Abstract
Caffeine, a nonspecific adenosine receptor (AR) antagonist is widely used to treat apnea of prematurity. Because adenosine modulates multiple biologic processes including inflammation, we hypothesized that AR blockade by caffeine would increase cytokine release from neonatal monocytes. Using cord blood monocytes (CBM), we investigated 1) the changes in AR mRNA profile by real time quantitative reverse-transcription polymerase-chain-reaction (qRT-PCR) and protein expression (western blot) after in vitro culture, caffeine or lipopolysaccharide (LPS) exposure, and 2) the modulation of cytokine release and cyclic adenosine monophosphate (cAMP) production by enzyme-linked immunosorbent assay (ELISA) induced by caffeine and specific AR antagonists: DPCPX(A1R), ZM241385(A2aR), MRS1754(A2bR), and MRS1220(A3R). After 48 h in culture, A2aR and A2bR gene expression increased 1.9 (p = 0.04) and 2.5-fold (p = 0.003), respectively. A1R protein expression directly correlated with increasing LPS concentrations (p = 0.01), with minimal expression preexposure. Only caffeine (50 μM) and DPCPX (10 nM) decreased tumor necrosis factor-alpha (TNF-α) release from LPS activated-CBM by 20 and 25% (p = 0.01) and TNF-α gene expression by 30 and 50%, respectively, in conjunction with a ≥2-fold increase in cAMP (p < 0.05). AR blockade did not modulate other measured cytokines. The induction of A1R after LPS exposure suggests an important role of this receptor in the control of inflammation in neonates. Our findings also suggest that caffeine, via A1R blockade, increases cAMP production and inhibits pretranscriptional TNF-α production by CBM.
Similar content being viewed by others
Main
Caffeine (1,3,11 trimethylxantine) is a stimulant widely used in neonatology to treat apnea of prematurity (1). At therapeutic range (5 to 15 μg/mL), caffeine blocks A1 and A2a adenosine receptors (ARs) stimulating ventilation (2–4). Recently, caffeine has also been linked to a decrease in the incidence of bronchopulmonary dysplasia and cerebral palsy in extremely premature infants (5,6), although the mechanisms explaining these findings have not been elucidated.
The natural ligand for ARs, adenosine, has a crucial role in multiple biologic processes including inflammation (7,8). The increase in tumor necrosis factor-alpha (TNF-α) release by adult peripheral blood monocytes (PBM) in response to lipopolysaccharide (LPS) exposure can be abolished by pretreatment with A2aR agonists (9,10). Adenosine binding to A1R (11,12) and A3R (13,14) also modulates TNF-α release from adult monocytes, whereas A2bR appears to have little effect (10).
Little is known about AR expression on neonatal monocytes and the role of caffeine in modulating cytokine release. We hypothesized that caffeine blockade of ARs on neonatal monocytes would increase the release of cytokines in response to LPS. To test this hypothesis, we used cord blood monocytes (CBM) from full-term infants to 1) characterize the changes in AR mRNA profile and protein expression after 48 h in culture, exposure to caffeine and activation with LPS; and 2) determine the effect of AR blockade by caffeine on the release of pro- and antiinflammatory cytokines with indirect confirmation of AR function via determination of changes in intracellular cyclic adenosine monophosphate (cAMP) levels and cytokine gene expression.
METHODS
Subjects.
This study complied with the Guidelines for Human Experimentation from the United States Department of Health and Human Services and received approval of The Johns Hopkins Medicine Institutional Review Board (NA_00002034). Informed consent was obtained from parents before inclusion in this study.
CBM from full-term infants (≥37 wk gestation at birth) and PBM from adult volunteers were used for experiments. Cord blood was collected after repeat cesarean section without labor or vaginal delivery without evidence of chorioamnionitis. We excluded births with known genetic disorder, intrauterine growth restriction or small for gestational age (birth weight ≤10th percentile for gestational age), and suspected viral infection (based on maternal serological or clinical findings). Infants who subsequently received antibiotics or had illness sufficient to be admitted to the neonatal intensive care unit (NICU) were removed from analysis. Healthy adult volunteers, who were not using theophylline, were included in the study. Caffeine levels were obtained on all blood samples.
Isolation of cord blood monocytes.
