Abstract
To evaluate bladder function in infants with antenatally diagnosed hydronephrosis (ANH) using dynamic ultrasound protocol. Forty consecutive male infants (mean, 0.25 y) with ANH and 33 age-matched normal controls (mean, 0.49 y) were recruited. Anteroposterior (AP) diameter of renal pelvis (RP) and hydronephrosis index [HI = anteroposterior diameter of RP of kidney divided by urinary bladder volume (BV)] were calculated. Maximum BV (MaxBV) was determined just before voiding. Residual volume (RV) and bladder wall thickness (BWT) were measured after spontaneous voiding. Thirty-one infants (77.5%) showed persistently dilated RP postnatally in which 12 (39%) showed significantly high HI. In general, ANH infants had smaller MaxBV (30.71 versus 52.45 mL), larger residual volume (2.47 versus 1.93 mL), and larger BWT (4.4 versus 3.7 mm) than normal (p < 0.05, Mann-Whitney test). Infants with abnormally high HI had significantly more disturbed bladder parameters [smaller MaxBV (23.33 versus 33.49 mL) and larger BWT (4.67 versus 3.79 mm)] than the normal HI group (p < 0.05, Mann-Whitney test). Abnormal functional bladder parameters were evident in ANH infants. We postulated that immature function in the pelviureteric junction was associated with bladder dysfunction in these infants. Dynamic ultrasound protocol might help to understand the underlying pathophysiology of urinary system in ANH infants.
Similar content being viewed by others
Main
There has been a broad interest in both antenatal and postnatal evaluation of infants with antenatally diagnosed hydronephrosis (ANH). A wide range of ultrasound studies have been published, which include discriminating the degree of renal pelvis (RP) dilatation between normal and pathologic condition; monitoring degree of hydronephrosis which remains static, resolved, or progressing; and detecting the presence of any obstruction or congenital anomalies of the urinary tract (1–6). Lots of effort has also been put on management of these infants (1–3).
Postnatal ultrasound is essential to confirm and determine whether ANH is related to urinary tract obstruction. In the postnatal assessment, a number of infants are found to have resolution of ANH, whereas in some infants, vesicoureteric reflux (VUR) is revealed as the contributory factor of ANH. The status of urinary bladder is found to be an important confounding factor in detection of pelvicalyceal dilatation both antenatally (4–6) and postnatally (7). Recently, we have established a nomogram of hydronephrosis index (HI) for fetal ultrasound examinations at different gestations. HI is defined as the anteroposterior (AP) diameter of the RP in a fetal kidney divided by the urinary bladder volume (BV). We propose that this index can be used as a new physiologic reference for assessment of fetal hydronephrosis by eliminating the confounding effect of a distended fetal bladder (8). Our group has also demonstrated that dynamic ultrasound protocol is useful in the assessment of bladder dysfunction in children with primary nocturnal enuresis (9–11) and urinary tract infection (UTI) (12). Recently, this protocol has been applied to study infants with UTI successfully (13). To the best of our knowledge, data on sonographic evaluation of bladder function in infants with ANH are lacking.
This study was set to compare the functional parameters of urinary bladder between normal infants and infants with ANH using dynamic ultrasound approach. We sought to investigate whether there was an association between pelvicalyceal dilatation and bladder dysfunction in infants.
METHODS
Subjects.
All infants with ANH were consecutively enrolled in this study between July 2007 and June 2008. The diagnosis of ANH was established with criteria of RP diameter larger than 7 mm in either side of the fetal kidney after 33 wk of gestation (14). Before enrollment into this study, postnatal ultrasound was performed to exclude any structural uropathy. In all subjects, normal development was documented by records from the Maternal Child Health Clinic. No subject was found to have aneuploidy clinically. In infants who were found to have persistently dilated RP postnatally, micturiting cystogram and nuclear medicine (diuretic MAG 3 renal scintigraphy) were performed to look for any VUR and obstruction, respectively, as part of the clinical protocol for ANH. There was no history of previous UTI or concurrent UTI in all recruited infants. No medication, including prophylaxis antibiotics, was given to these infants at the time of the study. Each infant was only recorded as a single entry in this study.
