Abstract
Neurofibromatosis type 1 (NF1) is a hereditary disease caused by mutations of the NF1 gene at 17q11.2. Loss of the NF1 gene product in Schwann cells leads to the development of benign nerve sheath tumors. These neurofibromas may occur at any time but tend to arise during periods of hormonal imbalance, suggesting that hormones influence neurofibroma growth. As steroid hormone levels rise during these times, we hypothesized that progesterone has proliferative effects on neurofibroma-derived Schwann cells. We chose specific medium conditions for selective proliferation of NF (+/−) and NF (−/−) cells from human neurofibromas. Genetic characterization was not performed, but former works have shown that under the conditions used (+/−) and (−/−) cells can be selected. Different progesterone concentrations were added at different days with BrdU-staining was performed to investigate proliferation rates and DAB-staining to identify a progesterone receptor. We could demonstrate that Schwann cells from human neurofibromas express progesterone receptors. These cells show elevated proliferation rates (highest in NF(−/−) cells) under progesterone, whereas normal human Schwann cells were not affected. These data suggest that progesterone plays an important role in the development of neurofibromas in NF1.
Similar content being viewed by others
Main
Neurofibromatosis type 1 (NF1) is caused by mutations of the NF1 gene at 17q11.2 and affects approximately 1 in 3000 individuals (1). Thus, NF1 represents one of the most frequent hereditary diseases. The disease is characterized by typical clinical symptoms that were summarized in a “diagnostic criteria list” at a National Institutes of Health consensus conference in 1988 (2). Despite a markedly variable clinical expression, the hallmark feature of the disease is benign peripheral nerve sheath tumors termed neurofibromas. These tumors consist of a mixed cellular population that mainly consists of Schwann cells and fibroblasts but also contains a significant proportion of mast cells, perineurial, and endothelial cells (3). All NF1 patients will inevitably develop neurofibromas during their lifetime (1) but the number, size, and age of onset of these tumors are entirely unpredictable.
Homozygous inactivation of the NF1 gene and subsequent loss of its gene product neurofibromin in Schwann cells has been identified as the primary step in a pathogenetic process that ultimately results in the development of neurofibromas (4,5). Many investigators (6,7) have stressed the fact that apart from mutations at NF1, various additional factors are necessary to enable neurofibroma formation. However, still very little is known about these additional factors, which might affect tumor development and growth in NF1.
From a clinical view, dermal neurofibromas tend to develop in adolescence as well as during pregnancy. These are times of increased hormonal influence and it has to be postulated that steroid hormones might affect neurofibroma development.
In this context, it is intriguing that progesterone—as a steroid hormone with numerous functions in the human body—has been shown to play an important role in myelin synthesis of Schwann cells. Jung-Testas et al. (8) demonstrated that human Schwann cells synthesize progesterone in an autocrine loop and express progesterone receptors. McLaughlin and Jacks (9) and Fishbein et al. (10) showed that progesterone receptors are expressed in human neurofibromas.
Here, we demonstrate that neurofibroma-derived Schwann cells are highly susceptible to progesterone and show elevated proliferation rates in the presence of this steroid hormone. The extent of this progesterone-induced proliferative effect appears to be dependent on the mutational status at NF1. These experimental data provide a convincing explanation as to why many NF1 patients observe stimulated neurofibroma growth during puberty or pregnancy and identify steroid hormones as one possible factor that drives neurofibromin-deficient Schwann cells toward tumor formation.
METHODS
Tissue sample collection and preparation of Schwann cell cultures.
After informed consent had been obtained from NF1 patients who underwent surgery, excised neurofibromas could be used for further studies. The following investigations were approved by our institutional review board for good clinical practice.