Full-term cord blood and adult peripheral blood were collected in EDTA for monocyte isolation and in no-additive tubes for caffeine level determination (BD Biosciences, Franklin Lakes, NJ). All blood samples were centrifuged at 2400× g for 10 min at 25°C, cellular portion was reconstituted in DPBS (pH 7.4; Mediatech, Inc., Herndon, VA) and monocytes were isolated using Ficoll-Hypaque gradient (GE Healthcare Biosciences AB, Uppsala, Sweden). Monocytes were then washed and reconstituted in 4 × RPMI 1640 media containing 8% (vol/vol) [Delta]H human AB serum, penicillin/streptomycin (400 IU/mL/400 μg/mL), and 8 mM l-glutamine (Sigma Chemical,Aldrich, St. Louis, MO). Viable monocytes were used in experiments as outlined below.
Real time quantitative reverse-transcription polymerase-chain reaction.
Total RNA was extracted from monocytes to determine changes in 1) A1R, A2aR, A2bR, and A3R gene expression after 48 h in culture (37°C/5% CO2), caffeine exposure (50 μM) and LPS activation (0, 100, and 200 ng/mL), and 2) TNF-α gene expression after caffeine and DPCPX (10 nM) treatments alone and combined. PureLink Micro-to-midi total RNA purification system (Invitrogen, Carlsbad, CA) was used according to specifications. Approximately 1 μg of total RNA was used for generation of complementary DNA (cDNA) using iScript cDNA synthesis kit (BioRad, Hercules, CA). Reverse transcription protocol included 5 min at 25°C; 30 min at 42°C; and 5 min at 85°C. cDNA was then used to amplify target genes by real time quantitative reverse-transcription polymerase-chain-reaction (qRT-PCR) using 300 nM concentration of primers [Table 1; (15–19)]. SYBR Green Supermix (BioRad) was used for signal detection by MyIQ PCR Thermocycler (BioRad). Two different amplification protocols were used 1) for ARs: 40 cycles of 15 s at 95.0°C, 1 min at 60.0°C, and 30 s at 72.0°C; and 2) for TNF-α: 40 cycles of 1 min at 94.0°C, 1 min at 60.0°C, and 2 min at 72.0°C. The fold difference in gene expression was corrected to GAPDH (human glyceraldehyde phosphate dehydrogenase; reference gene) using the Pfaffl method (20). Melting curves were used to ascertain purity of PCR products, which were also visualized by 1.3% gel electrophoresis.
Western blot analysis.
Changes in A1R and A2aR protein expression on CBM after 24 h LPS exposure (0, 100, and 200 ng/mL) were determined using western blot. Protein was extracted by manually homogenizing monocytes followed by ice-cold ethanol precipitation. Protein pellet was reconstituted in 0.01 M PBS (pH 7.4; Quality Biologic, Gaithersburg, MD) and concentration was determined using the Bradford method. Twenty five microgram of protein were diluted in loading buffer containing 20% (wt/vol) glycerol and loaded on to 12% SDS-PAGE. Proteins were transferred to nitrocellulose membrane, stained with Ponceau S, blocked with 2.5% nonfat dry milk with 0.1% Tween 20 in 50 mM Tris buffered saline (pH 7.4), and consecutively incubated overnight at 4°C with polyclonal rabbit anti-A1R (Sigma Chemical, Aldrich), or monoclonal mouse anti-A2aR antibodies (Upstate, Lake placid, NY) both at 1:1000, or mouse anti-β-actin MAb (Sigma Chemical, Aldrich) at 1:20,000. The membrane was then washed with milk, exposed to goat anti-rabbit or anti-mouse antibodies (Bio-Rad) at 1:10,000 for 1 h and then developed with enhanced chemiluminescence using SuperSignal kit (Thermo Scientific, Rockford, IL). To quantify protein immunoreactivity, films were scanned using Adobe Photoshop, and optical density (OD) was determined with IP Lab Gel H software adjusting for background (ΔOD). β-actin was used for protein loading correction. Protein levels are expressed as relative OD measurements (arbitrary density units; ADU).
Enzyme-linked immunosorbent assay.