Age-matched normal controls were recruited from patients admitted into the pediatric surgery ward during the same period. These controls were referred for minor nonurological problems, and they had no anatomical urinary tract abnormalities detected on both antenatal and postnatal ultrasound. Exclusion criteria included infants who had previous urinary tract surgery, previous history of UTI, or VUR.
Informed consents were obtained from the parents of all subjects. This study was approved by the institutional review committee of the Chinese University of Hong Kong.
Ultrasound examination.
The ultrasound examination was performed in all infants in a systemic approach as described in previous studies (13). An ATL HDI 5000 machine (Philips Ultrasound, Bothell, WA) was used with a 7.5 or 12 MHz probe. The total examination time required was ∼60 min.
The imaging protocol, in brief, is illustrated as follows:
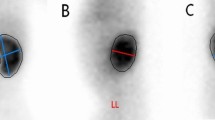
Infants were fed by milk ∼20 min before the examination to facilitate natural filling of the bladder and to ensure the infants were maximally hydrated. All infants were examined in the supine position. Both kidneys were scanned on a transverse (TS) view at which the maximum AP diameter of each RP was measured (Fig. 1), immediately followed by measurement of urinary BV, as described below. Along the longitudinal plane, the maximum length (L) of the bladder was measured from the fundus of the bladder to the internal opening of the urethra (Fig. 2A). The maximum TS section was then chosen along the TS plane. The depth (D) was measured along the midline from the anterior to posterior mucosal surface of the bladder. The width (W) was measured perpendicular to the midline at its mid point (Fig. 2B). The BV was calculated based on the ellipsoid volume formula: BV = L × W × D × 0.523 (15). HI was calculated as AP diameter of RP divided by BV to get a simultaneous relationship (8).
Measurement of bladder volume. A, LS view, the length (L) of the bladder is measured from the fundus of the bladder to the internal opening of the urethra. B, TS view, depth (D) is measured along the midline from the anterior to posterior mucosal surface of the bladder, width (W) is measured perpendicular to the midline at its mid point.
After that, the urinary bladder was scanned in the TS plane at an interval of every 5 min. The above strategy involved frequent checking and repeated measurement of all required parameters to ensure data were obtained when the bladder was at its state of maximum fullness before natural voiding occurred. The maximum BV (MaxBV) was taken as the data measured just before natural voiding occurred, whereas the residual volume (RV) was also measured using the same ellipsoid volume formula immediately after the natural voiding. The measurement of RV was repeated for three attempts in each infant. Bladder wall thickness (BWT) was measured from a zoomed image of the TS plane of the voided bladder at three points: anterolaterally, laterally, and posterolaterally, and the mean value were calculated of the above three measurements (Fig. 3).
All the ultrasound scanning was made by a single operator (J.-X.L.) who was experienced in ultrasound examination in children. Before the start of the study, the operator received intensive training of the study protocol set by the second operator (V.Y.F.L.).
To eliminate observer bias, images taken by the first operator (J.-X.L.) were saved in the ultrasound machine. The second operator (V.Y.-F.L.) did the measurement off-line after anonymization without any clinical knowledge of the infants. An intra- and interobserver error study was also set. The second and third operator (V.Y.-F.L. and W.C.-W.C.) performed all measurements off-line in 10 randomly selected infants for three times. Results showed good agreement of repeatability coefficient (interobserver: 9.52 cm3 and intraobserver: 1.24 cm3 in BV; interobserver: 1.2 mm and intraobserver: 0.7 mm in BWT).
Statistical analysis.
For statistical analysis, Mann-Whitney test was used to compare mean values between normal infants and infants with ANH and comparing infants with normal and abnormally high HI. p value of <0.05 was taken to indicate statistical significance. The results were expressed in mean ± 1 SD.
