Abstract
We used novel 3′-monoiodothyronine sulfate (3′-T1S) and 3,3′,5-triiodothyroacetic acid sulfate (TriacS) RIAs to characterize sulfation pathways in fetal thyroid hormone metabolism. 3′-T1S and TriacS levels were measured in serum samples obtained from fetal (n = 21, 94–145 d gestational age), newborn (NB, n = 5), and adult sheep (AD, n = 5) as well as from fetuses after total thyroidectomy (Tx), or sham-operated twin fetuses controls, conducted at gestational age 110–113 d (n = 5). Peak levels (expressed as ng/dL) of both 3′-T1S and TriacS occurred at 130 d gestation. These levels in fetuses were higher than those in NB and AD. In Tx fetuses, there was a significant decrease in the mean serum level of 3′-T1S, but not TriacS. The decrease in 3′-T1S in Tx is similar to that observed for thyroxine sulfate (T4S) and 3,3′,5′-triiodothyronine sulfate (rT3S), whereas TriacS levels were not altered in the hypothyroid state, similarly to 3,3′,5-triiodothyronine sulfate (T3S). These data demonstrate that 3′-T1S and TriacS are normal thyroid hormone metabolites in ovine serum and that TriacS is likely derived from T3S or from the same precursor(s) as T3S.
Similar content being viewed by others
Main
We have identified the sulfated iodothyronines, thyroxine sulfate (T4S), 3,3′,5-triiodothyronine (T3S), 3,3′,5′-triiodothyronine sulfate (reverse T3S) (rT3S), and 3,3′-diiodothyronine sulfate (3,3′-T2S), as important thyroid hormone metabolites in ovine and human fetal fluids (1–5). The relatively high concentrations of these sulfated iodothyronines in the developing fetus probably reflect the low type I deiodinase activities observed in fetal tissues (6,7). To further characterize metabolism of the sulfated iodothyronines in ovine fetuses, we developed sensitive and specific 3′-T1S and 3,3′-5-triiodothyroacetic acid sulfate (TriacS) RIAs to quantify 3′-T1S and TriacS levels in normal and hypothyroid fetal and maternal serum. In previous studies of hypothyroid fetuses, we found significant reductions of mean serum concentrations of T4S and rT3S, but not T3S, suggesting that T3, presumably the precursor of T3S, was derived from T3 in tissue. Hypothyroidism may result in a compensatory increase in activity of type II 5′-deiodinase, which tends to maintain tissue T3 in a relatively normal range (8,9). The present study is to determine whether serum levels of TriacS and/or 3′-T1S are reduced in fetal hypothyroidism.
MATERIALS AND METHODS
3′-T1S and TriacS RIAs.
3′-T1S and 3′-[125I]T1S as well as TriacS and [125I]TriacS were prepared by the method of Eelkman-Rooda and co-workers (10,11). 3′-T1S and TriacS in 0.025 N NaOH (4 mg/mL) were further purified and quantitatively recovered by reverse-phase HPLC with a preparative column (Biochrom 1010 ODS; Regis, Morton Grove, IL). The products were eluted isocratically with a mixture of acetonitrile and 20 mM ammonium acetate, pH 4.0 (22:78 vol/vol), at a solvent flow of 10 mL/min. 3′-T1S and TriacS were recovered with purity >99%, as assessed by HPLC.
The RIA for 3′-T1S used an anti-3′-T1S antibody 3A-2, obtained from one of three New Zealand rabbits immunized with a 3′-T1S-BSA conjugate (2). The RIA for TriacS used an antibody D1-3 against TriacS-BSA conjugants. Each rabbit was immunized with emulsions of 1 mL of a solution of the conjugate containing 2 mg BSA and an equal volume of complete Freund's adjuvant (Calbiochem, San Diego, CA) in multiple dorsal subcutaneous sites. Booster injections comprised of 1 mL conjugate and an equal volume of incomplete Freund's adjuvant were continued at 6-wk intervals. Moderate titer antibodies were detected in the sera of two of the three rabbits 3 wk after the fourth (3′-T1S) and sixth (TriacS) immunizations. At a final dilution of 1:12,000 (in 1 mL of 0.075 M barbital buffer, pH 8.6, and 0.12% normal rabbit serum), anti-3′-T1S antibody bound 35–45% of a tracer amount (= 8.3 fmol or 5 pg) of 3′-[125I]T1S (similarly for TriacS antibody, final dilution 1:5000). Ethanol did not appreciably inhibit the binding of 3′-[125I]T1S to 3A-2 antibody up to a final concentration of 22% (similarly for TriacS antibody D1-3). Therefore, we used ethanol (63%) extracts of sera to measure 3′-T1S and TriacS concentrations. The final ethanol concentration in the assays was 19%.
