Abstract
Eighty to 85% of the venous perfusion to the fetal liver is from the umbilical vein, the rest from the portal vein. Umbilical venous flow to the liver is essential for intrauterine growth, and is impaired in placental insufficiency. We hypothesized that in growth-restricted fetuses portal blood flow compensates for insufficient umbilical blood flow to the liver. In 29 fetuses with fetal growth restriction (estimated fetal weight ≤5th percentile), we used ultrasound to measure blood flows in the umbilical vein, ductus venosus, left portal vein, and main portal stem. Compared with normal fetuses, both absolute and normalized total venous liver blood flows were reduced in growth-restricted fetuses, related to the degree of placental compromise and equally affecting both liver lobes. However, portal replaced umbilical flow to the right lobe, in a manner graded according to placental vascular resistance; in extreme cases, the right lobe received no umbilical perfusion. In fetal growth restriction, the liver suffers from venous hypoperfusion, and portal blood partially replaces umbilical flow to the right lobe; this will result in right liver lobe hypoxemia. This striking prioritization in nutrient delivery of left over right lobes suggests an adaptive response to poor placental perfusion that may have functional consequences.
Similar content being viewed by others
Main
Placental insufficiency is a major cause of impaired fetal growth and premature birth, which, in turn, is associated with an increased risk of chronic disease in later life (1). Decreased placental function forces the fetus to redistribute its circulation to maintain oxygen and nutrient supply to the heart and brain, but these responses that are beneficial in the short term may have deleterious long-term effects. One of these adaptive changes is increased shunting of nutrient-rich umbilical venous blood through the ductus venosus thereby decreasing the amount of umbilical venous blood perfusing the liver (2,3). Experimental data indicate a direct relationship between venous liver perfusion and fetal organ growth (4). Thus, the study of fetal liver flow may further elucidate the pathogenesis of fetal growth restriction (FGR).
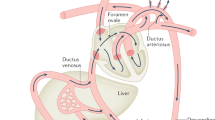
The venous perfusion of the fetal liver differs from that after birth in that, venous blood flows to it both from the splanchnic bed via the portal vein and from the placenta via the umbilical vein (Fig. 4A). Although the latter has been studied extensively, the role of portal perfusion during placental compromise is unknown. Therefore, we developed a technique for blood flow measurement in the main portal stem and established reference ranges for this flow, showing that in uncompromised fetuses 15–20% of the venous liver perfusion is of splanchnic origin (5).
Typical pattern of venous blood supply of the fetal liver with level of oxygenation, flow direction (blue arrows), and flow amount (arrow size) in the uncompromised (A) and growth-restricted fetus (B). Red, high oxygen saturation; purple, low oxygen saturation. UV, umbilical vein; DV, ductus venosus; LPV, left portal vein; RPV, right portal vein; PV, main stem of the portal vein; LeLL, left liver lobe; RiLL, right liver lobe; ST, stomach; SP, spine. Volume blood flow (mL/min) in brackets: Panel A, corresponding to the mean for 32 gestational wks in uncompromised pregnancies, panel B, one FGR case at 32 + 4 wk.
In this study, we have gone on to characterize hepatic venous perfusion in FGR and to test the hypothesis that portal blood flow may compensate for insufficient umbilical blood flow to the liver when the latter is diverted through the ductus venosus.
MATERIALS AND METHODS
In a cross-sectional study, we examined 31 growth-restricted (i.e., estimated fetal weight ≤5th percentile) singleton fetuses without malformations or chromosomal abnormalities. Gestational age was determined by ultrasound in the second trimester (6). The study protocol was approved by the local research ethics committee (Regional Committee for Research Ethics, Western Norway, REK-Vest 04/3837) and participants were recruited after informed written consent from April 2005 until March 2007. At delivery, gender and birth weight were recorded. Cord acid-base data from both the umbilical artery and vein were obtained by clamping a cord segment immediately after delivery. The cord blood samples were analyzed by an ABL 735 Radiometer (Bergman Diagnostica, Oslo, Norway) calculating base deficit of the extracellular fluid according to Siggaard-Andersen algorithm (7).
