Abstract
Intrauterine growth restriction (IUGR) is associated with an increased risk for short stature and diseases in adulthood thought to be inflicted by fetal programming. We hypothesized that placental endocrine systems involved in perinatal growth might also play a role in postnatal growth after IUGR. In a prospective controlled multicenter study, placental gene expression of IGF-binding protein-1 (IGFBP-1), leptin and 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2) were measured in 14 IUGR infants and 15 children born appropriate for gestational age (AGA) proven by serial ultrasound examinations. Postnatally, IUGR infants experienced a significantly higher growth velocity than AGA neonates (at 1 y: p = 0.001). Gene expression of 11β-HSD2 at birth correlated positively with birth length (r = 0.55, p = 0.04) and inversely with growth velocity in the first year of life (r = −0.69, p = 0.01) in the IUGR, but not in the AGA group. There was no correlation between gene expression of placental IGFBP-1, leptin and birth weight, length and growth velocity during the first year of life. AGA infants showed significantly higher concentrations of cortisone in venous cord blood after birth (p = 0.02) as a surrogate of a higher 11β-HSD2 activity in the fetoplacental unit. In conclusion, placental 11β-HSD2 gene expression might predict postnatal growth in IUGR.
Similar content being viewed by others
Main
Traditionally, the term “small for gestational age” (SGA) has been used to describe a fetus or a neonate whose birth weight or birth length is below the 3rd percentile or <−2 standard deviations below the mean for the infant's gestational age and sex. In contrast, intrauterine growth restriction (IUGR) implies an underlying pathologic process that prevents the fetus from achieving its growth potential. Being born SGA does not necessarily mean that IUGR has occurred. Although the term IUGR is often used synonymously with the term SGA, its use should be restricted to describing infants whose small size can be attributed to a specific cause and whose prenatal growth has been confirmed by several anomalous intrauterine growth assessments.
Low birth weight is associated with an increased risk for short stature, insulin resistance, childhood obesity, premature adrenarche, hypertension, and renal disease (1–3). The process leading to these diseases in later child- or adulthood is called perinatal programming (4).
Various placental endocrine regulators have been linked to IUGR, e.g., leptin, 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2) and IGF-binding protein-1 (IGFBP-1) (5–7). These markers might play a role as pathophysiological links between small birth size and obesity, short stature and hypertension, respectively, although this still needs to be shown in humans.
In SGA-neonates, the amount of adipose tissue is dramatically less than in neonates born appropriate for gestational age (AGA) (8). Additionally, body composition is unhealthy in SGA, as fat is generally deposited centrally (9). The concentrations of circulating leptin and adiponectin in individuals born SGA are lower than in AGA-infants, even after correction for body mass index (BMI), gender and hyperinsulinemia (10). A specific development of neurons in the hypothalamus could only be detected after a postnatal leptin infusion (11). One could therefore hypothesize that reduced adipose tissue in IUGR resulting in decreased leptin serum concentrations may potentially lead to altered hypothalamic programming of appetite regulation and tissue proliferation. It has also been shown that glucocorticoid excess during fetal life is thought to play an important role in early-life programming, promoting growth restriction and leading to adult metabolic, cardiovascular and neuroendocrine disease (12). In normal pregnancy, the placenta expresses high levels of 11β-HSD2 that builds a placental barrier by converting cortisol into the inactive form cortisone, thus protecting the fetus from excess maternal cortisol (13). MRNA expression of 11β-HSD2 is reduced in IUGR placenta (7). The impairment of this glucocorticoid barrier is thought to result in fetal cortisol excess (12). The hypothesis that excess exposure of the fetoplacental unit to maternal glucocorticoids reduces birth weight and programs subsequent metabolic and neuroendocrine development has been supported by studies using pharmacological blockade of 11β-HSD2 activity (14) and observations in human hypertension caused by 11β-HSD2 mutations (15). In the rat, blocking maternal corticosterone secretion during restraint stress also blocks stress-induced effects in the offspring (16). Therefore, elevated fetal cortisol concentrations promote growth restriction and seem to play an important role in programming of fetal cortisol metabolism, whereas moderate amounts of cortisol are important to facilitate fetal organ maturation (17).