The 3′-T1S and TriacS RIA procedures, developed using antisera 3A-2 and D1-3, respectively, were modifications of the RIA procedure described previously for the measurement of T4S and T2S in sera (2,4). The 3′-T1S and TriacS RIAs were carried out in duplicate or triplicate in 10 × 75-mm disposable glass tubes containing 0.053 M barbital buffer (pH 8.6), 0.07% sodium azide, 0.088% normal rabbit serum, and 19% ethanol (containing either the unknown or standard amounts of unlabeled 3′-T1S or TriacS). Standard curves were constructed with 1 pg to 1000 pg of 3′-T1S (2.1 fmol to 2.09 pmol) or TriacS (1.4 fmol to 1.43 pmol), optimal amount of 3A-2 or D1-3 antibody (final dilution 1:12,000 for 3A-2 and 1:5000 for D1-3), and 18,000–22,000 cpm 3′-[125I]T1S or [125I]TriacS, in a final volume of 1 mL. The tubes were thoroughly mixed and incubated at 4°C overnight. A sufficient amount of a previously titered goat anti-rabbit gamma globulin (second antibody) was then added. The tubes were mixed, incubated at 4°C overnight, and centrifuged at 2000 g for 20 min. Supernatants were carefully aspirated, and radioactivities in the precipitates were quantified using an Isodata 20/20 gamma counter (Isodata, Palatine, IL). Nonspecific binding (determined in tubes without added 3A-2 or D1-3 antibody) was <3% and was subtracted from counts of bound 3′-[125I]T1S or [125I]TriacS. Standard curve plots and other calculations were undertaken as described previously (5,8).
Animal preparation and samples.
Western mixed-breed time-dated pregnant ewes with twin pregnancies were obtained from the Nebeker Ranch (Lancaster, CA) and acclimated to our laboratory conditions and food. Animals were studied in the following periods of gestation: 94 d (n = 5), 110–111 d (n = 6), 130–131 d (n = 6), and 145 d (n = 6). These gestational ages were chosen because of differences in thyroid hormone secretion and metabolism during this period of development (12,13). In addition, sera from newborn (NB) (n = 5) and adult (AD) (n = 5) animals, including pregnant and nonpregnant ewes, were studied.
To assess the effect of fetal hypothyroidism on 3′-T1S and TriacS levels, a group of five ewes (110–113 d gestation) with twin fetuses were selected. The ewes were sedated (1.2 mg atropine and 700 mg ketamine i.m.), and a continuous infusion of ketamine (100 mg/h) was started via a jugular venous catheter. After local anesthesia of the abdominal wall (2% lidocaine), a midline incision was followed by palpation of the uterus and fetal parts and identification of the fetal head. A hysterotomy was performed over the fetal neck, which was exteriorized with attention to avoiding loss of amniotic fluid. The fetal neck was infiltrated with 1% lidocaine, followed by dissection and complete removal of the thyroid gland (Tx). The neck incision was closed, and the second fetus was handled in a similar manner, except that a sham operation was conducted, and the thyroid gland was left intact (control). The ewes were treated for 3 d postoperatively with oxacillin (2 g) and gentamicin (80 mg) given intramuscularly in divided doses. The fetuses were studied 13 d after the initial operation. The 13-d interval from Tx to death was chosen based on the serum half-life of T4 in the ovine fetus (24 h) and to avoid long-term effects of Tx on cell number and body weight (12–14).
To obtain serum from fetuses, the ewes were sedated as previously outlined and given spinal and epidural anesthesia (5 mL of 0.5% Marcaine and 5 mL of 2% lidocaine). After a paramedian abdominal incision and hysterotomy, each twin was delivered and immediately killed with an overdose of i.v. pentobarbital sodium. After obtaining samples from each fetus, the ewe was similarly killed. All experiments were approved by the Harbor-University of California at Los Angeles Medical Center Animal Use Committee.