All patients whose samples were included carried the diagnosis NF1, based on the clinical criteria list (2). Immediately after excision, the tumor samples were placed into RPMI medium (GIBCO), shipped at room temperature to our laboratory and processed within 24 h. The specimens were prepared as described by Rosenbaum et al. (11), dissected into small pieces and preincubated for 2 wk at 37°C and 10% CO2. Tumor pieces were then mechanically dissociated and transferred to fresh medium and 160 U/mL collagenase type 1 (Sigma Chemical Co.) and 0.8 U/mL dispase (Roche) added. After an incubation period of 18–20 h at 37°C and 10% CO2, tissue pieces were completely dissolved by trituration with a narrowed Pasteur pipette. Cell suspensions were transferred to a 50 mL Falcon tube containing DMEM with 10% FCS. After centrifugation at 5000 rpm for 10 min, the resulting pellet was resuspended in proliferation medium, containing DMEM with 10% FCS, 500 U/mL penicillin/streptomycin, 0.5 mM 3-iso-butyl-1-methylxanthine, 10 nM β1-Heregulin, 0.5 μM forskolin and 2.5 μg/mL insulin. To selectively expand NF (+/−) and NF (−/−) Schwann cells, half of the pellet was plated in the abovementioned culture medium to expand NF (+/−) cells and the other half was plated in culture medium without forskolin to expand NF (−/−) cell (4). We did not perform a genetic characterization of these cells. Former studies have shown that by using this method, NF (+/−) and NF(−/−) can be selectively expanded (12). In the following discussion, we will speak of NF(+/−) and NF(−/−) cells for simplicity reasons in spite of the lack of genetic characterization.
The cells were then seeded onto plastic six-well plates as well as labtek slides (Nunc) all coated with 1 mg/mL poly-l-lysine (Sigma Chemical Co.) and 4 μg/mL natural mouse laminin (GIBCO). The labteks were used for S100-staining in each passage. Cultures were incubated in a humidified atmosphere at 37°C and 10% CO2. Proliferation medium was changed twice a week and cells were passaged when cultures were 70–80% confluent. Cultured neurofibroma-derived Schwann cells were identified by S100 staining and were regarded pure once the percentage of contaminating fibroblasts (S100 negative) was lower than 5%. This was usually achieved by passage 3–5.
Proliferation of human neurofibroma-derived Schwann cells in the presence of progesterone.
Pure Schwann cell cultures from 10 different dermal neurofibromas were transferred to 8-well labtek slides in proliferation medium at a density of 2000 cells/cm2. Once they had adhered to the surface, the medium was changed to pure DMEM plus 10% FCS with 500 U/mL penicillin/streptomycin to arrest growth. We did not use the established N2 medium for this purpose because we found a high percentage of nonviable cell Schwann cell cultures after N2-treatment. After testing various different media conditions, pure DMEM plus 10% FCS appeared to be sufficient to rule out any proliferating effects of the culture medium After 24 h in this medium, progesterone was added in different, predetermined concentrations (0 nM, 50 nM, 100 nM, 500 nM) with the effect of each progesterone concentration studied in pairs. Each condition was tested for NF(+/−) and NF (−/−) cells, respectively. The first two wells of every (+/−) and (−/−) eight-well labtek slide were left without additional progesterone as a negative control. Two wells of each labtek slide were incubated with 50 nM, 2 wells with 100 nm, and the remaining 2 with 500 nM progesterone (Sigma Chemical Co.). To follow proliferation rates in the presence of progesterone over a period of time, proliferation rates were determined on four different days in a separate set of cultures each. Media were replaced every 2 d.
Determination of proliferation rates.
To determine the percentage of proliferating Schwann cells under these conditions, incorporation of 5`-bromo-2`-deoxyuridine-5`-monophosphate (BrdU, Roche) was visualized by immunochemistry. To achieve this, BrdU was added to the medium and cells were fixed 18 h later as described in several previous studies before (13–15). Immunocytochemical detection was performed as described by Bosse et al. (16). In that way, one 8-well-labtek of NF(+/−) and of NF(−/−) cells was stained at each of the preselected days 1, 3, 5 and 8. As a control neurofibroma-derived Schwann cells left in DMEM plus 10% FCS with 500 U/mL penicillin/streptomycin were used. To compare the influence of progesterone on the growth of normal and neurofibroma-derived human Schwann cells, we performed the same experiment with human Schwann cells derived from healthy multiorgan donors.
Proliferation rates of neurofibroma-derived and normal human Schwann cells at different days and under different conditions were subjected to a t test (two-tailed) for statistical analysis.
Detection of progesterone receptors in human neurofibroma-derived Schwann cells.