A Sonos 7500 ultrasound machine (Philips, Seattle, WA) with a 3.5 MHz (2–6 MHz) curved linear transducer including color Doppler (2.5 MHz) and pulsed Doppler (3 MHz) facilities was used for the study. The high-pass filter was set at 50 Hz. Vessel diameter and flow velocities [time-averaged maximum flow velocity (TAMXV)] were measured at the intra-abdominal portion of the umbilical vein, inlet of the ductus venosus, left portal vein, and main portal stem. Flow velocity was measured in an insonation along the vessel axis with the angle of insonation kept as low as possible and always lower than 30 degree. For diameter measurement, the vessel walls were insonated perpendicularly and the mean of the repeated measurements was used for flow calculation according to the formula: Q = π(D/2)2hTAMXV (h: velocity profile h = 0.7 for ductus venosus, h = 0.5 for the umbilical and portal veins). Further details on measurement techniques and calculation of volume blood flow (Q) are described elsewhere (5,8,9). The pulsatility index of the umbilical artery (PIumb artery) was measured in a free cord loop.
The total venous blood supply of the liver (Qliver) was calculated as: Qliver = Qumbilical vein − Qductus venosus + Qportal vein. The venous blood flow to the left liver lobe (Qleft liver) was calculated as: Qleft liver = Qumbilical vein − (Qductus venosus + Qleft portal vein) and to the right lobe (Qright liver) as: Qright liver = Qportal vein + Qleft portal vein. The umbilical and portal fraction of the total venous liver supply was calculated as (Qumbilical vein − Qductus venosus) × 100%/Qliver and Qportal vein × 100%/Qliver, respectively. Blood flow to the right and left lobes relative to the total venous liver flow was Qleft liver × 100%/Qliver and Qright liver × 100%/Qliver, respectively. The ductus venosus shunt fraction was calculated as Qductus venosus/Qumbilical vein × 100%. The blood flow was normalized for estimated fetal weight, calculated by Combs formula. The corresponding percentile was obtained using gender-specific reference ranges (10). Blood flow data from FGR were compared with a reference population (11) using z scores (SDS). The relationship between continuous variables was assessed by linear regression analysis. PIumb artery >97.5 percentile was considered representing a more severe degree of hemodynamic compromise than those below and the group was divided accordingly for subanalyses. Statistical analysis was performed using SPSS (Statistical Package for the Social Sciences, SPSS Inc, Chicago, IL).
RESULTS
Of the 31 fetuses with estimated fetal weight ≤5th percentile, 2 had a birth weight on the 25th percentile, giving 29 fetuses eligible for analysis. Sixteen of 29 fetuses had normal and 13 of 29 increased PIumb artery (>97.5 percentile). Further characteristics of the study population are shown in Table 1. Missing measurements (Table 2) were due to unfavorable fetal position, fetal movements, or time constraints. FGR fetuses shunted an increased fraction of umbilical blood through the ductus venosus (Table 2, Fig. 1A). Although absolute ductus venosus flow is decreased (Table 2, Fig. 1B), normalized flow is maintained compared with the reference population (Table 2, Fig. 1C). FGR fetuses had relatively low total venous liver flow (Table 2, Fig. 2A), even when normalized for fetal weight (Table 2, Fig. 2B). The low total venous blood flow was related to the degree of placental compromise and equally affected the left and right liver lobes (Table 2, Fig. 2C and D), thus maintaining the fractional distribution between left (60%) vs. right liver lobe (40%) as in the reference population (Table 2, Fig. 3A). However, growth-restricted fetuses substituted umbilical with portal blood to the right liver lobe depending on the degree of placental compromise, and in extreme cases the right lobe was exclusively perfused by portal blood (Table 2, Fig. 3B). There was a strong inverse relationship between the portal contribution to the right liver lobe flow and the time-averaged maximum blood velocity in the left portal vein (r = 0.73; p < 0.0001) (Fig. 3C). Furthermore, our data suggest a direct relationship between time-averaged maximum blood velocity in the left portal vein and the oxygen partial pressure in the umbilical artery sampled at birth (Fig. 3D).
Ductus venosus shunt fraction (A), absolute (B), and normalized (C). Ductus venosus blood flow in growth-restricted fetuses with normal (light blue bars, n = 16) and increased (dark blue bars, n = 7) umbilical artery pulsatility. Data presented in relation to gestational age with mean, 5th and 95th percentile of the reference population.