Additionally, fetal growth is influenced by the IGF signaling system (6). The circulating level of IGF-binding protein (IGFBP)-1, a secreted protein that binds to IGF in extracellular environments, is elevated in IUGR fetuses (18). In vitro, IGFBP-1 binds IGFs with high affinity and inhibits IGF activities on cell growth (19). In animal studies, IGFBP-1 overexpressing transgenic mice showed reduced birth weight (20). Hypoxia strongly induces the expression of IGFBP-1 (21). In zebrafish embryos, hypoxia treatment resulted in embryonic growth retardation and developmental delay, concomitant with a significant increase in IGFBP-1 mRNA and protein levels, inhibiting IGF-1 and IGF-2-stimulated cell proliferation (22). Therefore, in hypoxia-caused IUGR, IGFBP-1 seems to play a major role by binding fetal IGFs and inhibiting their growth-promoting activities.
We hypothesized that placental endocrine systems involved in perinatal growth and, potentially, in the process of fetal programming might play a role in postnatal growth after IUGR.
SUBJECTS AND METHODS
We enrolled 14 infants with prenatal ultrasound-proven IUGR (six males, eight females) and 15 healthy newborns (nine males, six females) in the study. Inclusion and exclusion criteria are presented in Table 1. All the patients were of Caucasian ethnicity and were born during 2 y of study period. Initially 16 IUGR babies were recruited, two of them had to be excluded from the study because of maternal preeclampsia. As we defined IUGR by anomalous placental Doppler velocimetry, all of the IUGR pregnancies in this study experienced placental complications and potential hypoxia. Fifty-seven percent of the IUGR and 40% of the AGA infants enrolled in this study were premature. The clinical characteristics of the two groups at birth are indicated in Table 2.
Auxological parameters were studied at birth and at 1 y of age in 14 IUGR infants and 15 children of the control group. Length, weight, BMI (kg/m2), and head circumference were expressed as a SD score (SDS) and corrected for gestational age and gender. Birth length and weight were calculated according to Voigt et al. (23), for head circumference and length at 1 y of age the reference values from Prader et al. (24) were used. BMI was calculated at 1 y of age according to Kromeyer-Hauschild et al. (25). Gestational age was determined from the date of the last menstrual period of the mother and was confirmed by ultrasound measurements before the 14th week of gestation. Prematurity was defined as a gestational age <37 wk.
Acquisition of umbilical cord blood.
Blood specimens from the umbilical vein of each placenta were collected after placental delivery in collaboration with the Department of Obstetrics and Gynaecology at the University of Erlangen-Nuremberg and were immediately put on ice for transport. After centrifugation at 5000 rpm for 10 min, aliquots of plasma were stored at −20°C until further analysis.
Measurement of cortisol and cortisone.
Plasma cortisol and cortisone were measured simultaneously according to a modified method of Vogeser et al. (26), using liquid chromatography-mass spectrometry/mass spectrometry with atmospheric pressure chemical ionization in the positive ion mode. The intraassay coefficients of variation were 2.6-4.0% for cortisone (concentration range 19-38 ng/mL) and 2.0-5.6% for cortisol (concentration range 55-206 ng/mL).
Measurement of leptin and IGF-1.
The concentration of leptin and IGF-1 in venous cord blood was determined using a commercial RIA (Mediagnost GmbH, Reutlingen, Germany).
Placental tissue acquisition.
Fresh samples of human placenta were obtained in a standardized procedure within 30 min after placental delivery. Using a sterile scalpel a quadrangular segment (approximately 2 × 2 cm) along the placental thickness from basal toward chorionic surface was excised, which was localized at the central region of the placenta. After a rinse of the samples with normal saline, the amniotic membranes and the maternal decidua were removed, and then the samples were snap frozen in liquid nitrogen and stored at −80°C until further processing.
RNA extraction and reverse transcription.
Total cellular RNA was extracted from the placental tissue by TRIzol® reagent (TRIzol®, Invitrogen GmbH, Karlsruhe, Germany). RNA concentrations were determined spectrophotometrically. One microgram of RNA was reversely transcribed in a volume of 20 μL at 37°C for 60 min to synthesize cDNA (chemicals from Boehringer Mannheim, Germany).