Progesterone receptors in neurofibroma-derived Schwann cells were visualized by DAB receptor staining. Cells were first fixed with 4% paraformaldehyde in Tris-buffered saline (TBS) for 5 min at room temperature and with 4% paraformaldehyde with 0.03% Triton X-100 for another 10 min. Subsequently, cells were rinsed three times in PBS and preincubated for 30 min with 10% normal goat serum in TBS. Cells were then rinsed a further three times in PBS and then blocked with H2O2/TBS for another 30 min. After again rinsing the cells three times, they were incubated overnight at 4°C with the primary antibody in the following dilution: mouse monoclonal antiprogesterone receptor IgG1 (Affinity Bioregents) 1:2500. Cells were again rinsed three times in TBS and incubated with a biotinylated secondary anti-rabbit antibody (1:200, Vector) for 1 h at room temperature. The Vectastain Elite ABC System (Vector) followed by DAB staining was used to detect bound antibodies. Cell bodies were visualized using Meyers Hämalaun (Merck). After a final wash, cultures were mounted using Aquatex (Merck).
RESULTS
Detection of a progesterone receptor in human neurofibroma-derived Schwann cells.
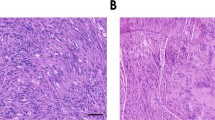
We were able to visualize a progesterone receptor in human neurofibroma-derived Schwann cells by DAB staining (Fig. 1) in 60% of the tumors. DAB staining is mainly concentrated intracellularly where we assumed the receptor localization. The progesterone receptor was not detectable in neurofibroma-derived fibroblasts. Therefore, we hypothesized that addition of progesterone to the culture medium might have an effect on the growth rates of neurofibroma-derived Schwann cells.
Proliferation of human Schwann cells.
It was known that proliferation of Schwann cells in vitro is crucially dependant on the presence of various growth factors. Leaving Schwann cell cultures without these factors leads to growth arrest. In experiments with rodent Schwann cells, the established N2 medium (17) is used to block Schwann cell proliferation. Usually a 24 h passage in N2 medium is sufficient to produce a quiescent but viable Schwann cell culture on which the proliferative effect of a given factor of interest can then be tested. Although we previously showed that this procedure also works for human Schwann cells (4,11), we found a high percentage of detached, nonviable human Schwann cell cultures after N2 treatment. Therefore, we tested various media conditions and finally used pure DMEM plus 10% FCS with 500 U/mL penicillin/streptomycin to keep human Schwann cells alive but induce growth arrest. Although this medium was different from the inert N2 medium and did contain certain growth factors, proliferation rates under these conditions were considerably low so that any proliferative effect of added progesterone was clearly visible. Adding progesterone to these cultures resulted in proliferation of all Schwann cells (Fig. 2). However, progesterone-induced proliferation of normal human Schwann cells was significantly lower as in the usual proliferation-promoting medium GFM.
Proliferation rates of normal human Schwann cells (n = 2). (A) NF 1 (+/−) Schwann cells (n = 8) (B) and NF1 (−/−) Schwann cells (n = 7) (C) in GFM compared with DMEM/10% FCS with 0, 50, 100, 500 nM added progesterone. Progesterone has only a minor proliferating effect on normal human Schwann cells. In NF (+/−), proliferation is accelerated by the addition of progesterone at day 3, 5, and 8 at concentrations of 50 and 100 nM. In NF (−/−) proliferation is elevated at day 3 and 5 at concentrations of 50 nM and especially at 100 nM. Although proliferation rates of both, NF 1 (+/−) and NF 1 (−/−) Schwann cells are elevated by progesterone, NF1 (−/−) Schwann cells appear to react more sensitive to the hormone ▪ day 3, □ day 5,  day 8.
day 8.
Proliferation of NF 1 (+/−) Schwann cells under different conditions.
After evaluation of proliferation rates on the preselected days 1, 3, 5, and 8 at different progesterone concentrations we could demonstrate that neurofibroma-derived Schwann cells exhibit an increased proliferation rate in response to progesterone compared with normal human Schwann cells (Fig. 2). Neurofibroma-derived Schwann cells proliferate in proliferation medium (GFM) with proliferation rates of approximately 15–20% at the different days. Once the proliferation medium was replaced by pure DMEM with FCS, those cells were merely proliferating at rates of 5–7%. By addition of progesterone to this medium, we were able to increase proliferation rates up to 15–20% in NF (+/−) cells. These proliferation rates were comparable with those achieved with our usual proliferation medium (including 3-iso-butyl-1-methylxanthine, Insulin, β-Heregulin and Forskolin). Proliferation rates of NF(±) Schwann cells were highly increased with progesterone concentrations of 50–100 nM. With higher concentrations, proliferation rates were still higher than in absence of progesterone but could not be further increased.