RESULTS
All subjects were Chinese infants with age ranged from 20 d to 12 mo old. A total of 47 infants with ANH (40 males, seven females, mean age of 0.25 ± 0.24 y) and 43 age-matched normal controls (33 males, 10 females, mean age 0.49 ± 0.35 y) were initially recruited. However, because of the small number of female infants with ANH (ANH is male predominance) and to avoid potential confounding bias from sex difference, female infants were excluded from the analysis. There was no significant difference in the age between the finalized ANH group (n = 40) and the normal controls (n = 33).
In this study, the mean MaxBV in male infants with ANH (30.71 mL) was significantly smaller than the normal male infants (52.45 mL) (p < 0.05, Mann-Whitney test). On the contrary, the mean RV in the ANH group (2.47 mL) was significantly larger than the normal group (1.93 mL) (p < 0.05, Mann-Whitney test) despite up to three voiding attempts. The mean BWT in the ANH group (4.40 mm) was also significantly larger than the normal group (3.70 mm) (p < 0.05, Mann-Whitney test) (Table 1, Fig. 4).
In 40 infants with ANH, the overall AP diameter of the RP ranged from 0 to 1.4 cm. A total of 31 infants were found to have persistently dilated RP postnatally (AP diameter >0.7 cm) in which four had bilateral and 27 had unilateral dilated RP. No definite mechanical cause was identified for 32/35 dilated renal systems. None of them was found to have urinary tract obstruction on MAG-3 studies. Three ureters were found to have grade II reflux on subsequent micturiting cystogram investigation.
Among those 31 infants with persistently dilated renal pelves postnatally, their corresponding HI ranged from 0.004 to 0.104. Twelve (12/31, 39%) of them showed abnormal HI >0.04 (which was >95th centile according to the previously published nomograms) (8) and 19 (19/31, 61%) of them showed normal HI.
When comparing the 12 infants with abnormal HI > 0.04 and the 19 infants with normal HI, the mean MaxBV in infants with abnormal HI (23.33 mL) was significantly smaller than the infant with normal HI (33.49 mL) (p < 0.05, Mann-Whitney test). The mean BWT in the abnormal HI group (4.67 mm) was significantly larger than the normal HI group (3.79 mm) (p < 0.05, Mann-Whitney test) (Table 2). However, there was no statistically difference between the mean RV in the abnormal HI group (2.59 mL) and the normal HI group (2.35 mL) (p = 0.65, Mann-Whitney test). The overall results showed that infants with abnormal HI had more disturbed bladder parameters than those with normal HI.
DISCUSSION
In our previous dynamic ultrasound study in infants, we have established HI as a new physiologic reference of fetal hydronephrosis by eliminating the confounding effect of a distended fetal bladder (8). We found that the HI showed a fairly constant value after 28 wk of gestation. We related this to the maturation of the “pacemaker” in the urinary tract during normal fetal development. We postulate that maturation of pelviureteric pacemaker and peristalsis starts around 28 wk of gestation, after which equilibrium is gradually established between pelvicalyceal filling and bladder filling/emptying in most fetuses.
Constantinou (16) first suggested the presence of pacemaker in the RP of pig in 1974. Afterward, Djurhuus and Constantinou (17) found that chronic ureteric obstruction might disrupt the pacemaker mechanism of the RP. However, the exact site, mechanism, or factors that affect such “pacemaker” are still not known, and it may exist anywhere along the urinary tract. It is proposed that the pacemaker might exist in the RP and is responsible for coordination of peristaltic activity of the RP and/or the ureter. This view is also supported by Mendelsohn (18), who has addressed the crucial role of RP as a regulator of peristalsis of the urinary outflow tract. The pacemaker could also exist in the urinary bladder and responsible for coordination of bladder contractility. The timing of maturation of these pacemakers is not exactly known; however, it is likely that these pacemakers mature at a similar pace. Therefore, immaturity of one might be associated with immaturity of the other. Therefore, we postulated that dilated RP might associate with bladder dysfunction, which could be reflected by abnormal bladder parameters such as MaxBV, RV, and BWT quantitatively. Our previous study on enuretic children has showed that there were significant correlations between abnormal thickness of the bladder wall and multiple abnormal parameters demonstrated by urodynamic studies (10).