Preparation of test serum.
Samples were extracted with two volumes of 95% ethanol before assay. Preliminary experiments showed that the extraction efficiencies of 3′-T1S and TriacS in serum were 90–98% and 88–95%, respectively (mean ± SE, 93 ± 4 and 91 ± 5, respectively, in 6–8 experiments with different, known amounts of unlabeled 3′-T1S or TriacS added). Final values of 3′-T1S and TriacS concentration were not corrected for recovery efficiency. The preliminary experiments demonstrated that the immunoreactive 3′-T1S and TriacS in ethanol extracts of fetal and maternal serum co-chromatographed with the corresponding synthetic compounds on HPLC.
Source of materials.
3,3′-T2, 3′,5′-T2, D-T3, T3, rT3, 3-monoiodothyronine (3-T1), 3′-T1, 3,5-diiodothyroacetic acid (Diac), 3,3′,5-triiodothyroacetic acid (Triac), 3,3′,5,5′-tetraiodothyroacetic acid (Tetrac), and thyroxine (T4) were purchased from Henning-Berlin (Berlin, Germany). Thyronine (To), 3,5-T2, 3,3′,5-triiodothyropropionic acid (Triprop), monoiodotyrosine (MIT), diiodotyrosine (DIT), BSA, and 1-ethyl-3-(3-dimethyl-aminopropyl)-carbodiimide were purchased from Sigma Chemical Co. (St. Louis, MO). 3′-[125I]T1 and [125I]TriacS were prepared by radioiodination of To and Diac, respectively, using the method described previously (15). T4S, T3S, and rT3S were synthesized by the method described previously (10,11). Chlorosulfonic acid, 99%, was purchased from Aldrich Chemical (Milwaukee, WI).
Statistical analysis.
ANOVA was used for multi-group comparisons. If significant differences were detected, Dunnett's multicomparison test was used to compare the control or baseline mean and the mean values of other groups (16). Significance was defined as p < 0.05. Results are reported as mean ± SE.
RESULTS
3′-T1S and TriacS RIAs: Specificity and sensitivity.
Of the various thyroid hormone analogs studied, only T4S and T3S cross-reacted significantly (0.3% and 0.01%, respectively) in the 3′-T1S RIA; T4, T3, rT3, and 3,3′-T2 showed <0.0002% cross-reaction with the antiserum. The antiserum to TriacS cross-reacted significantly with T4S (5.9%), 3,3′-T2S (3.0%), rT3S (1.1%), T3S (0.09%), and 3′,5′-T2S (0.001%); all other analogs cross-reacted <0.0001% (Table 1). The lower limit of detection was 2 ng/dL (or 41.8 pmol/L and 28.5 pmol/L, respectively, for 3′-T1S and TriacS) in a 300-μL ethanol extract.
Parallelism, recovery, and reproducibility.
The dose-response curves for inhibition of binding of 3′-[125I]T1S to antibody 3A-2 produced by the ethanol extracts of serum were compared with the standard curve. The mean deviation from predicted values in various dilutions was 6.2% and 4.1% in two mean concentrations studied, 86 ng/dL and 175 ng/dL, respectively. The mean recovery of nonradioactive 3′-T1S added to serum extracts in concentrations ranging from 20 to 200 ng/dL in six experiments averaged (±SE) 93 ± 4%. The mean coefficients of variation for different 3′-T1S concentrations in sera tested (n = 5) in the same assay were 9.7% for 25 ng/dL, 6.0% for 100 ng/dL, and 7.4% for 150 ng/dL. The mean interassay coefficients of variation of 3′-T1S concentrations were 16.5% at 25 ng/dL, 8.5% at 100 ng/dL, and 15.4% at 150 ng/dL.
The dose-response curves for inhibition of binding of 3′-[125I]TriacS to antibody D1-3 produced by the ethanol extracts of serum were compared with the standard curve. The mean deviations from predicted values in various dilutions were 3.9% and 15.4% in two mean concentrations studied, 8.7 ng/dL and 34.1 ng/dL, respectively. The mean recovery of nonradioactive TriacS added to serum extracts in concentrations ranging from 20 to 200 ng/dL in seven experiments averaged (±SE) 91 ± 5%. The mean within assay coefficient of variation for TriacS (concentration 25 ng/dL n = 5) was 9.9%. The mean interassay coefficient of variation of TriacS (concentrations 30 ng/dL, n = 6) was 15.6%.