Proliferation of NF 1 (−/−) Schwann cells under different conditions.
NF 1 (−/−) Schwann cells show higher proliferation rates than NF1 (+/−) Schwann cells under proliferation conditions (rates of 20–27%) (Fig. 2). In medium without proliferative stimuli, they tend to proliferate with a rate up to 10%. Even in these cells that showed high spontaneous proliferation rates, proliferation could further be increased by addition of progesterone. Although the addition of 50 nM progesterone showed an effect on day 3, with proliferation rates increasing to 30%, 100 nM progesterone elevated proliferation rates on day 3 and day 5 to over 36%.
DISCUSSION
Our studies show that neurofibroma-derived Schwann cells express progesterone receptors and that their proliferation can be enhanced by progesterone. The stimulating effect of hormones on neurofibroma growth has previously been studied by other groups. Most of these studies focused on the in vitro effects of hGH (18,19) whereas Fischbein et al. (10) recently examined expression of various steroid hormone receptors and ligand-mediated cell growth in cultured normal and neurofibroma-derived Schwann cells. From a clinical point of view, there are well-documented observations showing that neurofibromas tend to grow during adolescence and pregnancy. Although increased levels of growth hormone might be responsible for enhanced tumor growth during puberty, this cannot sufficiently explain the observed increase in tumor size and number during pregnancy. Here, profound changes in sex steroid levels, in particular progesterone, might play a crucial role in promoting neurofibroma growth.
Progesterone is a steroid hormone produced by the ovaries and adrenal glands, and is known to regulate reproductive functions and gonadotropin release. Progesterone is synthesized from cholesterol to pregnenolon, which is further transformed to androgens, and therefore plays an important role in the steroid hormonal cascade of both genders. Progesterone can also be synthesized by glial cells and some types of neurons and may thus also be considered a neurosteroid. This term refers to steroid hormones that can be synthesized within the central and peripheral nervous system. In the peripheral nervous system, progesterone is synthesized by Schwann cells. Robert et al. (20) showed that its actions are important for normal cell function and cell regeneration. Rat Schwann cells express progesterone receptors (8) and produce progesterone themselves in an autocrine loop, which seems to be important for myelination (8). These data suggest that adequate Schwann cell function is at least in part dependent on the presence of progesterone. Since Schwann cells play a key role in neurofibroma formation, this raises the question of whether elevated progesterone levels during pregnancy might interfere with Schwann cell function, predisposing for neurofibroma growth.
Schwann cells are the predominant cell type in neurofibromas (3) and harbor a “second hit” at the NF1 locus (4,5), which makes them different from all other cells present in neurofibromas. However, loss of NF1 in Schwann cells appears to be insufficient to cause neurofibroma formation. Early experiments with the first generation of NF1-knockout mice demonstrated that Schwann cells with a homozygous mutation of the NF1 gene do not hyperproliferate in vitro (21). This indicates that NF1 mutations as such cannot explain the cellular hyperproliferation seen in human NF1-associated tumors. In a conditional NF1 knockout mouse model loss of NF1 in Schwann cells resulted in cellular hyperproliferation in the presence of a haploinsufficient cellular environment (7). Finally, in vitro growth of neurofibromin-deficient human Schwann cells can be modulated by different culture conditions (4). These studies indicate that there might be a combined effect of genetic and environmental factors, which finally results in neurofibroma formation. Progesterone might be one of these environmental factors, which promotes neurofibroma growth under certain conditions.
Just recently, Fishbein et al. (10) published new data on the hormonal influence of progesterone in Schwann cell cultures of human neurofibromas. They showed significantly increased progesterone receptor expression in neurofibroma sections compared with Schwann cell cultures. On RNA level, it was almost 100% positive. They suggested that different cell types are involved in steroid receptor expression and that not mainly Schwann cells harbor the receptor.
