Abstract
Pulmonary surfactant is inactivated in meconium aspiration syndrome and neonatal pneumonia. Development of an exogenous surfactant less sensitive to inactivation might be useful for treating these diseases. We investigated in vitro whether addition of the cationic cyclic membrane cross-linking peptide polymyxin B (PxB) and/or calcium chloride (CaCl2) to modified porcine surfactant Curosurf increases resistance to meconium-induced inactivation of surface activity while antimicrobial activity of PxB is maintained. To study bacterial proliferation, Escherichia coli, group B streptococci (GBS), or Staphylococcus aureus were incubated 0–5 h in saline or in meconium in the presence or absence of Curosurf with or without PxB. PxB and CaCl2 improved spreading and adsorption of Curosurf. Curosurf plus CaCl2/PxB needed a 4-fold increase of meconium concentration to increase dynamic surface tension significantly compared with Curosurf plus CaCl2 alone, indicating that PxB further increases the resistance of Curosurf to meconium-induced inactivation. Meconium alone like meconium/Curosurf promoted growth of E. coli and GBS, but addition of Curosurf/PxB or PxB alone significantly reduced the growth of E. coli. Biophysical and antibacterial properties of Curosurf and PxB may be combined into a useful adjunct in the treatment of neonatal Gram-negative pneumonia and/or meconium aspiration syndrome.
Similar content being viewed by others
Main
MAS and pneumonia remain relevant causes of respiratory failure in neonates. In MAS, direct inhibition, inflammation, and, in some cases, secondary bacterial pneumonia contribute to surfactant dysfunction. This may result in hypoxia and acidosis, which further reduces surfactant activity. Meconium-induced surfactant inactivation in vitro was first described by Moses et al. (1). Minimum surface tension of bovine surfactant, measured in a PBS, increased when diluted human meconium was added. In a newborn rabbit model of meconium aspiration, surfactant treatment improved lung function and morphology (2). Pilot studies using intratracheal doses of 50–200 mg/kg of exogenous pulmonary surfactant as well as a controlled, randomized clinical study showed improved oxygenation of neonates with MAS receiving repeated high doses of surfactant (3). However, modified natural surfactants containing hydrophobic surfactant proteins B (SP-B) and C (SP-C) were more sensitive to inactivation by meconium than synthetic surfactant preparations based on artificial peptides such as SP-C analogues or the simplified peptide KL4 (4). Thus, development of surfactants that are resistant to inactivation might be useful (5).
Gram-positive bacteria like group B streptococci (GBS) or Staphylococcus aureus and Gram-negative microorganisms such as Escherichia coli, Klebsiella pneumoniae, or Enterobacter cloacae are common pathogens causing serious neonatal infections, including neonatal pneumonia (6–8). The infection leads to pulmonary inflammation with accumulation of cytokines in the lung, leakage of plasma proteins into the alveoli, and surfactant inactivation. In experimental neonatal GBS pneumonia in rabbits, treatment with modified natural surfactant [Curosurf poractant alfa); Nycomed Pharma GmbH, Unterschleissheim, Germany]) significantly reduced bacterial growth compared with control animals not receiving surfactant (9). Clinically, treatment of neonatal pneumonia includes endotracheal surfactant administration and antibiotic therapy (10).
PxB, a cationic polypeptide antibiotic against Gram-negative bacteria is in clinical use as a topical or, rarely, a systemic antimicrobial agent. Because of its potential neuro- and nephrotoxicity, PxB is used preferentially in case of bacterial resistance or intolerance to other antibiotics. PxB binds to membrane phospholipids and LPS of Gram-negative bacterial membranes, inducing pore formation, which ultimately leads to bacteriolysis (11).
PxB like SP-B may cross-link two juxtaposed phospholipid bi-layers, and addition of SP-B or PxB to synthetic pulmonary surfactant improved surface activity (12).
The aim of this study was to investigate in vitro the surface activity and the antimicrobial properties of PxB/surfactant mixtures in the presence of meconium.
MATERIALS AND METHODS
Surfactant.
Curosurf (80 mg/mL, batches 02705003, 194/09 and 200/05) was obtained from Nycomed Pharma GmbH. It contains approximately 41–48% saturated phosphatidylcholine (PC), 51–58% other phospholipids, and 1% SP-B and SP-C. PxB (8100 IU/mg) was from Sigma Chemical Co. Aldrich Chemicals, Steinheim, Germany. Curosurf and PxB were mixed at a ratio of 100:1 (wt/wt).
Meconium.
Human meconium from 40 healthy term neonates was collected in sterile tubes with parental consent at the University of Göttingen. The collection was approved by the local ethics committee of the medical school of Göttingen, Germany. Aliquots of the suspensions were serially diluted, transferred to agar plates, and incubated at 37°C for 24 h to investigate bacterial contamination. Samples containing <104 CFU/g were lyophilized and pooled. After lyophilization, the dry weight of the samples was 254 mg/g meconium.
Determination of surface activity.
Lyophilized meconium and surfactant were dissolved in saline in presence or absence of 1.5 mM calcium chloride (CaCl2). Final concentrations of Curosurf were 2.5 and 5 mg/mL and for meconium 0.08–5 mg/mL. Before measurement, the samples were incubated at 37°C for 30 min under mechanical agitation.