Caffeine and specific AR antagonists [Table 2; (4,21–24)] were used to investigate the role of ARs in modulating cytokine release. Concentrations were based on IC50 and Ki to maximize specificity to each AR. A total of 50 μM of caffeine was within therapeutic range for neonates treated for apnea of prematurity (5–15 μg/mL). Caffeine was prepared in acidic water (citric acid based, pH 4.7) and titrated to pH 7.3 before use. All other antagonists were reconstituted in DMSO (DMSO, cell-culture-concentration: 2.7 × 10−6 g/mL). ZM241385 was purchased from Tocris Bioscience (Ellisville, MO), and all other drugs were purchased from Sigma Chemical. Monocytes were exposed to caffeine, ZM241385, MRS1754, or MRS1220 in initial experiments and to caffeine and DPCPX alone and combined in additional experiments. After exposure to these conditions for 75 min (37°C/5% CO2), monocytes were activated using Escherichia coli K235 LPS (100 ng/mL; Sigma Chemical, Aldrich). After 24 h, supernatants were recovered for subsequent determination of cytokine (TNF-α, IL-1β, IL-6, and IL-10) concentrations by enzyme-linked immunosorbent assay (ELISA) using LINCOplex Multiplex kits (Millipore, Billerica, MA) according to manufacturer's protocol and concentrations were calculated using the Luminex detection system (Millipore).
Intracellular cAMP levels were measured to confirm functionality of ARs during exposure to caffeine and/or DPCPX. After exposure, monocytes were lysed using 2.5% dodecyltrimethylammonium bromide in assay buffer (pH 5.8; 0.05 M sodium acetate buffer and 0.02% BSA). Intracellular cAMP levels were measured using the Amersham cAMP EIA System (GE Healthcare, Little Chalfont, Buckinghamshire, UK) and results were reported as percentage change from baseline (no-treatment) corrected for 2 × (10)5 viable monocytes.
Statistical analysis.
Because of the non-normal distribution of the data, nonparametric statistics, including Wilcoxon signed rank test, Mann-Whitney test, Friedman analysis of variance (ANOVA), and Spearman Rank correlation were used. Results are reported as median with interquartile range (IQR, 25 to 75th percentile) and, in most cases, represented as box-and-whisker plots with outliers (boxes symbolize IQR). Additional analysis of the data, including correlations and multilevel analysis were performed to investigate the persistency of the effect attributed to caffeine on TNF-α release after correcting for the influence of other measured cytokines (supplemental material online, www.pedresearch.org). Significance was assigned by p < 0.05. SPSS 14.0 software was used.
RESULTS
Cord blood from 28 neonates (mean gestational age ± SD = 39 2/7 ± 1.3 wk; birth weight = 3312 ± 513 g; Table 3) and peripheral blood from eight adult volunteers (age = 40.9 ± 9.9 y) were used for in vitro experiments. Serum caffeine concentrations were below the therapeutic range (mean ± SD = 1.2 ± 1.4 μg/mL for neonates and 2.4 ± 2 μg/mL for adults) and were not statistically different between age groups.
Effect of culture and caffeine on AR gene expression in CBM.
A1R gene expression was undetected in full-term CBM whereas PCR products of alternative splicing variants expressed at baseline, and after 48 h in culture in adult PBM, showing alternative splicing products. In CBM, after 48 h in culture, A2aR mRNA expression increased 1.9-fold (IQR 0.5–11.8; p = 0.04) and A2bR mRNA increased 2.5-fold (IQR 1.6–3.5, p = 0.003). As a comparison, culture conditions did not change A2aR mRNA expression but did increase A2bR mRNA expression by 2.3-fold (IQR 1.3–7.2, p = 0.03) in adult PBM (Fig. 1). In vitro exposure to caffeine did not further modify the gene expression of any AR subtype.
Fold change in AR mRNA levels after 48 h in-culture. (A) Neonates (n = 12) and (B) Adults (n = 8). Solid line inside boxes represents median. ▴, Extremes; •, outliers. *p < 0.05 (Wilcoxon signed rank test) versus reference line (at 1, no change). ND, nondetected. Electrophoresis shows RT-PCR products before and after culture, arrows identify products after culture.
Effect of LPS exposure on AR expression in CBM.
Although A1R mRNA was not detected in CBM at baseline by real time qRT-PCR (Fig. 1A), 24 h exposure to LPS (100 ng/mL) induced the expression of this gene in 67% (n = 8/12) of the subjects with no further induction at higher LPS concentration (200 ng/mL). Electrophoresis (Fig. 2A) showed A1R PCR products at 308-bp, which corresponds to the two recognized transcript variants (NM_000674.2 and NM_001048230.1; NCBI database). Nontranslated products of alternative splicing are shown at 455-bp (Alternative Splicing and Transcript Diversity Database; www.ebi.ac.uk/astd). LPS exposure also increased A2aR mRNA expression by 4.2-fold (IQR 3.6–12.2; p = 0.02; Fig. 2B).