Total venous liver flow in absolute terms (A) and normalized for fetal weight (B); venous blood flow to the left (C) and right (D) liver lobe in growth-restricted fetuses with normal (light blue bars; n = 16) and increased (dark blue bars; n = 9 [A and B]; n = 10 [C]; n = 7 [D]) umbilical artery pulsatility. Data presented in relation to gestational age with mean, 5th and 95th percentile of the reference population.
The portal contribution to right liver lobe flow (A) and the contribution of right liver lobe flow to total venous liver flow (B) in growth-restricted fetuses with normal (light blue bars, n = 16) and increased (dark blue bars, n = 7) umbilical artery pulsatility. Data presented in relation to gestational age with mean, 5th and 95th percentile of the reference population. The two cases with a portal contribution >100% had reversed blood flow in the left portal vein. Relationship between left portal vein time-averaged maximum flow velocity (TAMXV) and the portal contribution to right liver lobe flow (r = 0.73, p < 0.0001) (C), and the cord artery oxygen tension at delivery (r = 0.52, p = 0.059) (D) with 95% confidence interval for the regression lines (curved lines).
DISCUSSION
This study demonstrates that in FGR the venous perfusion of the liver is reduced. To minimize the volume discrepancy, portal blood (with low oxygenation) increasingly substitutes oxygen- and nutrient-rich umbilical flow to the right liver lobe, graded according to umbilical artery compromise, worsening oxygenation of this part of the liver (Fig. 4).
In agreement with previous human studies (2,3), we found an increased ductus venosus shunt fraction in FGR as part of the circulatory adaptation to placental insufficiency.
Our findings also confirm microsphere blood flow measurements in fetal sheep (12) and a Doppler study in human FGR (3) showing a greater drop in umbilical blood flow to the right compared with the left lobe of the liver during placental compromise. The fraction of cardiac output directed to the placenta for reoxygenation is decreased in growth-restricted fetuses (13) with a correspondingly increased fraction recirculating within the fetal body. In fetal lambs, portal venous blood is largely oxygen depleted with a saturation of 30% (14). The relative increase in portal blood directed to the liver observed in this study thus constitutes part of this recirculation although it mainly affects the right lobe. This is reflected in the blood velocity in the left portal vein (Fig. 3C). A reduced or reversed blood velocity in this vessel has been shown to be a direct reflection of the altered distribution between umbilical and portal blood supply to the liver, the umbilicoportal watershed (Fig. 4) (15). The shift in this watershed toward the left liver lobe was related to the degree of hypoxemia at birth (Fig. 3D). The decreased splenic artery pulsatility reported in FGR with low cord Po2 (16) provides further support for the concept that splanchnic vessels contribute to increased recirculation during hypoxemia.
It is likely that a reduction in well-oxygenated umbilical venous flow will affect liver growth and function. Indeed, a direct relationship between venous liver perfusion and tissue proliferation was demonstrated in fetal sheep (4). Our findings suggest that this will affect predominantly the right lobe and, interestingly, fetal lambs exposed to reduced uterine blood flow selectively show reduced growth of the right lobe of the liver (17). Selective right liver lobe damage was also reported in human fetuses that have suffered intrauterine asphyxia (18). Furthermore, exposure of the liver to hypoxia per se may also induce reduced fetal growth: human hepatocytes cultured in hypoxic conditions increase their expression of IGF binding proteins, which inhibits the IGF action (19). Impaired liver growth has been previously demonstrated in human FGR (20,21) although differences between the lobes were not addressed.
Even in apparently uncompromised pregnancies differences in microarchitecture, enzyme function (22), and gene expression (23) between the fetal right and left liver lobes have been found and mainly ascribed to fetal venous perfusion patterns. Because differences between the liver lobes seem to persist into adulthood (24,25), the circulatory changes within the liver of growth-restricted fetuses may have long-term implications on liver function after birth. Growth-restricted neonates showed a decreased hepatic biotransformation compared with appropriately grown babies (26). Moreover, a recent case-control study of children with nonalcoholic fatty liver disease showed a 39% prevalence of being born small for gestational age, compared with 7% among controls (27).
