Abstract
The National Institute of Child Health and Human Development and the Digestive Diseases Interagency Coordinating Committee held a workshop, chaired by Dr. W. Allan Walker, on July 10–11, 2006, to identify promising leads in necrotizing enterocolitis (NEC) research. The goals of the workshop were to identify new approaches to the prevention and treatment of NEC, to define basic and translational mechanisms of potential approaches to NEC, and to develop recommendations for clinical studies to reduce the incidence of NEC. Workshop participants implicated prematurity, introduction of enteral feedings, gastrointestinal bacterial colonization, gut motility, proinflammatory cytokines, impaired gut blood flow, and various neonatal complications in the pathogenesis of NEC. They concluded that a unifying hypothesis encompassing these pathogenetic factors is the uncontrolled exuberant inflammatory response to bacterial colonization that characterizes the intestine of premature infants. The inflammatory cascade appears to offer multiple targets for interventions with a variety of anti-inflammatory agents, including human milk and probiotics. Because of the rapidity with which the inflammatory response gets out of control in infants with NEC, workshop participants agreed that searching for ways to prevent NEC will be more rewarding than trying to identify ways to treat the condition once it has become established.
Similar content being viewed by others
Main
Approximately 12% of premature infants weighing less than 1500 g will become afflicted with necrotizing enterocolitis (NEC), and about one-third of them will succumb to the disease. Despite many advances in the care of prematurely born infants, these rates have remained remarkably constant over the past four decades. While many potential causes of NEC have been suggested, the only clearly established risk factor is prematurity.
The goals of this workshop were to identify new approaches to the treatment or prevention of NEC, to define mechanisms underlying these new approaches, to discuss possible multicenter clinical trials, and to discuss the best ways to incorporate new treatment or prevention modalities into the clinical care of premature infants. The meeting participants were charged with developing recommendations to establish meaningful treatments or prevention options for NEC.
SESSION I: PREDICTORS AND OUTCOMES
Research and clinical trials on NEC have been limited by difficulties in clearly defining the condition and by our inability to identify subsets of premature infants at high risk of developing NEC, and subsets of infants with NEC at high risk of perforation and necrosis. Diagnosis of NEC is complicated by the overlap of its symptoms with those of a similar condition, spontaneous intestinal perforation (SIP). Some evidence suggests SIP is a distinct clinical syndrome with a different pathogenesis than NEC. Whether or not this is true, SIP presents in a fashion similar to NEC. However, SIP occurs in a population with a different epidemiologic profile than NEC and involves smaller amounts of intestine than NEC. Diagnosing NEC is particularly difficult in very premature infants. Postnatal steroids, often given to these infants, can mask inflammation and other symptoms while simultaneously increasing the risk of intestinal perforation. Certain factors, including exposures to surfactant, indomethacin, glucocorticoids, and vasopressors, as well as the diagnosis of patent ductus arteriosus (PDA), are more commonly observed in cases of SIP than in cases of NEC.
When NEC progresses to severe disease with necrotic or perforated intestine, the disease is treated surgically. Laparotomy with excision of necrotic bowel and primary peritoneal drainage (PPD) are the two most commonly used surgical treatments for NEC. A recently completed randomized trial that compared mortality, gastrointestinal morbidity, and length of hospital stay in infants receiving laparotomy or PPD for NEC found no significant difference in these short-term outcomes (1). These findings might lead to an increase in the use of PPD because this procedure is perceived to be less invasive and produces results similar to laparotomy in the sickest, weakest patients.
An observational study comparing long-term outcomes in infants treated with PPD or laparotomy found no difference in neonatal mortality before discharge between the procedures. However, when the short-term outcome of mortality was combined with the long-term outcome of neurodevelopmental impairment (cerebral palsy, vision impairment, and Bayley Mental Development Index and Psychomotor Development Index scores of <70), the study showed that, in a selected cohort of patients, the combined incidence of death and neurodevelopmental impairment was increased in infants undergoing PPD compared with those undergoing laparotomy (2). It is possible that the inflammatory environment created by allowing necrotic bowel to remain in place may have systemic effects that adversely affect growth and development.