Effect of LPS exposure on AR gene and protein expression. (A) RT-PCR products for A1R from CBM after LPS exposure (0, 100, and 200 ng/mL). Transcript variants were amplified as 308-bp products (455-bp product is result of alternative splicing). (B) Fold change in AR mRNA on CBM after 24 h LPS exposure at 100 ng/mL (n = 8). Solid line inside boxes represents median. *p < 0.05 (Wilcoxon signed rank test) versus reference line (at 1, no change). (C) Western blot showing A1R protein at ∼36 kD band in 12% SDS-PAGE, and (D) A1R protein levels expressed as arbitrary density units (ADU) adjusted to β-actin. Data as mean ± SEM (ADU) after exposure to LPS at 0, 100, and 200 ng/mL (n = 6). *p < 0.05 (Wilcoxon signed rank test); overall p = 0.001 (Friedman ANOVA).
Western blot analysis showed that A1R and A2aR protein expression directly correlated with LPS concentrations to which CBM were exposed (r = 0.64, p = 0.01 and r = 0.71, p = 0.001, respectively). LPS at 100 and 200 ng/mL, increased A1R protein by 35% (IQR 18 to 360%; p = 0.03) and 100% (IQR 41 to 785%; p = 0.03), respectively (p = 0.002, Friedman ANOVA; Fig. 2C and D) and increased A2aR protein by 174% (IQR 16 to 455%; p = 0.02) and 230% (IQR 36 to 621%; p = 0.02), respectively, versus no LPS exposure (p = 0.006, Friedman ANOVA).
Effect of AR antagonists on cytokine release from LPS-activated monocytes.
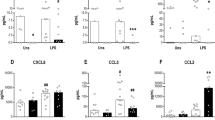
Exposure to LPS significantly increased the release of all measured cytokines (TNF-α, IL-1β, IL-6, and IL-10) from CBM and adult PBM compared with control conditions (p < 0.05). In response to LPS, cytokine release did not differ between neonates and adults, except for the antiinflammatory cytokine, IL-10, which showed 80% (IQR 69 to 96%, p = 0.04) less release from CBM compared with adults PBM (Fig. 3). Mode of delivery did not influence baseline cytokine levels or response to antagonists.
In LPS-activated CBM, caffeine (a nonspecific AR antagonist) down-regulated TNF-α release by 20% from baseline (IQR −40 to −9%, p = 0.015) whereas A2aR, A2bR, and A3R specific antagonists did not produce an effect. In experiments using adult PBM, there was a trend toward an increase in TNF-α release from baseline after exposure to all AR antagonists with MRS1220 (A3R antagonist) reaching significance (p = 0.03). The differences in TNF-α release between CBM and adult PBM after caffeine and ZM241385 reached significance (p = 0.02 and 0.03 for each treatment, respectively; Fig. 4A). Caffeine and specific antagonists did not affect IL-1β, IL-6, and IL-10 release from monocytes in either age group (data not shown). Because the observed decrease in TNF-α release by CBM following caffeine exposure (Fig. 4A) can result from 1) the blockade of ARs and/or 2) the interaction with other cytokines, we determined the influence of these two factors using a multilevel model. Correcting for interactions with other cytokines, the model supported our initial findings associating caffeine with down-regulation of TNF-α release (−56%, β = −303 ± 66 pg/mL; p = 0.01). The mechanism behind this effect was explored in the next series of experiments.
Effect of AR antagonists on TNF-α release. (A) Change from baseline in TNF-α (pg/mL) release from neonatal (gray boxes; n = 12) and adult (white boxes; n = 8) monocytes pretreated with caffeine and specific A2aR, A2bR, and A3R antagonists. (B) Change in TNF-α (pg/mL) release (n = 8); (C) Percentage change in intracellular cAMP concentrations (n = 6); and (D) Fold change in TNF-α gene expression (n = 8) in LPS-activated CBM in response to caffeine and DPCPX alone and combined. Electrophoresis showed 444-bp RT-PCR product for TNF-α. Reference lines (baseline conditions), positioned at 0 (A, B, and C) and 1 (D), represent no change. Line inside boxes represents median. *p < 0.05 (Wilcoxon signed rank test versus baseline and Mann-Whitney test, between age groups). ▴, extremes; •, outliers.
Role of A1R in the decrease of TNF-α release from CBM.
