CCA BF measurements. Finally, because we used normotensive newborn piglets to investigate the hemodynamic effects of vasoactive medications, our findings may not fully apply to a neonatal animal model with cardiovascular compromise (29). To address this concern, we have recently completed a study investigating the effects of these vasoactive agents in neonatal piglets with endotoxin-induced shock (manuscript in preparation).
In summary, we have identified novel interactions between systemic BP and systemic and organ BF in response to escalating doses of vasoactive medications in normotensive anesthetized neonatal piglets. At low to medium doses, systemic BF and BP increased with dopamine, epinephrine, and dobutamine and, in a narrow dose range, with norepinephrine. We also found that the renal and mesenteric hemodynamic effects of dopamine, epinephrine, and dobutamine are similar although not identical. Among the metabolic effects, the epinephrine-induced hyperglycemia and, especially, lactic acidosis are of clinical relevance as the drug-induced lactic acidosis (22–24) prevents the use of serial serum lactate levels as an indirect marker of changes in BF during epinephrine administration. Finally, because of the interspecies differences, caution must be exercised when extrapolating the findings on systemic and regional hemodynamics to the human neonate. Accordingly, although the information obtained in this study is useful for the design of interventional clinical trials, future studies using complex hemodynamic monitoring approaches (30) in critically ill neonates need to confirm these findings before the information provided here can be applied to the clinical practice.
Abbreviations
- BF:
-
blood flow
- BP:
-
blood pressure
- CBF:
-
cerebral blood flow
- CCA:
-
common carotid artery
- CrSO2 KrSO2 GrSO2 MrSO2:
-
regional tissue O2 saturation of brain, kidney, gut, and muscle, respectively
- NIRS:
-
near-infrared spectroscopy
- PaO2:
-
partial arterial O2 pressure
- Paco2:
-
partial arterial CO2 pressure
- rSO2:
-
regional tissue O2 saturation
- SpO2:
-
arterial O2 saturation
References
Seri I 2001 Circulatory support of the sick newborn infant. Semin Neonatol 6: 85–95
Tsuji M, Saul P, du Plessis A, Eichenwald E, Sobh J, Crocker R, Volpe J 2000 Cerebral intravascular oxygenation correlates with mean arterial pressure in critically ill premature infants. Pediatrics 106: 625–632
O'Leary H, Gregas MC, Limperopoulos C, Zaretskaya I, Bassan H, Soul JS, Di Salvo DN, du Plessis AJ 2009 Elevated cerebral pressure passivity is associated with prematurity-related intracranial hemorrhage. Pediatrics 124: 302–309
Seri I 1995 Cardiovascular, renal and endocrine actions of dopamine in neonates and children. J Pediatr 126: 333–344
Seri I, Noori S 2005 Diagnosis and treatment of newborn hypotension outside the transitional period. Early Hum Dev 81: 405–411
Rolfe P 2000 In vivo near-infrared spectroscopy. Annu Rev Biomed Eng 2: 715–754
Nagdyman N, Ewert P, Peters B, Miera O, Fleck T, Berger F 2008 Comparison of different near-infrared spectroscopic cerebral oxygenation indices with central venous and jugular venous oxygenation saturation in children. Paediatr Anaesth 18: 160–166
Toet MC, Lemmers PM, van Schelvenb LJ, van Bel F 2006 Cerebral oxygenation and electrical activity after birth asphyxia: their relation to outcome. Pediatrics 117: 333–339
Lemmers PM, Toet MC, van Bel F 2008 Impact of patent ductus arteriosus and subsequent therapy with indomethacin on cerebral oxygenation in preterm infants. Pediatrics 121: 142–147
Wong FY, Barfield CP, Horne RS, Walker AM 2009 Dopamine therapy promotes cerebral flow-metabolism coupling in preterm infants. Intensive Care Med 35: 1777–1782
Sakamoto T, Jonas RA, Stock UA, Hatsuoka S, Cope M, Springett RJ, Nollert G 2001 Utility and limitations of near-infrared spectroscopy during cardiopulmonary bypass in a piglet model. Pediatr Res 49: 770–776
Berne RM, Levy MN, Koeppen BM, Stanton BA 2004 Physiology. Elsevier-Mosby Co: St. Louis, MO pp 912–914
Greisen G 2005 Autoregulation of cerebral blood flow in newborn babies. Early Hum Dev 81: 423–428
Felder RA, Felder CC, Eisner GM, Jose PA 1989 The dopamine receptor in adult and maturing kidney. Am J Physiol 257: F315–F327