Large-scale studies to assess prevention strategies and treatment for NEC are inherently difficult because of the relatively small proportion of infants who develop NEC. Although prevention would be greatly preferable to treatment, there are few reliable predictors of NEC. Development of predictors of NEC would allow clinicians to conduct smaller, more precisely targeted trials that would expose fewer infants to the undefined risks inherent in the testing of new treatment or prevention modalities. A multivariate regression model to predict NEC found that PDA surgery and antenatal steroids were statistically significant predictors for increased risk of NEC for infants born weighing 400–1000 g. For infants born weighing 1001 to 1500 g, reaching full feeds by 14 d of age was associated with decreased risk (Donovan, unpublished data). A birth weight–stratified model to predict risk—instead of a model that includes all birth weights—reduces the numbers of infants that would be needed for a trial to prevent NEC. The birth weight–stratified model also provides more accurate predictions for individual infants, decreasing exposure to possible harm from an unproven intervention.
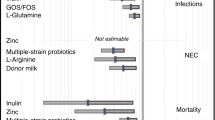
An attempt to fully understand the risk factors for NEC led to the analysis of feeding practices in newborn intensive care units at three hospitals within the same health care system in Utah. Results of this study showed that the institutions that widely practiced breast feeding, or feeding donor human milk to very low birth weight (VLBW) infants had improved survival and decreased incidence of NEC in comparison to institutions practicing lower rates of breast feeding (3). Enteral fasting also has been identified as an independent risk factor for NEC (Moss, unpublished data). It remains to be seen whether early enteral “priming” of the gut can reduce the incidence of NEC.
Swallowing amniotic fluid in utero is believed to be necessary for proper intestinal maturation, leading to efforts to prevent or treat small bowel atrophy by feeding infants simulated amniotic fluid. Such a fluid, named SAFEstart, has been developed, and contains erythropoietin (EPO) and granulocyte-colony stimulating factor (G-CSF). Receptors for these growth factors are found on luminal villus surfaces in the neonatal intestine; binding of G-CSF and EPO to their receptors induces an antiapoptotic effect. Infants receiving SAFEstart had higher caloric intakes and gained more weight, suggesting that supplementing at-risk infants with appropriate growth factors may help protect infants from developing NEC (4).
Epidermal growth factor (EGF) has been found to be a key factor in resection-induced adaptation (RIA), which occurs after removal of large amounts of small bowel. Exogenous EGF has been shown to enhance RIA in mouse models, causing increases in intestine length, villus height, and crypt depth. EGF stimulates enterocyte proliferation and limits apoptosis, tipping enterocyte homeostasis toward enhanced proliferation to facilitate mucosal growth (5). EGF also may be involved in the smooth muscle cell proliferation required for the bowel lengthening and the increase in intestinal circumference that occurs during RIA. The apparent role of EGF in RIA suggests that this growth factor could be used to treat or prevent NEC.
SESSION II: MECHANISMS OF DISEASE
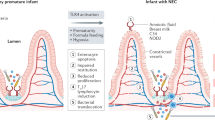
The newborn infant's innate immune response may be negatively affected by enteral fasting, which occurs commonly in the conventional care of VLBW infants. Lack of enteral feeding was associated with translocation of bacteria across the intestinal barrier in a mouse model (6). This finding indicates that feeding may play a role in maintaining this barrier. Protein deprivation in the lumen has been observed to stunt villus growth and cause villus atrophy in a gastrostomy-fed rat pup model (7). Supplementing total parenteral nutrition (TPN) with glucagon-like peptide-2 (GLP-2) stimulated mucosal growth to an extent similar to that observed during enteral feeding, perhaps due to GLP-2's ability to increase blood flow (8). TPN supplemented with butyrate was administered to piglets that had 80% intestinal resection. This treatment resulted in increased villus height, increased proliferation, decreased apoptosis, and an increase in GLP-2 plasma levels. The intestinotrophic effect of butyrate may be mediated through GLP-2 (9). Enteral feeding appears to stimulate the development of the barrier function of the infant intestine. Integrity of the intestinal epithelium is necessary to prevent bacterial translocation into the bloodstream. Epithelial integrity may be compromised by several conditions common to VLBW infants, such as stress, infections, and treatment with indomethacin and glucocorticoids. These factors affect epithelial integrity by weakening tight junctions. Feeding probiotics and other nutrients enhances barrier functions and helps control inflammation in the maturing intestine (10).