Because specific A2aR, A2bR, and A3R antagonists did not affect the release of TNF-α and LPS exposure induced A1R gene and protein expression, we hypothesized that A1R was involved in the reduction of TNF-α secretion after caffeine exposure. DPCPX, a specific A1R antagonist (10 nM), down-regulated TNF-α release by 25% from baseline (IQR −54 to −8%, p = 0.01) and the addition of caffeine to cells pretreated with DPCPX down-regulated TNF-α release by a total of 43% from baseline (IQR −70 to −26%, p = 0.01). The additional decrease in TNF-α release produced by caffeine after pretreatment with DPCPX was significant (p = 0.03 versus DPCPX alone; p = 0.001 by Friedman ANOVA; Fig. 4B).
In accordance with these findings outlined above, caffeine increased intracellular cAMP levels by 2.8-fold (IQR 2.3–3.8, p = 0.04) and DPCPX by 2.1-fold from baseline (IQR 1.6–2.3, p = 0.03). The addition of caffeine after pretreatment with DPCPX increased cAMP by 2-fold (IQR 1.7–2.2, p = 0.04) from baseline, similar to DPCPX alone (Fig. 4C). Caffeine inhibited TNF-α gene expression by 30% (IQR −10 to −40%, p = 0.01) whereas DPCPX inhibited the gene by 50% (IQR −40 to −50%, p = 0.04; Fig. 4D).
DISCUSSION
In this study, we determined the change in AR expression in full-term CBM compared with that on adult PBM after in vitro culture and exposure to caffeine or LPS. We also determined the effect of caffeine on cytokine release from CBM and indirectly confirmed the AR function by measuring the changes in cAMP production and TNF-α gene expression. We demonstrated that in contrast to adult PBM, A1R gene and protein expression in CBM is minimal until exposure to LPS, which also up-regulates A2aR. We showed that TNF-α release in response to LPS is similar in CBM and adult PBM, and exposure to caffeine or DPCPX (A1R antagonist) down-regulates TNF-α release only from CBM. In accordance with these findings, we found that intracellular cAMP concentration increases and TNF-α gene expression decreases as a result of caffeine or DPCPX exposure. These data suggest that the effect of caffeine on TNF-α release was in part mediated via A1R blockade.
AR gene expression in monocytes differs between neonates and adults. The dose-related induction of A1R protein expression in CBM after LPS exposure could explain the baseline A1R expression observed in adult PBM. This finding suggests that A1R induction occurs some time after birth following exposure to infections and also implies, similar to A2aR, a role in the modulation of inflammation (8,25).
Because of the nonspecific effect of caffeine on ARs, the identification of the particular AR subtype(s) involved in the modulation of TNF-α release is challenging. We used specific AR antagonists to evaluate the effects of endogenous adenosine. We believe that this approach is most applicable to studying the effects of caffeine, a nonspecific AR antagonist, compared with the use of agonists.
A1R dysregulation leads to a proinflammatory state (11) and because of the high affinity of A1R (coupled to Gi-protein) for adenosine, A1R blockade could potentially increase cAMP not only directly but also indirectly by displacing adenosine toward A2aR (coupled to Gs-protein), which ultimately will down-regulate TNF-α release (26,27). A1R is directly induced in response to infection and oxidative stress in multiple cells (28). Our experiments using DPCPX demonstrate that the blockade of A1R on LPS-activated CBM significantly down-regulates TNF-α release, via a pretranscriptional mechanism, to levels similar to that after caffeine exposure. These findings strongly suggest that, at least in part, A1R blockade mediates the effect of caffeine on TNF-α release, which is also associated with concurrent changes in cAMP levels and TNF-α gene expression.
A2aR agonists down-regulate TNF-α release; (10,29) however, specific A2aR antagonists (ZM 241385) failed to increase TNF-α release from adult PBM (30) or CBM (our data). This inconsistency may be related to the multiple AR subtypes differentially modulating cAMP production. As demonstrated by our data, LPS-activated CBM express the full spectrum of ARs identified to date, making possible that specific A2aR blockade displaces adenosine to bind to other available receptors, including A2bR and A3R, which will increase cAMP production. Studies targeting the A2bR are less abundant and conclusive and most show that in response to specific A2bR agonists, TNF-α is up-regulated probably as a result of the concomitant IL-19 release (10,31). In our experiments, MRS1754 (A2bR antagonist) appears to have a role in down-regulating TNF-α release (multilevel analysis, supplemental material online, www.pedresearch.org), however, its effect is abolished by the influence of other cytokines (Fig. 4A). Because caffeine simultaneously blocks A2aR and A2bR and the blockade of the latter could potentially have an effect in the TNF-α release, we cannot rule out that the mechanism of caffeine could also be directly or indirectly mediated by A2bR.