To our best knowledge, there is very little information available concerning the bladder function in infants with ANH. This paucity of information probably relates to absence of a systematic and noninvasive imaging tool. Dynamic ultrasound protocol serves as such a tool for understanding bladder physiology in infants with ANH.
This study showed that infants with ANH had abnormal bladder parameters including smaller MaxBV, larger mean volume of residual urine, and significant increase in the thickness of bladder wall, when compared with normal group. The deranged bladder parameters were statistically more significant in those with abnormally high HI compared with those had normal HI. Infants with high HI did have a smaller BV, thickened bladder wall, and larger residual BV than those with normal HI. The above findings suggested that infants with ANH might be sharing the same bladder dysfunction or bladder immaturity as in infants with UTI as reported in our previous study (13). However, whether infants with ANH are more susceptible to UTI is not within the scope of this study and longitudinal follow-up is required.
Limitations and implications of the study.
In this study, we have excluded the female infants from analysis in view of the small number (only seven) recruited. This is probably an intrinsic problem of ANH as it is male predominance. It is also well known that male infants, in general, have higher incidence of detrusor immaturity and higher voiding pressure than female (19). To avoid the confounding effect of sex difference and the unequal distribution of both sexes, only male infants are included in the analysis.
The intrinsic limitations of this study are similar to the study we have reported previously (13). Because of the young age of the subjects who had immature neural control of bladder emptying, the measurement of MaxBV was a tedious and time-consuming task, which required extreme patience of the operator. The average examination time lasts for 60 min, which may be too long for daily clinical practice; however, on research-based study, this is the best available noninvasive method to study the bladder dynamics in infants. To our knowledge, there has been no ultrasound study to evaluate bladder dynamics in infants with ANH. We have applied the same ultrasound protocol in the study of children with primary nocturnal enuresis (9–11) and in children and infants with UTI (12,13). Previous results showed good correlations with urodynamic findings and treatment outcome. Urodynamic studies were not considered in this cohort of infants because of invasive nature of the investigation.
The aim of this study was to investigate the extended potential of using ultrasound to study the physiology of urinary tract in infants with ANH. This study did provide objective parameters specifically for infants with ANH, which suggested there was a close association between immaturity in the function of pelviureteric junction and the urinary bladder. However, the exact mechanism of such a relationship has not been determined in this study and remains to be answered. A larger prospective study was warranted to evaluate whether the abnormal bladder parameters would affect the size of the RP in infants with ANH. If established, this might provide an explanation for nonobstructive pelvicalyceal dilatation, which is commonly observed in infancy. Serial studies on a subset of patients have been planned to assess stability of the bladder findings, especially in relationship to persistence versus resolution of the hydronephrosis. It would also be of interest to know whether family history of renal or urological disease would correlate with the severity of the bladder dysfunction in this group of infants. Counting ureteric peristalsis is an appealing approach to further evaluate the “pacemaker” mechanism along the urinary tract and should be considered to be implemented in future studies.
CONCLUSIONS
Abnormal bladder parameters were demonstrated by dynamic ultrasound in infants with antenatally diagnosed dilated RP. We postulated that there was a close association between functional immaturity of the pelviureteric junction and urinary bladder.
Dynamic ultrasound provides a noninvasive and physiologic method to study the sonographic characteristics of bladder function in infants with ANH, which might be helpful for understanding the underlying physiology of nonobstructed dilated renal systems.