FGR fetuses with normal umbilical artery pulsatility index are commonly considered to be at minor risk for perinatal complications. Interestingly, this study demonstrated that such fetuses not only may show brain sparing (28,29) with potential consequences for postnatal development (30), but also an impaired and distorted liver perfusion.
In conclusion, both mild and severe placental insufficiency cause venous hypoperfusion of the fetal liver and an increased contribution of poorly oxygenated blood from the portal vein. This predominantly affects the right liver lobe (Fig. 4). The reduced oxygenation of the right lobe may have longer-term consequences, ranging from the right liver necrosis occasionally described in the perinatal period to alterations in metabolic processes, which may predispose to later disease.
Abbreviations
- FGR:
-
fetal growth restriction
References
Godfrey KM, Lillycrop KA, Burdge GC, Gluckman PD, Hanson MA 2007 Epigenetic mechanisms and the mismatch concept of the developmental origins of health and disease. Pediatr Res 61: 5R–10R
Kiserud T, Kessler J, Ebbing C, Rasmussen S 2006 Ductus venosus shunting in growth-restricted fetuses and the effect of umbilical circulatory compromise. Ultrasound Obstet Gynecol 28: 143–149
Bellotti M, Pennati G, De Gasperi C, Bozzo M, Battaglia FC, Ferrazzi E 2004 Simultaneous measurements of umbilical venous, fetal hepatic, and ductus venosus blood flow in growth-restricted human fetuses. Am J Obstet Gynecol 190: 1347–1358
Tchirikov M, Kertschanska S, Sturenberg HJ, Schroder HJ 2002 Liver blood perfusion as a possible instrument for fetal growth regulation. Placenta 23: S153–S158
Kessler J, Rasmussen S, Kiserud T 2007 The fetal portal vein: normal blood flow development during the second half of human pregnancy. Ultrasound Obstet Gynecol 30: 52–60
Johnsen SL, Rasmussen S, Sollien R, Kiserud T 2004 Fetal age assessment based on ultrasound head biometry and the effect of maternal and fetal factors. Acta Obstet Gynecol Scand 83: 716–723
Siggaard-Andersen O 1971 An acid-base chart for arterial blood with normal and pathophysiological reference areas. Scand J Clin Lab Invest 27: 239–245
Kiserud T, Rasmussen S, Skulstad S 2000 Blood flow and the degree of shunting through the ductus venosus in the human fetus. Am J Obstet Gynecol 182: 147–153
Haugen G, Kiserud T, Godfrey K, Crozier S, Hanson M 2004 Portal and umbilical venous blood supply to the liver in the human fetus near term. Ultrasound Obstet Gynecol 24: 599–605
Johnsen SL, Rasmussen S, Wilsgaard T, Sollien R, Kiserud T 2006 Longitudinal reference ranges for estimated fetal weight. Acta Obstet Gynecol Scand 85: 286–297
Kessler J, Rasmussen S, Godfrey K, Hanson M, Kiserud T 2008 Longitudinal Study of Umbilical and Portal Venous Blood flow to the Fetal Liver: low pregnancy weight gain is associated with preferential supply to the fetal left liver lobe. Pediatr Res 63: 315–320
Edelstone DI, Rudolph AM, Heymann MA 1980 Effect of hypoxemia and decreasing umbilical flow on liver and ductus venosus blood flows in fetal lambs. Am J Physiol 238: H656–H663
Kiserud T, Ebbing C, Kessler J, Rasmussen S 2006 Fetal cardiac output, distribution to the placenta and impact of placental compromise. Ultrasound Obstet Gynecol 28: 126–136
Rudolph AM 1983 Hepatic and ductus venosus blood flows during fetal life. Hepatology 3: 254–258
Kessler J, Rasmussen S, Kiserud T 2007 The left portal vein as an indicator of watershed in the fetal circulation: development during the second half of pregnancy and a suggested method of evaluation. Ultrasound Obstet Gynecol 30: 757–764
Capponi A, Rizzo G, Arduini D, Romanini C 1997 Splenic artery velocity waveforms in small-for-gestational-age fetuses: relationship with pH and blood gases measured in umbilical blood at cordocentesis. Am J Obstet Gynecol 176: 300–307