A3R activation has been linked to pro- and anti-inflammatory effects, from pro- to antiinflammatory (14,26,32). Recently, A3R has been described as positively coupled to adenylyl cyclase, thereby increasing cAMP production upon activation (33,34). Because caffeine, at 50 μM, blocks all ARs except for A3R, we speculate that the down-regulation of TNF-α release associated with caffeine is also mediated by the indirect increase in A3R affinity for adenosine when the other ARs are blocked. This mechanism could explain our results showing an additional decrease in TNF-α release after caffeine is added to DPCPX pretreated CBM.
The synchronized increase of intracellular cAMP levels and decrease of TNF-α gene expression after caffeine exposure suggest that the decrease in TNF-α release is likely a pretranscriptional process. Several pathways related to accumulation of intracellular cAMP such as cAMP/protein kinase A (35) and modifications in NF-κB (36) are targets of our future experiments.
Although TNF-α release is modulated by caffeine via AR blockade, that is not the case for all cytokines. Multiple studies using adult PBM show that IL-1β (14,35), IL-6 (29), and IL-10 (35) release from these cells are not modulated by ARs. A recent study using neonatal monocytes reported similar finding related to IL-6 (32). We have extended this finding by also showing that IL-1β and IL-10 release are also independent of ARs in CBM.
In this article, we demonstrate the significant differences in AR mRNA profile between monocytes from neonates and adults, the induction of A1R gene and protein expression after LPS exposure and also the suppressive effect of caffeine on TNF-α release by LPS-activated CBM via mechanisms involving ARs. Our data suggest that A1R blockade is operative in mediating the effect of caffeine. Although the direct applicability of our findings to premature infants is still unclear, we believe that our results are clinically relevant to neonates exposed to caffeine either throughout gestation or postnatally. Whether the suppression of TNF-α release produced by caffeine has a beneficial effect by decreasing chronic inflammation or has a detrimental effect by increasing the risk for infection is unclear. Nevertheless, reduction in the incidence of chronic lung disease and periventricular leukomalacia in premature infants has been attributed to the use of caffeine citrate (5). Our data suggest a potential biologic mechanism to explain these clinical observations and add to the body of evidence characterizing the differences in inflammatory response between neonates and adults as it relates to ARs on monocytes.
Abbreviations
- AR:
-
adenosine receptor
- CBM:
-
cord blood monocytes
- IQR:
-
interquartile range
- LPS:
-
lipopolysaccharide
- PBM:
-
peripheral blood monocytes
References
Herlenius E, Lagercrantz H, Yamamoto Y 1997 Adenosine modulates inspiratory neurons and the respiratory pattern in the brainstem of neonatal rats. Pediatr Res 42: 46–53
Bona E, Aden U, Fredholm BB, Hagberg H 1995 The effect of long term caffeine treatment on hypoxic-ischemic brain damage in the neonate. Pediatr Res 38: 312–318
Ferre S, Ciruela F, Borycz J, Solinas M, Quarta D, Antoniou K, Quiroz C, Justinova Z, Lluis C, Franco R, Goldberg SR 2008 Adenosine A1–A2A receptor heteromers: new targets for caffeine in the brain. Front Biosci 13: 2391–2399
Fredholm BB, Irenius E, Kull B, Schulte G 2001 Comparison of the potency of adenosine as an agonist at human adenosine receptors expressed in Chinese hamster ovary cells. Biochem Pharmacol 61: 443–448
Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, Solimano A, Tin W 2006 Caffeine therapy for apnea of prematurity. N Engl J Med 354: 2112–2121
Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, Solimano A, Tin W 2007 Long-term effects of caffeine therapy for apnea of prematurity. N Engl J Med 357: 1893–1902
Krump E, Lemay G, Borgeat P 1996 Adenosine A2 receptor-induced inhibition of leukotriene B4 synthesis in whole blood ex vivo. Br J Pharmacol 117: 1639–1644