We could demonstrate that neurofibroma-derived Schwann cells but not neurofibroma-derived fibroblasts express progesterone receptors. Only 60% of the analyzed cultures were receptor-positive without any significant difference between NF(+/−) and NF(−/−) cells, which corresponds with the results of Fishbein et al. They suggested that other cells and not Schwann cells alone must have influence on progesterone receptor RNA expression.
Our data now clearly show that human neurofibroma-derived Schwann cells are in a certain percentage progesterone receptor-positive and respond to elevated progesterone levels with increased proliferation. In NF1 (−/−) Schwann cells, this effect was significantly higher than in NF1 (+/−) or even normal human Schwann cells.
It remains to be investigated whether this might be caused by a qualitative change of the receptor or due to a higher autocrine progesterone production.
Furthermore, it will be of interest to see whether NF Schwann cell proliferation is reduced under the influence of antiprogesterones, which could lead the way to innovative treatment strategies. Fishbein et al. (10) have already done initial studies on this topic, but could only retrieve controversial results. Further studies on the subject are needed. Our study, with its close look at NF (−/−) cells, supports the hypothesis that antiprogesterones should have an effect.
Another question that arises is whether hormonal contraceptives are safe enough to be prescribed for NF patients. Lammert et al. (22) performed a retrospective analysis of NF patients and did not find a significant relationship between tumor growth and intake of hormonal contraceptives. This might be due to relatively low doses of gestagen in most combined estrogen-gestagen preparations. Nevertheless, they found that in patients treated with depot gestagens and thus higher hormone dosages there was a significant rise in tumor growth. This supports our experimental data and underlines the necessity of further investigation on this relevant topic.
Abbreviations
- NF1:
-
neurofibromatosis type 1
References
Friedman JM, Riccardi VM 1992 Clinical and epidemiologic features in NF1. Friedman JM, Gutman DH, McCollin M, Riccardi VM Neurofibromatosis. Phenotype, Natural History and Pathogenesis, 2nd ed. The Johns Hopkins University Press, Baltimore 29–86
Mulvihill JJ, Parry DM, Sherman JL, Pikus A, Kaiser-Kupfer ML, Eldridge R 1990 NIH conference: neurofibromatosis 1 (Recklinghausen disease) and neurofibromatosis 2 (bilateral acoustic neurofibromatosis). An Update. Ann Intern Med 113: 39–52
Peltonen J, Jaakkola S, Lebwohl M, Renvall S, Risteli L, Virtanen I, Uitto J 1988 Cellular differentiation and expression of matrix genes in type 1 neurofibromatosis. Lab Invest 59: 760–771
Serra E, Rosenbaum T, Winner U, Aledo R, Ars E, Estivill X, Lenard HG, Lázaro C 2000 Schwann cells harbor the somatic NF1 mutation in neurofibromas: evidence of two different schwann cell subpopulations. Hum Mol Genet 9: 3055–3064
Kluwe L, Friedrich R, Mautner VF 1999 Loss of NF1 allele in schwann cells but not in fibroblasts derived from an NF1-associated neurofibroma. Genes Chromosomes Cancer 24: 283–285
Cichowski K, Tyler J 2001 NF1 tumor suppressor gene function: narrowing the GAP. Cell 104: 593–604
Zhu Y, Ghosh P, Charnay P, Burns DK, Parada LF 2002 Neurofibromas in NF1: schwann cell origin and role of tumor environment. Science 296: 920–922
Jung-Testas I, Schumacher M, Robel P, Baulieu E 1996 Demonstration of progesterone receptors in rat schwann cells. J Steroid Biochem Mol Biol 58: 77–82
McLaughlin ME, Jacks T 2003 Progesterone receptor expression in neurofibromas. Cancer Res 63: 752–755
Fishbein L, Zhang X, Fisher LB, Li H, Campbell-Thompson M, Yachnis A, Rubenstein A, Muir D, Wallace MR 2007 In vitro studies of steroid hormones in neurofibromatosis 1 tumors and schwann cells. Mol Carcinog 46: 512–523