3′-T1S and TriacS concentrations.
Serum 3′-T1S and TriacS concentrations measured in each age group of fetuses, newborns, and adults are shown in Figure 1. Fetal serum 3′-T1S and TriacS levels increased progressively from 94 to 130 d, reaching a peak of 229 ± 24 ng/dL and 114 ± 13 ng/dL, respectively. These 130-d values were significantly higher than values in near-term animals (145 d gestation; 68 ± 3.8 and 44 ± 8, respectively; p < 0.05) and in newborns (105 ± 5 and 12.5 ± 2.8, respectively; p < 0.05).
Serum concentration of 3′-T1S (open) and TriacS (shaded) in ng/dL of fetuses of different gestational ages (in days), of NB and AD sheep. Vertical bars and lines represent means ± SE. Each bar represents mean of 5–6 animals. For conversion to nmol/L 3′-T1S, multiply by 0.0209; nmol/L TriacS, multiply by 0.0140.
The mean serum level of 3′-T1S in NB sheep was 105 ± 5 ng/dL, compared with 41 ± 4.4 ng/dL in AD (p < 0.0005); in contrast, TriacS levels were similar in NB (12.5 ± 2.8 ng/dL) and AD (9.3 ± 1.2 ng/dL; p > 0.05).
Effect of Tx on 3′-T1S and TriacS concentrations.
Figure 2 summarizes the effect of Tx on 3′-T1S and TriacS concentrations in serum from ovine fetuses (125 d of gestation). Serum 3′-T1S was found to be significantly decreased in Tx fetuses (49 ± 6.8 ng/dL) compared with sham-operated control fetuses (92 ± 15 ng/dL; p < 0.02). No such decrease was observed in TriacS levels between Tx fetuses (39 ± 6.0 ng/dL) and sham-operated control fetuses (50 ± 9.5 ng/dL, p > 0.05).
DISCUSSION
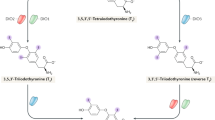
There is general agreement that sequential monodeiodination is the major mechanism regulating the bioavailability of thyroid hormones in tissues. However, the alternate pathways may also play a role in some circumstances. Sulfoconjugation of iodothyronines, for example, is an important pathway in developing animals (7,17) (Fig. 3), and sulfated iodothyronines can also be deiodinated, at an even faster rate (7). Likewise, iodothyroacetates can be sulfated and further deiodinated.
Postulated metabolic pathways for ovine fetal thyroid hormones. D1, D2, and D3: type I, type II, and type III iodothyronine deiodinases; ST: iodothyronine sulfotransferases; LAO: L-amino acid oxidase; AT: thyroid hormone aminotransferase. LAO and AT are postulated to convert T3S to TriacS. Heavy solid lines indicate pathways that are more active in fetuses than in adults; thin solid lines, pathways that are less active in fetuses. The upper horizontal light dotted line depicts T4 of maternal origin moving to the fetal compartment in the first trimester, before the fetal thyroid begins functioning. Other broken lines represent unconfirmed pathways. Numbers in parentheses indicate published production rates (μg/kg/d).
Previously, we demonstrated high levels of T4S, T3S, rT3S, and 3,3′-T2S in ovine fetal serum, bile, and amniotic fluid (1–5). Our present results indicate that 3′-T1S and TriacS are normal metabolites in thyroid hormone metabolism in sheep and that the sulfation pathway is more prominent in ovine fetuses than in adults. In earlier studies of the effect of Tx on thyroid metabolism in fetal sheep, serum T4 and T3 levels fell to <0.7 μg/dL and <10 ng/dL, respectively (18). Labeled hormone kinetic studies showed minimal maternal to fetal transfer of T4 or T3 and estimated T4 and T3 turnover rates approximating 0.6 and 0.7 μg/24 h. These amounts represent <0.2% and <50% of the mean daily T4 and T3 turnover rates of 46 and <1.5 μg/kg in euthyroid third trimester fetal sheep (18). Reductions in T4S, rT3S, and 3,3′-T2S were also observed in later studies but there was no decrease in serum T3S levels (5,8). The relatively constant levels of T3S in these animals led to the postulation that T3S may be derived from T3 in tissue. Hypothyroidism results in a compensatory increase in activity of type II 5′-deiodinase, which would tend to maintain tissue T3 in a relative normal range, important for brain development. There is also a limited maternal to fetal placental transfer of T3 (13). In addition, Tx may reduce the clearance of T3S by a decrease in type I deiodinase activity (8). The Tx fetus would presumably have a severe reduction of type I deiodinase activity in a state analogous to targeted disruption of the type 1 selenodeiodinase gene (Dio1) in mice, in which the serum T3 levels remained unchanged compared with wild-type animals (9).