Hasko G, Pacher P, Deitch EA, Vizi ES 2007 Shaping of monocyte and macrophage function by adenosine receptors. Pharmacol Ther 113: 264–275
Bshesh K, Zhao B, Spight D, Biaggioni I, Feokistov I, Denenberg A, Wong HR, Shanley TP 2002 The A2A receptor mediates an endogenous regulatory pathway of cytokine expression in THP-1 cells. J Leukoc Biol 72: 1027–1036
Zhang JG, Hepburn L, Cruz G, Borman RA, Clark KL 2005 The role of adenosine A2A and A2B receptors in the regulation of TNF-alpha production by human monocytes. Biochem Pharmacol 69: 883–889
Mayne M, Shepel PN, Jiang Y, Geiger JD, Power C 1999 Dysregulation of adenosine A1 receptor-mediated cytokine expression in peripheral blood mononuclear cells from multiple sclerosis patients. Ann Neurol 45: 633–639
Sipka S, Kovacs I, Szanto S, Szegedi G, Brugos L, Bruckner G, Jozsef Szentmiklosi A 2005 Adenosine inhibits the release of interleukin-1beta in activated human peripheral mononuclear cells. Cytokine 31: 258–263
Martin L, Pingle SC, Hallam DM, Rybak LP, Ramkumar V 2006 Activation of the adenosine A3 receptor in RAW 264.7 cells inhibits lipopolysaccharide-stimulated tumor necrosis factor-alpha release by reducing calcium-dependent activation of nuclear factor-kappaB and extracellular signal-regulated kinase 1/2. J Pharmacol Exp Ther 316: 71–78
Sajjadi FG, Takabayashi K, Foster AC, Domingo RC, Firestein GS 1996 Inhibition of TNF-alpha expression by adenosine: role of A3 adenosine receptors. J Immunol 156: 3435–3442
Linden J, Taylor HE, Robeva AS, Tucker AL, Stehle JH, Rivkees SA, Fink JS, Reppert SM 1993 Molecular cloning and functional expression of a sheep A3 adenosine receptor with widespread tissue distribution. Mol Pharmacol 44: 524–532
Stehle JH, Rivkees SA, Lee JJ, Weaver DR, Deeds JD, Reppert SM 1992 Molecular cloning and expression of the cDNA for a novel A2-adenosine receptor subtype. Mol Endocrinol 6: 384–393
Thiele A, Kronstein R, Wetzel A, Gerth A, Nieber K, Hauschildt S 2004 Regulation of adenosine receptor subtypes during cultivation of human monocytes: role of receptors in preventing lipopolysaccharide-triggered respiratory burst. Infect Immun 72: 1349–1357
Tucker AL, Linden J 1993 Cloned receptors and cardiovascular responses to adenosine. Cardiovasc Res 27: 62–67
Stoltz DA, Nelson S, Kolls JK, Zhang P, Bohm RP, Murphey-Corb M, Bagby GJ 2002 Effects of in vitro ethanol on tumor necrosis factor-alpha production by blood obtained from simian immunodeficiency virus-infected rhesus macaques. Alcohol Clin Exp Res 26: 527–534
Pfaffl MW 2001 A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45
Jacobson KA, Park KS, Jiang JL, Kim YC, Olah ME, Stiles GL, Ji XD 1997 Pharmacological characterization of novel A3 adenosine receptor-selective antagonists. Neuropharmacology 36: 1157–1165
Kim YC, Ji X, Melman N, Linden J, Jacobson KA 2000 Anilide derivatives of an 8-phenylxanthine carboxylic congener are highly potent and selective antagonists at human A(2B) adenosine receptors. J Med Chem 43: 1165–1172
Kim YC, Ji XD, Jacobson KA 1996 Derivatives of the triazoloquinazoline adenosine antagonist (CGS15943) are selective for the human A3 receptor subtype. J Med Chem 39: 4142–4148
Ongini E, Dionisotti S, Gessi S, Irenius E, Fredholm BB 1999 Comparison of CGS 15943, ZM 241385 and SCH 58261 as antagonists at human adenosine receptors. Naunyn Schmiedebergs Arch Pharmacol 359: 7–10
Murphree LJ, Sullivan GW, Marshall MA, Linden J 2005 Lipopolysaccharide rapidly modifies adenosine receptor transcripts in murine and human macrophages: role of NF-kappaB in A(2A) adenosine receptor induction. Biochem J 391: 575–580
Takahashi HK, Iwagaki H, Hamano R, Wake H, Kanke T, Liu K, Yoshino T, Tanaka N, Nishibori M 2007 Effects of adenosine on adhesion molecule expression and cytokine production in human PBMC depend on the receptor subtype activated. Br J Pharmacol 150: 816–822
Takahashi HK, Kanke T, Liu K, Yoshino T, Sendo T, Tanaka N, Nishibori M 2007 Adenosine A2A-receptor stimulation inhibits lipopolysaccharide-induced interleukin-18 production in monocytes. J Pharmacol Sci 104: 183–186