TaqMan real-time PCR.
TaqMan real-time PCR (Perkin Elmer, Foster City, CA) was used to quantify the expression of genes in placenta of IUGR and AGA. The mRNA expression was normalized to two different housekeeping genes, hypoxanthine guanine phosphoribosyl transferase (HPRT) and β2-microglobulin (β2MG), that have been shown not to respond to hypoxia and that can be detected pseudogene free. The method is established and validated in our group and has been applied successfully for the quantification of gene expression in placental biopsies (7,27).
Commercial reagents (TaqMan PCR reagent kit, Perkin-Elmer) and conditions were applied according to the manufacturer's protocol. A 2.5 μL of cDNA (reverse transcription mixture) and oligonucleotides at a final concentration of 300 nmol/L (HPRT, 11βHSD2, IGFBP1 forward) or 600 nM [β2MG, IGFBP1 reverse, 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1), leptin] of primers and 200 nmol/L of TaqMan hybridization probe were analyzed in a 25 μL volume. All of the primers and probes were purchased from Eurogentec (Belgium) and Sigma Chemical Co. (Germany). The thermocycler parameters were 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C and 60°C for 1 min. Serial dilutions of one of the samples served as reference providing relative quantification of the unknown samples. Primers and probes are shown in Table 3.
The study was reviewed and approved by the Ethical Committee of the Medical Faculty of the University of Erlangen-Nuremberg. It was explained to each parent who signed a written consent.
Statistical analysis.
Results were expressed as mean ± standard error of the mean and median, minimum, maximum. Statistical analyses were performed using GraphPad Prism® Version 4.0c for Apple Macintosh, GraphPad Software, San Diego, CA (www.graphpad.com). The normal distribution of the data was determined using the Kolmogorov-Smirnov test. The data were analyzed using the unpaired parametric (t test) and nonparametric tests (Mann Whitney test). The correlation analysis was performed using Pearson's or Spearman's linear regression analyses as required. Fisher's exact test was used for the analysis of categorical data. The limit of significance was set at a p value of <0.05.
RESULTS
At birth, there was a significant difference in length, weight, and head circumference between IUGR and AGA infants as expected. This difference persisted after 1 y for head circumference but not for BMI and body length (Table 4).
After IUGR, children experienced a significantly higher growth velocity within their first year of life than control infants (p = 0.001, Fig. 1). Comparing growth velocity between male and female patients, there was no difference neither in the IUGR (male: 29.7 ± 1.1 cm/y vs. female: 29.9 ± 1.6 cm/y, p = 0.93) nor in the AGA group (male: 25.6 ± 0.9 cm/y vs. female: 24.8 ± 1.5 cm/y, p = 0.63), indicating that gender did not influence this finding. Gestational age showed an inverse correlation with growth velocity in IUGR infants (r = −0.77, p = 0.001) and in AGA infants (r = −0.58, p = 0.02). When analyzing all patients together, this inverse correlation between gestational age and growth velocity persisted (r = −0.69, p < 0.0001).
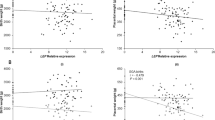
Considering placental endocrine systems, we found a positive correlation between gene expression of 11β-HSD2 and birth length in the IUGR group (r = 0.55, p = 0.04) but not in AGA infants (r = 0.06, p = 0.82, Fig. 2). Furthermore, the gene expression of 11β-HSD2 at birth correlated inversely with growth velocity in the first year of life only in children after IUGR (r = −0.69, p = 0.01, Fig. 3). There was an inverse correlation between growth velocity and gestational age but no relationship was seen for 11β-HSD2 gene expression and gestational age neither in the IUGR (r = 0.16, p = 0.61) nor in the AGA group (r = −0.30, p = 0.28).