Abbreviations
- ANH:
-
antenatally hydronephrosis
- AP:
-
anteroposterior
- BWT:
-
bladder wall thickness
- HI:
-
hydronephrosis index
- MaxBV:
-
maximal bladder volume
- RV:
-
residual volume
- UTI:
-
urinary tract infection
- VUR:
-
vesicoureteric reflux
References
McIlroy PJ, Abbott GD, Anderson NG, Turner JG, Mogridge N, Wells JE 2000 Outcome of primary vesicoureteric reflux detected following fetal renal pelvic dilatation. J Paediatr Child Health 36: 569–573
Morin L, Cendron M, Crombleholme TM, Garmel SH, Klauber GT, D'Alton ME 1996 Minimal hydronephrosis in the fetus: clinical significance and implications for management. J Urol 155: 2047–2049
Joseph VT 2006 The management of renal conditions in the perinatal period. Early Hum Dev 82: 313–324
Petrikovsky BM, Cuomo MI, Schneider EP, Wyse LJ, Cohen HL, Lesser M 1995 Isolated fetal hydronephrosis: beware the effect of bladder filling. Prenat Diagn 15: 827–829
Persutte WH, Hussey M, Chyu J, Hobbins JC 2000 Striking findings concerning the variability in the measurement of the fetal renal collecting system. Ultrasound Obstet Gynecol 15: 186–190
Damen-Elias HA, Stigter RH, De Jong TP, Visser GH 2004 Variability in dilatation of the fetal renal pelvis during a bladder filling cycle. Ultrasound Obstet Gynecol 24: 750–755
Gill WB, Curtis GA 1977 The influence of bladder fullness on upper urinary tract dimensions and renal excretory function. J Urol 117: 573–576
Leung VY, Chu WC, Metreweli C 2009 Hydronephrosis index: a better physiological reference in antenatal ultrasound for assessment of fetal hydronephrosis. J Pediatr 154: 116–120
Yeung CK, Sreedhar B, Leung VT, Metreweli C 2004 Ultrasound bladder measurements in patients with primary nocturnal enuresis: a urodynamic and treatment outcome correlation. J Urol 171: 2589–2594
Leung VY, Chu WC, Yeung CK, Metreweli C 2006 Ureteric jet Doppler waveform and bladder wall thickness in children with nocturnal enuresis. Pediatr Res 60: 582–586
Sreedhar B, Yeung CK, Leung VY, Chu CW 2008 Ultrasound bladder measurements in children with severe primary nocturnal enuresis: pretreatment and posttreatment evaluation and its correlation with treatment outcome. J Urol 179: 1568–1572; discussion 1572
Yeung CK, Sreedhar B, Leung YF, Sit KY 2007 Correlation between ultrasonographic bladder measurements and urodynamic findings in children with recurrent urinary tract infection. BJU Int 99: 651–655
Liu JX, Leung VY, Chu WC, Sreedhar B, Metreweli C, Yeung CK 2008 Characteristics of the bladder in infants with urinary tract infections: an ultrasound study. Pediatr Radiol 38: 1084–1088
Corteville JE, Gray DL, Crane JP 1991 Congenital hydronephrosis: correlation of fetal ultrasonographic findings with infant outcome. Am J Obstet Gynecol 165: 384–388
Dicuio M, Pomara G, Fabris FM, Ales V, Dahlstrand C, Morelli G 2005 Measurements of urinary bladder volume: comparison of five ultrasound calculation methods in volunteers. Arch Ital Urol Androl 77: 60–62
Constantinou CE 1974 Renal pelvic pacemaker control of ureteral peristaltic rate. Am J Physiol 226: 1413–1419
Djurhuus JC, Constantinou CE 1982 Chronic ureteric obstruction and its impact on the coordinating mechanisms of peristalsis (pyeloureteric pacemaker system). Urol Res 10: 267–270
Mendelsohn C 2004 Functional obstruction: the renal pelvis rules. J Clin Invest 113: 957–959
Wen JG, Yeung CK, Chu WC, Shit FK, Metreweli C 2001 Video cystometry in young infants with renal dilation or a history of urinary tract infection. Urol Res 29: 249–255
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Leung, VF., Rasalkar, D., Liu, JX. et al. Dynamic Ultrasound Study on Urinary Bladder in Infants With Antenatally Detected Fetal Hydronephrosis. Pediatr Res 67, 440–443 (2010). https://doi.org/10.1203/PDR.0b013e3181d22b91
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/PDR.0b013e3181d22b91