Seri I, Abbasi S, Wood DC, Gerdes JS 1998 Regional hemodynamic effects of dopamine in the sick preterm infant. J Pediatr 133: 728–734
Pearson RJ, Barrington KJ, Jirsch DW, Cheung PY 1996 Dopaminergic receptor-mediated effects in the mesenteric vasculature and renal vasculature of the chronically instrumented newborn piglet. Crit Care Med 24: 1706–1712
Dagal A, Lam AM 2009 Cerebral autoregulation and anesthesia. Curr Opin Anaesthesiol 22: 547–552
Seri I, Eklöf AC, Aperia A 1987 Role of dopamine2 receptors in mediating the renal vascular response to low-dose dopamine infusion in the rat. Acta Physiol Scand 130: 563–569
Seri I 2006 Management of hypotension and low systemic blood flow in the very low birth weight neonate during the first postnatal week. J Perinatol 26: S8–S13
Hausdorff WP, Caron MG, Lefkowitz RJ 1990 Turning off the signal: desensitization of beta-adrenergic receptor function. FASEB J 4: 2881–2889
Reverte M, Garcia-Barrado MJ, Moratinos J 1991 Changes in plasma glucose and lactate evoked by α- and β2-adrenoceptor stimulation in conscious fasted rabbits. Fundam Clin Pharmacol 5: 663–676
Totaro RJ, Raper RF 1997 Epinephrine-induced lactic acidosis following cardiopulmonary bypass. Crit Care Med 25: 1693–1699
Cheung PY, Barrington KJ, Pearson RJ, Bigam DL, Finer NN, Van Aerde JE 1997 Systemic, pulmonary and mesenteric perfusion and oxygenation effects of dopamine and epinephrine. Am J Respir Crit Care Med 155: 32–37
Pellicer A, Valverde E, Elorza MD, Madero R, Gayá F, Quero J, Cabañas F 2005 Cardiovascular support for low birth weight infants and cerebral hemodynamics: a randomized, blinded, clinical trial. Pediatrics 115: 1501–1512
Bhatt-Mehta V, Nahat MC 1989 Dopamine and dobutamine in pediatric therapy. Pharmacotherapy 9: 303–314
Movsesian MA, Alharethi R 2002 Inhibitors of cyclic nucleotide phosphodiesterase PDE3 as adjunct therapy for dilated cardiomyopathy. Expert Opin Investig Drugs 11: 1529–1536
Akita T, Joyner RW, Lu C, Kumar R, Hartzell HC 1994 Developmental changes in modulation of calcium currents of rabbit ventricular cells by phosphodiesterase inhibitors. Circulation 90: 469–478
Hoffman TM, Wernovsky G, Atz AM, Kulik TJ, Nelson DP, Chang AC, Bailey JM, Akbary A, Kocsis JF, Kaczmarek R, Spray TL, Wessel DL 2003 Efficacy and safety of milrinone in preventing low cardiac output syndrome in infants and children after corrective surgery forcongenital heart disease. Circulation 107: 996–1002
Joynt C, Bigam DL, Charrois G, Jewell LD, Korbutt G, Cheung PY 2010 Milrinone, dobutamine or epinephrine use in asphyxiated newborn pigs resuscitated with 100% oxygen. Intensive Care Med 36: 1058–1066
Soleymani S, Borzage M, Seri I 2010 Hemodynamic monitoring in neonates: advances and challenges. J Perinatol 30: S38–S45
Acknowledgements
We thank C. Jane Tavare, M.S., for her help with the statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by an independent research grant from Somanetics Corporation (Somanetics, Troy, MI), an independent educational grant from the Clinica Alemana de Santiago (Santiago de Chile, Chile), and the Center for Fetal and Neonatal Medicine, Children's Hospital of Los Angeles and the Keck School of Medicine, University of Southern California, Los Angeles, CA.
The authors report no conflicts of interest.
Rights and permissions
About this article
Cite this article
Nachar, R., Booth, E., Friedlich, P. et al. Dose-Dependent Hemodynamic and Metabolic Effects of Vasoactive Medications in Normotensive, Anesthetized Neonatal Piglets. Pediatr Res 70, 473–479 (2011). https://doi.org/10.1203/PDR.0b013e31822e178e
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/PDR.0b013e31822e178e
This article is cited by
-
Consequence of insertion trauma – effect on early measurements when using intracerebral devices
Scientific Reports (2019)
-
Dobutamine treatment reduces inflammation in the preterm fetal sheep brain exposed to acute hypoxia
Pediatric Research (2018)
-
Splanchnic NIRS monitoring in neonatal care: rationale, current applications and future perspectives
Journal of Perinatology (2018)
-
Hemodynamic and metabolic effects of a new pediatric dobutamine formulation in hypoxic newborn pigs
Pediatric Research (2017)
-
Baroreflex dysfunction in sick newborns makes heart rate an unreliable surrogate for blood pressure changes
Pediatric Research (2016)