A direct comparison of 11β-HSD2 gene expression between male and female patients did not show any significant differences both in the IUGR (male: 0.56 ± 0.17 vs. female: 0.53 ± 0.16, relative gene expression, p = 0.92) and the AGA group (male: 0.81 ± 0.13 vs. female: 0.60 ± 0.06, p = 0.23). In the AGA group, where eight from 15 children were born by spontaneous delivery, there was no difference in 11β-HSD2 gene expression between spontaneous and caesarean section delivery (spontaneous delivery: 0.71 ± 0.13 vs. section: 0.74 ± 0.11, relative gene expression, p = 0.83).
No correlation was found between gene expression of placental IGFBP-1, leptin and birth weight, length and growth velocity during the first year of life (Table 5). Furthermore, there was no correlation between gene expression of placental 11β-HSD2 and leptin, 11β-HSD2 and IGFBP-1, and leptin and IGFBP-1 in IUGR and AGA infants (data not shown). This finding indicates that gene expression of 11β-HSD2, leptin and IGFBP-1 are not influenced by each other, which means that they are regulated each in a separate way.
The direct comparison of gene expression data between IUGR and AGA neonates showed increased mRNA expression of leptin (IUGR: 2.19 ± 0.84, AGA: 1.38 ± 0.74, relative gene expression, p = 0.47) and IGFBP-1 in IUGR neonates (IUGR: 0.11 ± 0.06, AGA: 0.05 ± 0.04, relative gene expression, p = 0.0.06), which was not statistically significant. MRNA expression of 11β-HSD2 (IUGR: 0.30 ± 0.05, AGA: 0.33 ± 0.04, relative gene expression, p = 0.62) and 11β-HSD1 (IUGR: 0.63 ± 0.09, AGA: 1.11 ± 0.41, relative gene expression, p = 0.31) was decreased in the IUGR group without reaching statistical significance.
All data for placental gene expression are presented as relative gene expression normalized for HPRT. The use of the alternative housekeeping gene β2MG does not change the main results (data not shown).
To analyze a surrogate of 11β-HSD2 activity in the fetoplacental unit, cortisol and cortisone was determined in the umbilical vein after birth. Although the comparison of the cortisol level yielded no differences between IUGR and AGA children (IUGR: 25.0 ± 12.4 ng/mL; AGA: 19.4 ± 3.0 ng/mL, p = 0.62), AGA infants showed significantly higher concentrations of cortisone in venous cord blood (p = 0.02, Fig. 4). When analyzing the cortisol/cortisone ratio, there was no difference between IUGR and AGA (IUGR: 0.20 ± 0.04; AGA: 0.16 ± 0.02, p = 0.33). Placental gene expression of 11β-HSD2 did not correlate to plasma cortisol (IUGR: r = −0.18, p = 0.62; AGA: r = −0.11, p = 0.72), plasma cortisone (IUGR: r = −0.22, p = 0.55; AGA: r = 0.25, p = 0.39), or the cortisol/cortisone ratio (IUGR: r = 0.11, p = 0.76; AGA: r = −0.44, p = 0.11). Neither in the IUGR nor in the control group, we could detect any correlation between the plasma concentration of cortisol or cortisone and birth weight SDS (cortisol: IUGR: r = 0.25, p = 0.49; AGA: r = −0.16, p = 0.59; cortisone: IUGR: r = 0.57, p = 0.08; AGA: r = 0.51, p = 0.06), birth length SDS (cortisol: IUGR: r = −0.10, p = 0.78; AGA: r = 0.01, p = 0.97; cortisone: IUGR: r = 0.53, p = 0.12; AGA: r = 0.45, p = 0.10) and growth velocity in the first year of life (cortisol: IUGR: r = 0.45, p = 0.19; AGA: r = −0.47, p = 0.09; cortisone: IUGR: r = −0.03, p = 0.94; AGA: r = −0.38, p = 0.18).