Jhaveri KA, Toth LA, Sekino Y, Ramkumar V 2006 Nitric oxide serves as an endogenous regulator of neuronal adenosine A1 receptor expression. J Neurochem 99: 42–53
Le Vraux V, Chen YL, Masson I, De Sousa M, Giroud JP, Florentin I, Chauvelot-Moachon L 1993 Inhibition of human monocyte TNF production by adenosine receptor agonists. Life Sci 52: 1917–1924
Harada N, Okajima K, Murakami K, Usune S, Sato C, Ohshima K, Katsuragi T 2000 Adenosine and selective A(2A) receptor agonists reduce ischemia/reperfusion injury of rat liver mainly by inhibiting leukocyte activation. J Pharmacol Exp Ther 294: 1034–1042
Zhong H, Wu Y, Belardinelli L, Zeng D 2006 A2B adenosine receptors induce IL-19 from bronchial epithelial cells, resulting in TNF-alpha increase. Am J Respir Cell Mol Biol 35: 587–592
Levy O, Coughlin M, Cronstein BN, Roy RM, Desai A, Wessels MR 2006 The adenosine system selectively inhibits TLR-mediated TNF-alpha production in the human newborn. J Immunol 177: 1956–1966
Ezeamuzie CI, Philips E 2003 Positive coupling of atypical adenosine A3 receptors on human eosinophils to adenylyl cyclase. Biochem Biophys Res Commun 300: 712–718
Palmer TM, Harris CA, Coote J, Stiles GL 1997 Induction of multiple effects on adenylyl cyclase regulation by chronic activation of the human A3 adenosine receptor. Mol Pharmacol 52: 632–640
Horrigan LA, Kelly JP, Connor TJ 2004 Caffeine suppresses TNF-alpha production via activation of the cyclic AMP/protein kinase A pathway. Int Immunopharmacol 4: 1409–1417
Majumdar S, Aggarwal BB 2003 Adenosine suppresses activation of nuclear factor-kappaB selectively induced by tumor necrosis factor in different cell types. Oncogene 22: 1206–1218
Acknowledgements
The authors acknowledge Dr. Frances Northington and Dr. Lawrence Nogee for their support and helpful critiques; Mr. Reed Cooper for his initial assistance with RT-PCR; Mrs. Alyssa Sproles for her technical assistance with ELISA and monocyte isolation techniques; Mrs. Debra Flock, Ms. Ariel Mason, Mr. Wayne Hickok, and Mrs. Myra Black for their administrative assistance and the obstetric team at Johns Hopkins Hospital for their kind support with patient enrollment. They are also indebted with our patients and their families for their willingness to participate in this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by The Johns Hopkins University School of Medicine General Clinical Research Center grant M01-RR00052; The National Center for Research Resources/NIH; The National Institutes of Health grant HL-072748 (E.B.G); The Johns Hopkins University Institutional Research Grant; and The Thomas Wilson Sanitarium for Children of Baltimore City.
This article contains supplemental material, which is available online at www.pedresearch.org.
ArticlePlus
Click on the links below to access all the ArticlePlus for this article.
Please note that ArticlePlus files may launch a viewer application outside of your web browser.
Rights and permissions
About this article
Cite this article
Chavez-Valdez, R., Wills-Karp, M., Ahlawat, R. et al. Caffeine Modulates TNF-α Production by Cord Blood Monocytes: The Role of Adenosine Receptors. Pediatr Res 65, 203–208 (2009). https://doi.org/10.1203/PDR.0b013e31818d66b1
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/PDR.0b013e31818d66b1
This article is cited by
-
Caffeine ameliorates the metabolic syndrome in diet-induced obese mice through regulating the gut microbiota and serum metabolism
Diabetology & Metabolic Syndrome (2023)
-
Relation Between the Negative Cognitive Triad, Perceived Everyday Discrimination, Depressive Symptoms, and TNF-⍺ in Adolescents
Child Psychiatry & Human Development (2023)
-
Antioxidative effects of caffeine in a hyperoxia-based rat model of bronchopulmonary dysplasia
Respiratory Research (2019)
-
Loss of CD73-mediated extracellular adenosine production exacerbates inflammation and abnormal alveolar development in newborn mice exposed to prolonged hyperoxia
Pediatric Research (2017)
-
Mechanisms of modulation of cytokine release by human cord blood monocytes exposed to high concentrations of caffeine
Pediatric Research (2016)