Rosenbaum T, Rosenbaum C, Winner U, Müller HW, Lenard HG, Hanemann CO 2000 Long-term culture and characterization of human neurofibroma-derived schwann cells. J Neurosci Res 61: 524–532
Maertens O, Prenen H, Debiec-Rychter M, Wozniak A, Sciot R, Pauwels P, de Wever I, Vermeesch JR, de Raedt T, de Paepe A, Speleman F, van Oosterom A, Messiaen L, Legius E 2006 Molecular pathogenesis of multiple gastrointestinal stromal tumors in NF1 patients. Hum Mol Genet 15: 1015–1023
Rutkowski JL, Kirk CJ, Lerner MA, Tennekoon GI 1995 Purification and expansion of human Schwann cells in vitro. Nat Med 1: 80–83
Hanemann CO, Rosenbaum C, Kupfer S, Wosch S, Stoegbauer F, Muller HW 1998 Improved culture methods to expand schwann cells with altered growth behaviour from CMT1A patients. Glia 23: 89–98
Rosenbaum C, Kluwe L, Mautner VF, Friedrich RE, Muller HW, Hanemann CO 1998 Isolation and characterization of schwann cells from neurofibromatosis type 2 patients. Neurobiol Dis 5: 55–64
Bosse F, Zoidl G, Wilms S, Gillen CP, Kuhn HG, Muller HW 1994 Differential expression of two mRNA species indicates a dual function of peripheral myelin protein PMP22 in cell growth and myelination. J Neurosci Res 37: 529–537
Bottenstein JE, Sato GH 1979 Growth of a neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad Sci USA 76: 514–517
Bartolami S, Auge C, Travo C, Venteo S, Knipper M, Sans A 2003 Vestibular schwann cells are a distinct subpopulation of peripheral glia with specific sensitivity to growth factors and extracellular matrix components. J Neurobiol 57: 270–290
Mashour GA, Driever PH, Hartmann M, Drissel SN, Zhang T, Scharf B, Felderhoff-Muser U, Sakuma S, Friedrich RE, Martuza RL, Mautner VF, Kurtz A 2004 Circulating growth factor levels are associated with tumorigenesis in neurofibromatosis type 1. Clin Cancer Res 10: 5677–5683
Robert F, Guennoun R, Désarnaud F, Do-Thi A, Benmessahel Y, Baulieu EE, Schumacher M 2001 Synthesis of progesterone in schwann cells: regulation by sensory neurons. Eur J Neurosci 13: 916–924
Kim HA, Rosenbaum T, Marchionni M, Ratner N, DeClue JE 1995 Schwann cells from neurofibromin deficient mice exhibit activation of p21ras, inhibition of cell proliferation and morphological changes. Oncogene 11: 325–335
Lammert M, Mautner VF, Kluwe L 2005 Do hormonal contraceptives stimulate growth of neurofibromas? A survey on 59 NF1 patients. BMC Cancer 5: 16
Acknowledgements
We thank Dr. Conxi Lázaro and Dr. Eduard Serra, Medical and Molecular Genetics Center-IRO, Hospital Duran i Reynals, L'Hospitalet de Llobregat, Barcelona/Spain for continuous intellectual and molecular genetic support as well as critical reading and comments on the manuscript. We thank Murray Hartley, Great Britain, for proof reading our manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by Deutsche Krebshilfe (grant no. 50–2713).
Rights and permissions
About this article
Cite this article
Overdiek, A., Winner, U., Mayatepek, E. et al. Schwann Cells From Human Neurofibromas Show Increased Proliferation Rates Under the Influence of Progesterone. Pediatr Res 64, 40–43 (2008). https://doi.org/10.1203/PDR.0b013e31817445b8
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/PDR.0b013e31817445b8
This article is cited by
-
Cutaneous neurofibromas in the genomics era: current understanding and open questions
British Journal of Cancer (2018)
-
Receptor of ghrelin is expressed in cutaneous neurofibromas of individuals with neurofibromatosis 1
Orphanet Journal of Rare Diseases (2017)
-
Treatment of Neurofibromatosis Type 1
Current Treatment Options in Neurology (2015)
-
Clinical characteristics predicting internal neurofibromas in 357 children with neurofibromatosis-1: results from a cross-selectional study
Orphanet Journal of Rare Diseases (2012)
-
Genotype-phenotype associations in neurofibromatosis type 1 (NF1): an increased risk of tumor complications in patients with NF1splice-site mutations?
Human Genomics (2012)