Furthermore, we measured cord levels of plasma leptin and IGF-1 as one factor whose active concentration is regulated by IGFBP-1. The direct comparison of leptin and IGF-1 plasma concentration yielded no difference between IUGR and AGA infants (leptin: IUGR: 5.3 ± 1.1, AGA: 2.9 ± 0.7, p = 0.11; IGF-1: IUGR: 60.6 ± 16.8, AGA: 86.0 ± 7.6, p = 0.13). The concentration of leptin and IGF-1 in venous cord blood as opposed to placental mRNA did neither correlate to birth weight (leptin: IUGR: r = −0.52, p = 0.37; AGA: r = 0.01, p = 0.97; IGF-1: IUGR: r = 0.12, p = 0.82; AGA: r = 0.30, p = 0.32) nor to growth velocity (leptin: IUGR: r = 0.53, p = 0.36; AGA: r = −0.39, p = 0.19; IGF-1: IUGR: r = −0.14, p = 0.79; AGA: r = −0.42, p = 0.16).
DISCUSSION
There are multiple studies investigating growth patterns after IUGR. In most previous studies, IUGR is defined exclusively by birth weight below the 3rd or 10th percentile or <−2 SDS, without further differentiation between IUGR and infants born SGA. In this study, we defined IUGR by pathological uterine or umbilical artery Doppler velocimetry (28), shift of fetal growth with a reduction of abdominal circumference in addition to a birth weight below the 10th percentile according to Voigt et al. (23). By considering these pre- and perinatal data, we ensure that only children with IUGR and born SGA are enrolled and examined in our study.
It is well known that infancy is the fastest growing period of childhood. One third of total growth occurs in the child's first 2 y, and this is mainly nutrient dependant. About 90% of newborns with low birth weight show catch-up growth until 2 y of age. The remaining 10% of these children show short stature in later life (29). Catch-up growth is considered to be a compensatory mechanism for prenatal growth restriction by accelerating postnatal growth velocity. Premature cardiovascular and metabolic diseases in adults seem to be enhanced by a combination of IUGR and later catch-up growth and obesity (30).
Our data show that gene expression of 11β-HSD2 at birth correlates positively with birth length and inversely with growth velocity at 1 y of age in the IUGR group. There is a potential explanation for this observation. Attenuated placental 11β-HSD2 activity results in excessive amounts of maternal glucocorticoids reaching the fetus. Increased corticosteroids lead to impaired fetal growth and low birth weight. When this excess is stopped after birth, postnatal catch-up growth can take place. This is in line with the observation of impaired growth in children before and after renal transplantation, despite satisfactory organ function, because of the effects of steroids used for immunosuppression (31). Moreover, corticosteroids induce a hyporesponsiveness to the action of growth hormone. In this context, supraphysiological doses of growth hormone or IGF-1 in vitro and in vivo can partially overcome the growth-inhibiting effects of glucocorticoid treatment (32).
Glucocorticoid excess during fetal life is thought to play an important role in early-life programming and may contribute to adult metabolic, cardiovascular, and neuroendocrine disease (12). Because our data show a correlation of placental gene expression of 11β-HSD2 with postnatal growth only in IUGR and not AGA infants during the first year of life, we speculate that placental 11β-HSD2 gene expression can be used as a predictive value for the development of disease in later life.
Considering birth characteristics of our patients, there is a marked difference in the rate of spontaneous and caesarean section delivery between the two groups, which might be a potential confounding factor. However, we analyze data from IUGR and AGA infants separately and perform correlations between 11β-HSD2 gene expression and birth length and growth velocity only within each group. In this context, the mode of delivery should not influence the results. Moreover, we find no difference in 11β-HSD2 gene expression between infants born by caesarean section and infants born by spontaneous delivery in the AGA group, where the number of patients for each subgroup is almost equal. The effect of gender on 11β-HSD2 gene expression as another potential confounding factor was also analyzed and did not change the results. As we did not obtain information on postnatal nutrition from the parents, we could not evaluate the potential influence of different feeding practices on the lack of difference in BMI at 1 y of age between IUGR and AGA infants.