No particular bacterial species have been shown to be necessary for NEC to develop. However, 33–48% of infants with NEC have positive blood cultures (11). NEC is marked by immature expression and regulation of nuclear factor kappa B (NFκB) (Nanthakumar and Walker, unpublished data). The inability to distinguish and respond appropriately to commensal and pathogenic microorganisms (9) and the aberrant regulation of other effector molecules such as platelet activating factor (PAF) (12) lead to a proinflammatory environment that could contribute to the pathology seen in NEC. Host defense depends on maintaining an appropriate balance between proinflammatory processes and apoptosis. Immaturity of proinflammatory pathways could increase susceptibility to apoptotic activation, upsetting this balance. Absence of an inflammatory response, or the inability to respond, could result in increased apoptotic tissue damage during bacterial infection, as shown in a neonatal rat model (13).
The intestinal lumen contains large amounts of bacteria and bacterial products that can provoke an immune response, such as bacterial lipopolysaccharide or Salmonella flagellin, and that are proinflammatory in most cells. These stimuli, however, do not activate overt inflammation when interacting with the apical aspect of mature gut epithelial cells. Colonization of the intestine by commensal microorganisms is a key step in intestinal maturation. However, the inappropriate reaction of the immature intestine to colonization might be involved in the pathogenesis of NEC. Mature intestinal enterocytes can distinguish between commensal and pathogenic microorganisms (9). When exposed to commensal organisms, activation of the innate immune response is self-limited. However, a stronger, IL-8-driven proinflammatory response is provoked in immature intestinal cells when exposed to commensal organisms (14). Microarray analysis shows that expression of genes involved in up-regulation of inflammation is increased while expression of negative regulators of inflammation is decreased in the immature intestine (Walker, unpublished data).
Analysis of signal transduction pathways has shown that the NFκB signaling pathway in connection with Toll-like receptors (TLR) is involved in the intestinal immune response to the presence of bacteria. Commensal microorganisms in the gut interact with members of the TLR family to modulate NFκB signaling. The interaction of intraluminal bacteria with aberrantly overexpressed TLR4 in enterocytes may explain the exuberant inflammatory response of the immature intestine to bacterial colonization in neonatal rodent models. The importance of TLR4 in the pathogenesis of NEC is indicated by the decreased incidence of NEC in TLR4 mutant mice (15). Immature enterocytes have enhanced NFκB DNA binding, leading to increased transcription of genes encoding inflammatory cytokines such as IL-8 and TNF-α. The increased NFκB binding can be attributed to enhanced degradation of the NFκB inhibitor, IκB-α. Under normal conditions, commensal microorganisms stabilize IκB-α, thus moderating NFκB-driven transcription of genes encoding proinflammatory cytokines. Ubiquitination of IκB-α is a mechanism by which IκB-α is marked for degradation. A number of nonpathogenic bacteria mediate deactivation of the ubiquitin ligase, E3-SCFβ-TrCP, by promoting deneddylation of the Cul-1 regulatory subunit of E3-SCFβ-TrCP, thus inactivating its ubiquitin ligase activity and limiting degradation of IκB-a (16).
PAF is a phospholipid proinflammatory mediator observed to cause acute intestinal injury in animals, and may play a role in the etiology of NEC. Plasma PAF levels are high in NEC patients, and levels of PAF-acetylhydrolase (PAF-AH), which degrades PAF, are lower in patients than in controls. Activation of the PAF receptor by PAF is associated with apoptosis through activation of caspases, loss of tight junctions, and increased activation of the TLR4 promoter. TLR4 is necessary for modulation of the processes that eliminate pathogenic microorganisms, but in infants with NEC, this pathway is aberrantly activated, leading to exaggerated production of proinflammatory mediators (17).
SESSION III: PREVENTION AND TREATMENT
Preterm infants fed breast milk or colostrum appear to have a lower incidence of NEC than those fed formula (18). The mechanisms by which this apparent protective effect is mediated are under investigation. Milk from mothers of preterm infants differs from that of mothers of term infants. IL-10, which down-regulates inflammation and the production of proinflammatory cytokines, is undetectable in milk from mothers whose VLBW infants develop NEC. Although banked breast milk is an alternative for preterm infants whose mothers are unable to provide sufficient milk, analysis of the complement of cytokines present in breast milk before and after pasteurization shows that pasteurization differentially affects the stability of the cytokines. This observation may explain why banked breast milk is not as beneficial as mother's own milk (19).