In the present study, fetal Tx decreased 3′-T1S while TriacS levels were unchanged. The similar trends for T3S and TriacS suggest that TriacS is likely derived from T3S or from the same precursors as T3S (8). There is evidence to indicate that T3S can be converted to TriacS by oxidative degradation, possibly involving two enzymes, L-amino acid oxidase (LAO) and thyroid hormone aminotransferase (AT) (Fig. 3) (7,17). Alternatively, the relatively stable level of TriacS could be due to a reduction in the removal of TriacS by outer-ring deiodination, mediated by type I deiodinase, to 3,3′-DiacS (19–21). The role of glucuronidation of Triac is less well characterized in sheep (21,22); in addition, the enzyme activity is significantly less in ovine fetal liver (23). The observed rapid reduction of serum TriacS levels in newborns compared with fetuses (Fig. 1) may reflect a maturation of glucuronosyltransferase in the clearance of Triac.
The formation of TriacS may have clinical relevance because Triac has a higher affinity than T3 to thyroid hormone receptors TRβ1 and TRβ2 in tissue (7). In humans, Triac is found to derive mainly from T3 (21). TriacS has been found in mammalian circulation following the intravenous infusion of Triac, which is known to be a preferred substrate for sulfotransferase (SULT) 1A1 (21). In humans, SULT1A1 shows the highest affinity for both iodothyronines and 3′-phosphoadenosine 5′-phosphosulfate (PAPS), the universal sulfonate donor. The role of SULT1A1 in ovine tissue is not known. Sulfation of Triac would presumably reduce its binding to thyroid receptors, similar to sulfated T3 (21). As there is some evidence that TriacS can be desulfated to yield Triac (21), TriacS may serve as a reservoir for Triac even though its physiologic role in the fetus is not known.
The decrease in 3′-T1S in the Tx fetuses in the present study resembles the earlier observed trends in T4S, rT3S, and 3,3′-T2S (5,8). The precursor for 3′-T1S likely is 3,3′-T2S. Although outer-ring deiodination to 3-T1S is known (21), the conversion of 3,3′-T2S to 3′-T1S has not been demonstrated. Alternatively, 3′-T1S could be a sulfated product of 3′-T1, which could be derived from inner-ring deiodination of 3,3′-T2; the type III deiodinase is known to be active in fetal tissue. From the high potency of 3′-T1 in inhibiting sulfoconjugation of 3,3′-T2 in human SULT1A1 and SULT1A3, 3′-T1 may be an excellent substrate to be sulfated (24). After the infusion of outer-ring-labeled T3 in the euthyroid fetus, 3,3′-T2S was found to be the predominant metabolite, followed by lesser amounts of 3,3′-T2 and T3S; but no 3′-T1 or its sulfoconjugate was seen (25). The kinetics of labeled T3 in Tx ovine fetuses have not been studied with HPLC or other available RIAs specific to sulfated iodothyronines developed recently (7).