Placental 11β-HSD2 activity and expression are reduced in pregnancies complicated with fetal growth restriction (33). In parallel, gene expression of 11β-HSD1 is also decreased in children born SGA (7). Our data are in line with these findings; however, our results do not reach significance presumably due to the limited number of patients. Moreover, it has been shown that 11β-HSD2 mRNA expression paralleled 11β-HSD2 activity and protein expression (34). Changes in the activity of 11β-HSD2 and the consequent disequilibrium between cortisol and cortisone are thought to be a key mechanism for metabolic and cardiovascular disorders in later adult life after IUGR. Analyzing the concentrations of cortisol and cortisone in the umbilical vein after birth, we found no alterations between the cortisol/cortisone ratios of IUGR and AGA children. This is in line with data showing that serum cortisol/cortisone ratio does not necessarily reflect potential alterations in 11β-HSD2 activity (35). However, in our patients, there was a significantly higher plasma concentration of cortisone in AGA infants. This is consistent with a higher placental 11β-HSD2 expression in the AGA placenta, allowing for more conversion of cortisol to cortisone (7).
We could, however, find no relation between postnatal growth and umbilical vein steroid concentrations. There are a number of possible interpretations. One explanation might be the mode of delivery: Although in the IUGR group, in 93% of all cases, caesarean section was performed, eight from 15 children of the control group were born by spontaneous delivery. Thus, placental 11β-HSD2 activity regulating the amount of maternal glucocorticoids reaching the fetus might be concealed by maternal and fetal cortisol synthesis during the stress of delivery. Moreover, confounding factors like prenatal glucocorticoid exposure could influence the results, especially when a ratio between two separate values is analyzed. The combination of these influences might result in the absent sensitivity of cord blood steroid concentration for predicting growth in the first year of life.
As a consequence, only the measurement of placental 11β-HSD2 gene expression seems to allow a prediction model for postnatal growth after IUGR. It was the primary goal of our study to investigate whether the placental expression of specific genes related to fetal growth and metabolism might be of predictive value for postnatal growth. In addition, 11β-HSD2 gene expression is easy and fast to determine and would therefore be a parameter for early prediction of later events in the child's life that is easier to handle than determinations of cortisol/cortisone concentrations in umbilical blood.
In contrast to 11β-HSD2, there was no correlation between gene expression of placental IGFBP-1, leptin and birth weight, length and growth velocity during the first year of life. Although potentially involved in perinatal growth and the process of fetal programming, placental IGFBP-1 and leptin gene expression do not seem to be adequate predictors for postnatal growth in the first year of life. Potentially, their mechanism of influence on programming of metabolic disease after birth is not reflected by placental gene expression. They might act via different mechanisms.
In conclusion, our data show that gene expression of 11β-HSD2 at birth correlated inversely with growth velocity in the first year of life in the IUGR, but not in the AGA group. Further investigations in a large cohort are needed to demonstrate whether postnatal growth and programmed metabolic disorders could be predicted by gene expression of placental endocrine factors.
Abbreviations
- AGA:
-
appropriate for gestational age
- 11β-HSD2:
-
11β-hydroxysteroid dehydrogenase type 2
- IGFBP-1:
-
insulin-like growth factor-binding protein-1
- SGA:
-
small for gestational age
References
Ibanez L, Potau N, Francois I, de Zegher F 1998 Precocious pubarche, hyperinsulinism, and ovarian hyperandrogenism in girls: relation to reduced fetal growth. J Clin Endocrinol Metab 83: 3558–3562