Because of their beneficial effects on the gut, probiotics have been proposed as a possible treatment for NEC. Recently published results of a randomized clinical trial performed in Taiwan showed that a mixture of Lactobacillus acidophilus and Bifidobacterium infantis, marketed as Infloran—an over-the-counter food additive—decreased the incidence of death or NEC in preterm infants from 12.8% in the control group to 5% in the group fed the probiotic mixture (20). Randomized clinical trials in other populations are being planned to ascertain the generalizability of this observation and to explore the efficacy of other probiotics in the prevention of NEC.
Research to determine how probiotics exert their effects indicates that probiotic bacteria and their products can inhibit pathogen adherence and strengthen tight junctions, which prevents gut bacteria from entering the circulation. Probiotics also stimulate the expression of genes which encode immune protective factors, such as IgA, largely through modulation of the TLR family of signaling molecules. Other processes that protect against NEC and that are stimulated by probiotics include induction of a balanced T helper cell response, increased production of factors participating in repair (21), increased mucus production, and down-regulation of proinflammatory genes (Walker, unpublished data).
EGF is of interest as a protective agent because of its role in gut maturation and function. Infants with NEC have decreased levels of salivary EGF, as do very premature infants. A prospective observational study disclosed significant differences in salivary EGF levels across gestational and postnatal age. EGF levels were lowest in the most premature infants, increasing through gestation and during the first 3 wk after birth. Variables found to be associated with lower salivary EGF were younger gestational age, Caucasian race, and use of antibiotics. Variables that were associated with higher salivary EGF levels included female sex, receiving breast milk, and increasing chronologic age. Infants who developed NEC had lower salivary EGF levels at wk 1 than infants who did not develop NEC (22). Lower levels of EGF in NEC patients at wk 1 may have predisposed them to the disease. EGF levels increase between wk 1 and 2 in these patients, which may indicate a response to injury caused by NEC.
A preterm rat model of experimental NEC shows that administration of oral EGF results in reduction of NEC (23), normalization of villi structure, decreases in the overproduction of the proinflammatory cytokine IL-18, and increased production of the anti-inflammatory cytokine IL-10 (24). EGF also shifts the balance of pro- and antiapoptotic proteins in favor of cell survival (25).
Heparin-binding epidermal growth factor (HB-EGF) has similar effects (26) and has been shown to reduce inflammation in a rat model (27). Trials to test EGF and HB-EGF in premature infants at risk of NEC are planned, if problems can be overcome in regards to obtaining EGF and HB-EGF suitable for administration to preterm infants.
Vasoconstriction and ischemia are observed in patients with NEC and may contribute to the pathology of the disease. Endothelin-1 (ET-1) is a vasoactive peptide that causes vasoconstriction and ischemia. ET-1 exerts a vasoconstrictor effect by binding to the ETA receptor, which induces angiogenesis, up-regulates endothelial surface adhesion molecules, and increases vascular permeability. ET-1 levels are increased in the intestines of NEC patients with moderate inflammatory damage. Expression of the ETA receptor in human intestine is normally low, but is up-regulated in damaged intestine. Examination of intestinal subserosal arteriolar sections from affected intestine shows that the diameters of arterioles are reduced with a consequent diminution in blood flow. If the arterioles are treated with the ETA receptor antagonist BQ610, recovery of flow is observed. This recovery indicates that the vasoconstriction observed in NEC is mediated in part by ET-1 (28). ET-1 blocking agents thus may represent an additional candidate for novel therapy for NEC. Because cytokine-induced up-regulation of ET-1 appears to be an early event, and because ET-1 appears to participate in generating the vascular pathophysiology of NEC that contributes to disease progression, early blockage of ET-1 action—i.e. at the first signs of Bell's stage I NEC—might be the most effective way to use ET-1 blockade to treat this condition.
Clinical prevention trials targeted to infants at high risk for developing NEC and clinical treatment trials targeted to infants with NEC who are at high risk of progression will be necessary to improve outcomes in this disease. The National Institutes of Health can facilitate NEC prevention and treatment trials by increasing funding for clinical research, encouraging cross-disciplinary and collaborative research, and supporting multicenter collaborative research such as assisting in the development of a NEC network. Taking the pediatric cancer clinical trials enterprise as a model, effective management of NEC could result from a multicenter cooperative effort to develop a combination of approaches, rather than a single “magic bullet” intervention. The gains made in survival in childhood cancer were helped by the cancer clinical trials mechanism, which permitted most pediatric cancer patients to be enrolled in trials of experimental chemotherapy, radiation, adjuvant, or combination therapy. NEC researchers should emulate this model by creating a research network that permits all practitioners with NEC patients to enroll high-risk infants in NEC prevention clinical trials. Development of databases, such as the Glaser Pediatric Research Network Surgical Database, will assist with research aimed at understanding the clinical and biologic factors that increase the risk of NEC by determining risk factors that predict progression from intestinal inflammation to perforation or necrosis. Trials are also needed to analyze the relationship between feeding practices and NEC, and to assess current practice patterns regarding type and duration of antibiotic therapy in nonsurgical NEC to ascertain the impact of antibiotics on disease progression. The Glaser Pediatric Research Network Surgical Database could be instrumental in collecting samples for use in family studies and genomic and proteomic analyses that will advance our understanding of the pathogenesis and prevention of NEC.