A proposed scheme for thyroid hormone metabolism in developing sheep is outlined in Figure 3. The high production rate (PR) for sulfated iodothyronines reflects the dominant pathway, sulfoconjugation, in ovine fetuses. The PR for T4 and T3 are 46 and <1.5 μg/kg/d, respectively, in the euthyroid third trimester fetus while the PR for rT3S, T4S, and T3S are 12, 10, and 2 μg/kg/d, respectively (18,26). The kinetic studies predicted that 3,3′-T2S also is a major thyroid hormone metabolite and this was later confirmed qualitatively (25). 3,3′-T2S was also found to be the major metabolite in maternal circulation following the fetal infusion of radioactive T3 (26); a similar immunoreactive product, Compound W, was found peaked at term in pregnant women disappearing 7–10 d after delivery (4,27). Kinetic studies on the PR of 3,3′-T2S, 3′-T1S, and TriacS would be important to quantitatively evaluate the fetal thyroid hormone metabolism and fetal-maternal transfer of thyroid hormone metabolites that may be an invaluable tool in the noninvasive detection of fetal thyroid function. The elucidation of fetal and maternal exchange of thyroid hormone and/or its metabolites may have significant clinical implications.
Abbreviations
- AD:
-
adult
- NB:
-
newborn
- PR:
-
production rate
- rT3S:
-
3,3′,5′-triiodothyronine sulfate (reverse T3S)
- SULT:
-
sulfotransferase
- Tx:
-
thyroidectomy
- Triac:
-
3,3′,5-triiodothyroacetic acid
- TriacS:
-
3,3′,5-triiodothyroacetic acid sulfate
- T3:
-
3,3′,5-triiodothyronine
- T3S:
-
3,3′,5-triiodothyronine sulfate
- T4S:
-
thyroxine sulfate
- 3′-T1:
-
3′-monoiodothyronine
- 3′-T1S:
-
3′-monoiodothyronine sulfate
- 3,3′-T2S:
-
3,3′-diiodothyronine sulfate
References
Chopra IJ, Wu SY, Chua Teco GN, Santini F 1992 A radioimmunoassay for measurement of 3,5,3′-triiodothyronine sulfate: studies in thyroidal and nonthyroidal diseases, pregnancy, and neonatal life. J Clin Endocrinol Metab 75: 189–194
Wu S, Polk D, Wong S, Reviczky A, Vu R, Fisher DA 1992 Thyroxine sulfate is a major thyroid hormone metabolite and a potential intermediate in the monodeiodination pathways in fetal sheep. Endocrinology 131: 1751–1756
Wu SY, Huang WS, Polk D, Chen WL, Reviczky A, Williams J, Chopra IJ, Fisher DA 1993 The development of a radioimmunoassay for reverse triiodothyronine sulfate in human serum and amniotic fluid. J Clin Endocrinol Metab 76: 1625–1630
Wu SY, Polk DH, Chen WL, Fisher DA, Huang WS, Yee B 1994 A 3,3′-diiodothyronine sulfate cross-reactive compound in serum from pregnant women. J Clin Endocrinol Metab 78: 1505–1509
Wu SY, Polk DH, Fisher DA, Huang WS, Reviczky AL, Chen WL 1995 Identification of 3,3′-T2S as a fetal thyroid hormone derivative in maternal urine in sheep. Am J Physiol 268: E33–E39
Bianco AC, Larsen PR 2005 Intracellular pathways of iodothyronine metabolism. In: Braverman LE, Utiger RD (eds) Werner and Ingbar's The Thyroid: A Fundamental and Clinical Text, 9th Ed. JB Lippincott, New York, pp 109–133
Wu SY, Green WL, Huang WS, Hays MT, Chopra IJ 2005 Alternate pathways of thyroid hormone metabolism. Thyroid 15: 943–958
Wu SY, Polk DH, Huang WS, Reviczky A, Wang K, Fisher DA 1993 Sulfate conjugates of iodothyronines in developing sheep: effect of fetal hypothyroidism. Am J Physiol 265: E115–E120
Schneider MJ, Fiering SN, Thai B, Wu SY, St. Germain E, Parlow AF, St. Germain DL, Galton VA 2006 Targeted disruption of the type 1 selenodeiodinase gene (Dio1) results in marked changes in thyroid hormone economy in mice. Endocrinology 147: 580–589
Eelkman-Rooda SJ, Kaptein E, van Loon M, Visser TJ 1988 Development of a radioimmunoassay for triiodothyronine sulfate. J Immunoassay 9: 125–134