Levy-Marchal C, Czernichow P 2006 Small for gestational age and the metabolic syndrome: which mechanism is suggested by epidemiological and clinical studies?. Horm Res 65: 123–130
Plank C, Östreicher I, Dittrich K, Waldherr R, Voigt M, Amann K, Rascher W, Dötsch J 2007 Low birth weight, but not postnatal weight gain, aggravates the course of nephrotic syndrome. Pediatr Nephrol 22: 1881–1889
Plagemann A 2006 Perinatal nutrition and hormone-dependent programming of food intake. Horm Res 65: 83–89
Dötsch J, Nusken KD, Knerr I, Kirschbaum M, Repp R, Rascher W 1999 Leptin and neuropeptide Y gene expression in human placenta: ontogeny and evidence for similarities to hypothalamic regulation. J Clin Endocrinol Metab 84: 2755–2758
Abuzzahab MJ, Schneider A, Goddard A, Grigorescu F, Lautier C, Keller E, Kiess W, Klammt J, Kratzsch J, Osgood D, Pfaffle R, Raile K, Seidel B, Smith RJ, Chernausek SD 2003 IGF-I receptor mutations resulting in intrauterine and postnatal growth retardation. N Engl J Med 349: 2211–2222
Struwe E, Berzl GM, Schild RL, Beckmann MW, Dörr HG, Rascher W, Dötsch J 2007 Simultaneously reduced gene expression of cortisol-activating and cortisol-inactivating enzymes in placentas of small-for-gestational-age neonates. Am J Obstet Gynecol 197: 43.e1–43.e6
Lapillonne A, Peretti N, Ho PS, Claris O, Salle BL 1997 Aetiology, morphology and body composition of infants born small for gestational age. Acta Paediatr Suppl 423: 173–176, discussion 177
Ibanez L, Lopez-Bermejo A, Suarez L, Marcos MV, Diaz M, de Zegher F 2008 Visceral adiposity without overweight in children born small for gestational age. J Clin Endocrinol Metab 93: 2079–2083
Jaquet D, Gaboriau A, Czernichow P, Levy-Marchal C 2001 Relatively low serum leptin levels in adults born with intra-uterine growth retardation. Int J Obes Relat Metab Disord 25: 491–495
Bouret SG, Draper SJ, Simerly RB 2004 Trophic action of leptin on hypothalamic neurons that regulate feeding. Science 304: 108–110
Seckl JR 2004 Prenatal glucocorticoids and long-term programming. Eur J Endocrinol 151: U49–U62
Benediktsson R, Calder AA, Edwards CR, Seckl JR 1997 Placental 11 beta-hydroxysteroid dehydrogenase: a key regulator of fetal glucocorticoid exposure. Clin Endocrinol (Oxf) 46: 161–166
Lindsay RS, Lindsay RM, Edwards CR, Seckl JR 1996 Inhibition of 11-beta-hydroxysteroid dehydrogenase in pregnant rats and the programming of blood pressure in the offspring. Hypertension 27: 1200–1204
Mune T, White PC 1996 Apparent mineralocorticoid excess: genotype is correlated with biochemical phenotype. Hypertension 27: 1193–1199
Barbazanges A, Piazza PV, Le Moal M, Maccari S 1996 Maternal glucocorticoid secretion mediates long-term effects of prenatal stress. J Neurosci 16: 3943–3949
Murphy VE, Clifton VL 2003 Alterations in human placental 11beta-hydroxysteroid dehydrogenase type 1 and 2 with gestational age and labour. Placenta 24: 739–744
Giudice LC, de Zegher F, Gargosky SE, Dsupin BA, de las Fuentes L, Crystal RA, Hintz RL, Rosenfeld RG 1995 Insulin-like growth factors and their binding proteins in the term and preterm human fetus and neonate with normal and extremes of intrauterine growth. J Clin Endocrinol Metab 80: 1548–1555
Firth SM, Baxter RC 2002 Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev 23: 824–854
Crossey PA, Pillai CC, Miell JP 2002 Altered placental development and intrauterine growth restriction in IGF binding protein-1 transgenic mice. J Clin Invest 110: 411–418
Popovici RM, Lu M, Bhatia S, Faessen GH, Giaccia AJ, Giudice LC 2001 Hypoxia regulates insulin-like growth factor-binding protein 1 in human fetal hepatocytes in primary culture: suggestive molecular mechanisms for in utero fetal growth restriction caused by uteroplacental insufficiency. J Clin Endocrinol Metab 86: 2653–2659
Kajimura S, Aida K, Duan C 2005 Insulin-like growth factor-binding protein-1 (IGFBP-1) mediates hypoxia-induced embryonic growth and developmental retardation. Proc Natl Acad Sci USA 102: 1240–1245