NEXT STEPS FOR NEC RESEARCH
Workshop participants concluded that the onset of an excessive and uncontrolled inflammatory response by the neonatal intestine to exposure to luminal bacteria is a unifying hypothesis that encompasses many of the factors that have been associated with the development of NEC. Therefore, future research efforts should focus on identifying the underlying causes and molecular mechanisms contributing to the development of NEC, including processes occurring early in the pathogenic process. Understanding the impact of inflammation, various signaling pathways, and apoptosis should be a priority and will require analysis of multiple molecular signaling systems. Additionally, proteomic approaches could be used to compare children who develop NEC with those who do not to identify protective factors and to develop predictive biomarkers, such as serum levels of PAF, ET-1, and proinflammatory cytokines. Interested investigators should consider banking specimens from all preterm infants to use for prospective studies. To this end, banking and analysis of biospecimens, such as blood, peritoneal fluid, and tissue samples, should be part of NEC clinical trials. Investigators should also pursue collaborations with neonatal surgeons to obtain samples of resected human intestine. To share samples effectively, a centralized location for storing and annotating specimens is needed.
Clinical trials to prevent or treat NEC should include trials of growth factors, particularly EGF. Probiotics or conditioned media also should be tested, alone or in combination with EGF. Additives to human milk and substances such as colostrum, butyrate, anti-inflammatory drugs, and omega-3 fatty acids also should be considered for trials. Because NEC is relatively rare, trial designers should consider using prediction models to enrich trial enrollment with preterm infants at highest risk of NEC. Therefore, unified and consistent methods for identifying the risks for the onset of NEC and for the likely progression of NEC are needed. Although knowledge of the mechanisms and molecular pathways involved in NEC is in its infancy, trials testing potential preventive therapy or treatments should not be delayed for lack of this knowledge. NEC trials should include long-term outcomes in addition to early mortality and length of hospital stay.
Because breast feeding appears to decrease the risk of NEC, hospitals should consider providing support for mothers of infants in newborn intensive care units who wish to breast feed. Research is also needed on alternative ways to process donor milk that preserve as many of the anti-inflammatory components in human milk as possible. Methods should be developed to create breast milk concentrates that contain all protective components, not just single, selected protective components. Common clinical practices, such as antibiotic use, prolonged TPN, and aggressive or insufficient feeding, should be examined to determine whether these practices are beneficial or harmful.
Because NEC frequently progresses from early signs of intestinal inflammation to extensive necrosis within a matter of hours, the workshop participants concluded that research on approaches to prevent NEC should be emphasized rather than approaches to treatment. This conclusion underscores the need for more sensitive algorithms to identify preterm infants at highest risk of developing intestinal inflammation that is likely to progress to NEC.
MEETING PARTICIPANTS
Duane Alexander, M.D., Carol Berseth, M.D., Gail E. Besner, M.D., Martin Blakely, M.D., Michael S. Caplan, M.D., Erika C. Claud, M.D., Robert Dennis Christensen, M.D., Edward F. Donovan, M.D., Bohuslav Dvorak, Ph.D., Frank Hamilton, M.D., M.P.H., Phillip V. Gordon, M.D., Ph.D., Gilman D. Grave, M.D., Stephen Groft, Pharm.D, Larry Grylack, M.D., Ethan D. Hausman, M.D., FAAP, FCAP, Rosemary Higgins, M.D., Terry H. Huang, Ph.D., M.P.H., Stephen P. James, M.D., Susan McCune, M.D., Lawrence R. Moss, M.D., FACS, Andrew S. Neish, M.D., Josef Neu, M.D., Philip T. Nowicki, M.D., William Oh, M.D., Tonse N.K. Raju, M.D., William Rodriguez, M.D., Richard J. Schanler, M.D., Hong Tang, M.D., FACP, W. Allan Walker, M.D., Barbara K. Warner, M.D., Brad W. Warner, M.D., Anne Willoughby, M.D., M.P.H.