McLellan KC, Bocking AD, White SE, Han VK 1995 Placental and fetal hepatic growth are selectively inhibited by prolonged reductions of uterine blood flow in pregnant sheep. Reprod Fertil Dev 7: 405–410
Gruenwald P 1949 Degenerative changes in the right-half of the liver resulting from intra-uterine anoxia. Am J Clin Pathol 19: 801–813
Popovici RM, Lu M, Bhatia S, Faessen GH, Giaccia AJ, Giudice LC 2001 Hypoxia regulates insulin-like growth factor-binding protein 1 in human fetal hepatocytes in primary culture: suggestive molecular mechanisms for in utero fetal growth restriction caused by uteroplacental insufficiency. J Clin Endocrinol Metab 86: 2653–2659
Boito S, Struijk PC, Ursem NT, Fedele L, Wladimiroff JW 2003 Fetal brain/liver volume ratio and umbilical volume flow parameters relative to normal and abnormal human development. Ultrasound Obstet Gynecol 21: 256–261
Latini G, De Mitri B, Del Vecchio A, Chitano G, De Felice C, Zetterstrom R 2004 Foetal growth of kidneys, liver and spleen in intrauterine growth restriction: “programming” causing “metabolic syndrome” in adult age. Acta Paediatr 93: 1635–1639
Emery JL 1963 Functional asymmetry of the liver. Ann NY Acad Sci 111: 37–44
Cox LA, Schlabritz-Loutsevitch N, Hubbard GB, Nijland MJ, McDonald TJ, Nathanielsz PW 2006 Gene expression profile differences in left and right liver lobes from mid-gestation fetal baboons: a cautionary tale. J Physiol 572: 59–66
Jacobsson H, Jonas E, Hellstrom PM, Larsson SA 2005 Different concentrations of various radiopharmaceuticals in the two main liver lobes: a preliminary study in clinical patients. J Gastroenterol 40: 733–738
Barbaro B, Palazzoni G, Prudenzano R, Cina A, Manfredi R, Marano P 1999 Doppler sonographic assessment of functional response of the right and left portal venous branches to a meal. J Clin Ultrasound 27: 75–80
Boehm G, Teichmann B, Krumbiegel P 1995 Hepatic biotransformation capacity in low-birth-weight infants as measured with the [15N]methacetin urine test: influences of gestational age, postnatal age, and intrauterine growth retardation. Biol Neonate 68: 19–25
Nobili V, Marcellini M, Marchesini G, Vanni E, Manco M, Villani A, Bugianesi E 2007 Intrauterine growth retardation, insulin resistance, and nonalcoholic fatty liver disease in children. Diabetes Care 30: 2638–2640
Hershkovitz R, Kingdom JC, Geary M, Rodeck CH 2000 Fetal cerebral blood flow redistribution in late gestation: identification of compromise in small fetuses with normal umbilical artery Doppler. Ultrasound Obstet Gynecol 15: 209–212
Severi FM, Bocchi C, Visentin A, Falco P, Cobellis L, Florio P, Zagonari S, Pilu G 2002 Uterine and fetal cerebral Doppler predict the outcome of third-trimester small-for-gestational age fetuses with normal umbilical artery Doppler. Ultrasound Obstet Gynecol 19: 225–228
Eixarch Meler E, Iraola A, Illa M, Crispi F, Hernandez-Andrade E, Gratacos E, Figueras F 2008 Neurodevelopmental outcome in 2-year-old infants who were small-for-gestational age term fetuses with cerebral blood flow redistribution. Ultrasound Obstet Gynecol 32: 894–899
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National health council of Norway and the Western Norway Regional Health Authority (J.K.) and by British Heart Foundation (M.H.).
Rights and permissions
About this article
Cite this article
Kessler, J., Rasmussen, S., Godfrey, K. et al. Fetal Growth Restriction Is Associated With Prioritization of Umbilical Blood Flow to the Left Hepatic Lobe at the Expense of the Right Lobe. Pediatr Res 66, 113–117 (2009). https://doi.org/10.1203/PDR.0b013e3181a29077
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/PDR.0b013e3181a29077