Voigt M, Schneider KT, Jahrig K 1996 [Analysis of a 1992 birth sample in Germany. 1: New percentile values of the body weight of newborn infants]. Geburtshilfe Frauenheilkd 56: 550–558
Prader A, Largo RH, Molinari L, Issler C 1989 Physical growth of Swiss children from birth to 20 years of age. First Zurich longitudinal study of growth and development. Helv Paediatr Acta Suppl 52: 1–125
Kromeyer-Hauschild K, Wabitsch M, Kunze D, Geller F, Geib HC, Hesse V, von Hippel A, Jaeger U, Johnsen D, Korte W, Menner K, Muller G, Muller JM, Niemann-Pilatus A, Remer T, Schaefer F, Wittchen HU, Zabransky S, Zellner K, Ziegler A, Hebebrand J 2001 [Percentiles for the body mass index for the child and young adult under consulting different German samples]. Monatsschr Kinderheilkd 149: 807–818
Vogeser M, Briegel J, Jacob K 2001 Determination of serum cortisol by isotope-dilution liquid-chromatography electrospray ionization tandem mass spectrometry with on-line extraction. Clin Chem Lab Med 39: 944–947
Trollmann R, Klingmuller K, Schild RL, Rascher W, Dötsch J 2007 Differential gene expression of somatotrophic and growth factors in response to in vivo hypoxia in human placenta. Am J Obstet Gynecol 197: 601.e601–601.e606
Gudmundsson S, Korszun P, Olofsson P, Dubiel M 2003 New score indicating placental vascular resistance. Acta Obstet Gynecol Scand 82: 807–812
Ong KK, Ahmed ML, Emmett PM, Preece MA, Dunger DB 2000 Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. BMJ 320: 967–971
Barker DJ 2006 Adult consequences of fetal growth restriction. Clin Obstet Gynecol 49: 270–283
Fuqua JS 2006 Growth after organ transplantation. Semin Pediatr Surg 15: 162–169
Ulinski T, Cochat P 2006 Longitudinal growth in children following kidney transplantation: from conservative to pharmacological strategies. Pediatr Nephrol 21: 903–909
McTernan CL, Draper N, Nicholson H, Chalder SM, Driver P, Hewison M, Kilby MD, Stewart PM 2001 Reduced placental 11beta-hydroxysteroid dehydrogenase type 2 mRNA levels in human pregnancies complicated by intrauterine growth restriction: an analysis of possible mechanisms. J Clin Endocrinol Metab 86: 4979–4983
Homan A, Guan H, Hardy DB, Gratton RJ, Yang K 2006 Hypoxia blocks 11beta-hydroxysteroid dehydrogenase type 2 induction in human trophoblast cells during differentiation by a time-dependent mechanism that involves both translation and transcription. Placenta 27: 832–840
Plank C, Meissner U, Rauh M, Wollmann H, Dörr HG, Rascher W, Dötsch J 2007 Cortisol-cortisone ratios in small for gestational age (SGA) children without postnatal catch-up growth. Clin Endocrinol (Oxf) 67: 304–309
Acknowledgements
The authors thank the members of the FIPS-study group protocol committee, namely H. Böhles, M.D. (Frankfurt), E. Landmann, M.D. (Giessen), U. Lang, M.D. (Graz), L. Gortner, M.D., S. Zabransky, M.D., K. Ertan, M.D. (Homburg/Saar), M. Kirschbaum, M.D., J. Möller, M.D. (Saarbrücken). We also thank Mrs. Bitterer, Mrs. Allabauer, and Mrs. Birkner for measurements of placental gene expression and technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by Pfizer and Novo Nordisk (Educational Grant).
Rights and permissions
About this article
Cite this article
Tzschoppe, A., Struwe, E., Blessing, H. et al. Placental 11β-HSD2 Gene Expression at Birth Is Inversely Correlated With Growth Velocity in the First Year of Life After Intrauterine Growth Restriction. Pediatr Res 65, 647–653 (2009). https://doi.org/10.1203/PDR.0b013e31819e7337
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/PDR.0b013e31819e7337
This article is cited by
-
Influence of labor on direct and indirect determinants of placental 11beta-hydroxysteroid dehydrogenase activity
Archives of Gynecology and Obstetrics (2021)
-
Mechanisms for establishment of the placental glucocorticoid barrier, a guard for life
Cellular and Molecular Life Sciences (2019)
-
Fetale Programmierung und spätere Nierenfunktionsstörungen nach intrauteriner Wachstumsrestriktion
Der Nephrologe (2009)