Mol JA, Visser TJ 1985 Synthesis and some properties of sulfate esters and sulfamates of iodothyronines. Endocrinology 117: 1–7
Erenberg A, Omori K, Menkes JH, Oh W, Fisher DA 1974 Growth and development of the thyroidectomized ovine fetus. Pediatr Res 8: 783–789
Fisher DA, Dussault JH, Sack J, Chopra IJ 1976 Ontogenesis of hypothalamic–pituitary–thyroid function and metabolism in man, sheep, and rat. Recent Prog Horm Res 33: 59–116
Wu SY, Merryfield ML, Polk DH, Fisher DA 1990 Two pathways for thyroxine 5′-monodeiodination in brown adipose tissue in fetal sheep: ontogenesis and divergent responses to hypothyroidism and 3,5,3′-triiodothyronine replacement. Endocrinology 126: 1950–1958
Nakamura Y, Chopra IJ, Solomon DH 1977 Preparation of high specific activity radioactive iodothyronines and their analogues. J Nucl Med 18: 1112–1115
Kirk RE 1982 Experimental Design, Procedures for the Behavioral Sciences. Brooks/Cole, Belmont, pp 112–114
Siegrist-Kaiser CA, Burger AG 1994 Modification on the side chain of thyroid hormones. In: Wu SY, Visser TJ (eds) Thyroid Hormone Metabolism: Molecular Biology and Alternate Pathways. CRC Press, Ann Arbor, MI, pp 175–198
Erenberg A, Omori A, Oh W, Fisher DA 1973 The effect of fetal thyroidectomy on thyroid metabolism in maternal and fetal sheep. Pediatr Res 7: 870–877
Burrow GN, Fisher DA, Larsen PR 1994 Maternal and fetal thyroid function. N Engl J Med 331: 1072–1078
Cortelazzi D, Morpurgo PS, Zamperini P, Fisher DA, Beck-Peccoz P, Wu SY 1999 Maternal compound W serial measurements for the management of fetal hypothyroidism. Eur J Endocrinol 141: 570–578
Visser TJ 1994 Sulfation and glucuronidation pathways of thyroid hormone metabolism. In: Wu SY, Visser TJ (eds) Thyroid Hormone Metabolism: Molecular Biology and Alternate Pathways. CRC Press, Ann Arbor, MI, pp 85–117
Rutgers M, Heusdens FA, Bonthuis F, Rooda SJ, Visser TJ 1990 Identification of 3,3′-diiodothyroacetic acid sulfate: a major metabolite of 3,3′,5-triiodothyronine in propylthiouracil-treated rats. Endocrinology 127: 1617–1624
Wang LH, Zakim D, Rudolph AM, Benet LZ 1986 Developmental alterations in hepatic UDP-glucuronosyltransferase. A comparison of the kinetic properties of enzymes from adult sheep and fetal lambs. Biochem Pharmacol 35: 3065–3070
Kester MH, Kaptein E, Roest TJ, van Dijk CH, Tibboel D, Meinl W, Glatt H, Coughtrie MW, Visser TJ 1999 Characterization of human iodothyronine sulfotransferases. J Clin Endocrinol Metab 84: 1357–1364
Wu SY, Polk DH, Huang WS, Green WL, Thai B, Fisher DA 2006 Fetal-to-maternal transfer of thyroid hormone metabolites in late gestation in sheep. Pediatr Res 59: 102–106
Polk DH, Reviczky A, Wu SY, Huang WS, Fisher DA 1994 Metabolism of sulfoconjugated thyroid hormone derivatives in developing sheep. Am J Physiol 266: E892–E896
Wu SY, Huang WS, Ho E, Wu ES, Fisher DA 2007 Compound W, a 3,3′-diiodothyronine sulfate cross-reactive substance in serum from pregnant women—a potential marker for fetal thyroid function. Pediatr Res 61: 307–312
Acknowledgements
The authors thank Becky Thai, Melissa Jordan, Hien On, Kathy Wang, Peter Do, and Tina Huang for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by the Department of Veterans Affairs, by National Institutes of Health grants R15-GM-41949 and HD-04270, and by National Science Council (Republic of China) Grant NSC 94-2623-7-4006-007.
Rights and permissions
About this article
Cite this article
Wu, SY., Polk, D., Huang, WS. et al. 3′-Monoiodothyronine Sulfate and Triac Sulfate Are Thyroid Hormone Metabolites in Developing Sheep. Pediatr Res 63, 149–153 (2008). https://doi.org/10.1203/PDR.0b013e31815f6551
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/PDR.0b013e31815f6551