Abbreviations
- ET-1:
-
endothelin-1
- GLP-2:
-
glucagon-like peptide-2
- HB-EGF:
-
heparin-binding epidermal growth factor
- NEC:
-
necrotizing enterocolitis
- PAF:
-
platelet activating factor
- PPD:
-
primary peritoneal drainage
- RIA:
-
resection-induced adaptation
- SIP:
-
spontaneous intestinal perforation
- TLR:
-
Toll-like receptor
- VLBW:
-
very low birth weight (<1500 g)
References
Moss RL, Dimmitt RA, Barnhart DC, Sylvester KG, Brown RL, Powell DM, Islam S, Langer JC, Sato TT, Brandt ML, Lee H, Blakely ML, Lazar EL, Hirschl RB, Kenney BD, Hackam DJ, Zelterman D, Silverman BL 2006 Laparotomy versus peritoneal drainage for necrotizing enterocolitis and perforation. N Engl J Med 354: 2225–2234
Blakely ML, Tyson JE, Lally KP, McDonald S, Stoll BJ, Stevenson DK, Poole WK, Jobe AH, Wright LL, Higgins RD 2006 Laparotomy versus peritoneal drainage for necrotizing enterocolitis or isolated intestinal perforation in extremely low birth weight infants: outcomes through 18 months adjusted age. Pediatrics 117: e680–e687
Wiedmeier SE, Henry E, Baer VL, Stoddard RA, Eggert LD, Lambert DK, Christensen RD 2007 Necrotizing enterocolitis in three level III NICUs within one healthcare system. Am J Perinatol (in press)
Christensen RD, Havranek T, Gerstmann DR, Calhoun DA 2005 Enteral administration of a simulated amniotic fluid to very low birth weight neonates. J Perinatol 25: 380–385
Warner BW, Erwin CR 2006 Critical roles for EGF receptor signaling during resection-induced intestinal adaptation. J Pediatr Gastroenterol Nutr 43: S68–S73
Wildhaber BE, Yang H, Spencer AU, Drongowski RA, Teitelbaum DH 2005 Lack of enteral nutrition—effects on the intestinal immune system. J Surg Res 123: 8–16
Li N, Lassman BJ, Liu Z, Liboni K, Neu J 2004 Effects of protein deprivation on growth and small intestinal morphology are not improved by glutamine or glutamate in gastrostomy-fed rat pups. J Pediatr Gastroenterol Nutr 39: 28–33
Bartholome AL, Albin DM, Baker DH, Holst JJ, Tappenden KA 2004 Supplementation of total parenteral nutrition with butyrate acutely increases structural aspects of intestinal adaptation after an 80% jejunoileal resection in neonatal piglets. JPEN J Parenter Enteral Nutr 28: 210–222
Claud EC, Savidge T, Walker WA 2003 Modulation of human intestinal epithelial cell IL-8 secretion by human milk factors. Pediatr Res 53: 419–425
Neu J, Chen M, Beierle E 2005 Intestinal innate immunity: how does it relate to the pathogenesis of necrotizing enterocolitis?. Semin Pediatr Surg 14: 137–144
Uauy RD, Fanaroff AA, Korones SB, Phillips EA, Philllips JB, Wright LL 1991 Necrotizing enterocolitis in very low birth weight infants: biodemographic and clinical correlates. J Pediatr 119: 630–638
Martin CR, Walker WA 2006 Intestinal immune defences and the inflammatory response in necrotizing enterocolitis. Semin Fetal Neonatal Med 11: 369–377
Jilling T, Lu J, Jackson M, Caplan MS 2004 Intestinal epithelial apoptosis initiates gross bowel necrosis in an experimental rat model of neonatal necrotizing enterocolitis. Pediatr Res 55: 622–629
Claud EC, Lu L, Anton PM, Savidge T, Walker AW, Cherayil BJ 2004 Developmentally regulated IkappaB expression in intestinal epithelium and susceptibility to flagellin-induced inflammation. Proc Natl Acad Sci U S A 101: 7404–7408
Jilling T, Simon D, Lu J, Meng FJ, Li D, Schy R, Thomson RB, Soliman A, Arditi M, Caplan MS 2006 The roles of bacteria and TLR4 in rat and murine models of necrotizing enterocolitis. J Immunol 177: 3273–3282
Collier-Hyams LS, Sloane V, Batten BC, Neish AS 2005 Cutting edge: bacterial modulation of epithelial signaling via changes in neddylation of cullin-1. J Immunol 175: 4194–4198
Caplan MS, Simon D, Jilling T 2005 The role of PAF, TLR, and the inflammatory response in neonatal necrotizing enterocolitis. Semin Pediatr Surg 14: 145–151
Schanler RJ, Lau C, Hurst NM, Smith EO 2005 Randomized trial of donor human milk versus preterm formula as substitutes for mothers' own milk in the feeding of extremely premature infants. Pediatrics 116: 400–406
Heiman H, Schanler RJ 2006 Benefits of maternal and donor human milk for premature infants. Early Hum Devel Epub October 19:2006
Lin HC, Su BH, Chen AC, Lin TW, Tsai CH, Yeh TF, Oh W 2005 Oral probiotics reduce the incidence and severity of necrotizing enterocolitis in very low birth weight infants. Pediatrics 115: 1–4
Kalliomaki MA, Walker WA 2005 Physiologic and pathologic interactions of bacteria with gastrointestinal epithelium. Gastroenterol Clin North Am 34: 383–399
Shin CE, Falcone RA Jr, Stuart L, Erwin CR, Warner BW 2000 Diminished growth factor levels in infants with necrotizing enterocolitis. J Pediatr Surg 35: 173–176
Dvorak B, Halpern MD, Holubec H, Williams CS, McWilliam DL, Dominguez JA, Stepankova R, Payne CM, McCuskey 2002 Epidermal growth factor reduces the development of necrotizing enterocolitis in a neonatal rat model. Am J Physiol Gastrointest Liver Physiol 282: G156–G164
Halpern MD, Dominguez JA, Dvorakova K, Holubec H, Williams CS, Meza YG, Ruth MC, Dvorak B 2003 Ileal cytokine dysregulation in experimental necrotizing enterocolitis is reduced by epidermal growth factor. J Pediatr Gastroenterol Nutr 36: 126–133
Clark JA, Lane RH, Maclennan NK, Holubec H, Dvorakova K, Halpern MD, Williams CS, Payne CM, Dvorak B 2005 Epidermal growth factor reduces intestinal apoptosis in an experimental model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 288: G755–G762
Feng J, El-Assal ON, Besner GE 2006 Heparin-binding epidermal growth factor-like growth factor reduces intestinal apoptosis in neonatal rats with necrotizing enterocolitis. J Pediatr Surg 41: 742–747
Feng J, El-Assal ON, Besner GE 2006 Heparin-binding epidermal growth factor-like growth factor decreases the incidence of necrotizing enterocolitis in neonatal rats. J Pediatr Surg 41: 144–149
Nowicki PT, Dunaway DJ, Nankervis CA, Giannone PJ, Reber KM, Hammond SB, Besner GE, Caniano DA 2005 Endothelin-1 in human intestine resected for necrotizing enterocolitis. J Pediatr 146: 805–810
Author information
Authors and Affiliations
Corresponding author
Additional information
The workshop was sponsored by the National Institute of Child Health and Development and the NIDDK.
Rights and permissions
About this article
Cite this article
Grave, G., Nelson, S., Walker, W. et al. New Therapies and Preventive Approaches for Necrotizing Enterocolitis: Report of a Research Planning Workshop. Pediatr Res 62, 510–514 (2007). https://doi.org/10.1203/PDR.0b013e318142580a
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/PDR.0b013e318142580a
This article is cited by
-
Initial surgical treatment of necrotizing enterocolitis: a meta-analysis of peritoneal drainage versus laparotomy
European Journal of Pediatrics (2022)
-
IL-17-related signature genes linked to human necrotizing enterocolitis
BMC Research Notes (2021)
-
Surfactant protein A reduces TLR4 and inflammatory cytokine mRNA levels in neonatal mouse ileum
Scientific Reports (2021)
-
Does red blood cell irradiation and/or anemia trigger intestinal injury in premature infants with birth weight ≤ 1250 g? An observational birth cohort study
BMC Pediatrics (2018)
-
Timing of developmental reduction in epithelial glutathione redox potential is associated with increased epithelial proliferation in the immature murine intestine
Pediatric Research (2017)