Surface tension was determined with a PBS (Electronetics Corp., New York, NY) using bubbles with maximal surface area of 3.8 mm2. After bubble formation, surface tension at 37°C was first recorded under static conditions, and measurements obtained 2 s and 10 s after creation of the bubble were used as parameters of surface adsorption. After a period of 1 min, the bubble underwent 50% cyclic area compression at a frequency of 20/min for 5 min. The pressure gradient across the bubble wall was recorded continuously and dynamic surface tension at minimum and maximum bubble size (γmin, γmax) was calculated after 5 min of pulsation (13). For each PBS measurement, approximately 40 μL of sample fluid was filled in a new sample chamber. Each sample was measured five times.
Spreading rates of the various surfactant samples were evaluated using a modified Wilhelmy balance (Biegler, Mauerbach, Austria). The trough was filled with saline heated to 37°C. The surface area was 20 cm2 and each sample was applied as a single droplet on the hypophase 4 cm from the dipping sensor plate. The resulting change in surface tension was recorded during 5 min after application of the test material. Each applied sample contained the amount required to coat the surface with two monolayers of saturated phospholipids, calculated with a hypothetical area of approximately 40 A˚2 per saturated PC molecule (14).
Based upon a pilot study (unpublished data), we chose a meconium concentration that inactivated Curosurf/CaCl2 but not Curosurf/PxB/CaCl2. At this concentration, we studied the effects of CaCl2, PxB, or CaCl2 plus PxB on surface activity of Curosurf in the presence or absence of meconium. These experiments were conducted in parallel in the PBS and Wilhelmy balance to confirm the results with different biophysical assays.
Bacterial growth.
Bacterial growth experiments were conducted at final concentrations of 20 mg/mL meconium and 10 mg/mL Curosurf both in saline. Aliquots containing bacterial suspensions (1 mL) of E. coli (strain ATTC 25922), GBS (strain 090 Ia Colindale, a high-density variant that lacks a polysaccharide capsule, a kind gift from Stellan Håkanson, University of Umeå, Sweden), or S. aureus (strain ATTC 25923), stored at –80°C, were transferred to 11.5-mL nutrient broth (Standard I, Merck, Darmstadt, Germany: 15 mg/mL peptone, 6 mg/mL sodium chloride, 3 mg/mL yeast extract, 1 mg/mL D (+)-glucose) and incubated at 37°C for 16 h. The bacteria were then diluted 1:7 in freshly warmed broth and incubated, E. coli for 1 h and S. aureus or GBS for 3 h, at 37°C to reach the mid-logarithmic growth phase. The bacteria were centrifuged at 1800 × g for 10 min, washed twice, and thereafter resuspended in saline. Bacterial concentrations were adjusted by measuring OD of the suspensions at 595 nm to transfer approximately 108 CFU to sterile tubes containing finally 1 mL volume of either 1) meconium, 2) Curosurf and meconium, 3) 0.1 mg/mL PxB and meconium, 4) Curosurf with 0.1 mg/mL PxB and meconium, or 5) saline. After incubation periods of 0, 1, 3, and 5 h at 37°C under agitation at 180 rpm, 100 μL aliquots of each sample were serially diluted, transferred to Petri dishes, and mixed with warm blood agar. CFU were counted after incubation for 24 h at 37°C. Five repeats of each experiment were done.
Statistical analysis.
The results of surface activity studies and bacterial growth experiments are expressed as mean + SD. Statistical analysis was done using one-way ANOVA for repeated measures. Static surface tension, γmin, and γmax from meconium-containing samples were compared with control samples without meconium using Dunnett's post test. Bonferroni's post test was used to compare static surface tension of surfactants alone versus addition of CaCl2, PxB, or CaCl2 plus PxB in presence or absence of meconium. In bacterial growth studies, Dunnett's post test compared bacterial numbers at 1, 3, and 5 h with values at 0 h as control. Statistical calculations were conducted with GraphPad Prism 4.02 software (San Diego, CA).
RESULTS
Surface activity.
Static surface tension in the PBS showed that adsorption was significantly retarded in the most diluted surfactant samples (2.5 mg/mL) containing the larger amounts of meconium (1 mg/mL), as reflected by high values for static surface tension 10 s after bubble formation (Table 1). Furthermore, meconium significantly increased γmax and, at Curosurf 2.5 mg/mL, γmin after 5 min of pulsation (Table 2). This indicates meconium induced surfactant inactivation. Presence of CaCl2 and/or CaCl2 plus PxB reduced or counter-balanced effects of meconium on surfactant (Tables 1 and 2).
In spreading studies using small amounts of surfactant equivalent to approximately two monolayers of saturated PC, we found meconium and surfactant dose induced delay of adsorption (Table 3). Addition of CaCl2 and/or CaCl2 plus PxB accelerated spreading especially when given to Curosurf 5 mg/mL as reflected by values close to those obtained with Curosurf 80 mg/mL after 10 s (33 versus 27 mN/m; Table 3). Meconium-induced delay of static surface tension after 5 min was improved, but was not totally counter-balanced by addition of CaCl2 and/or CaCl2 plus PxB.
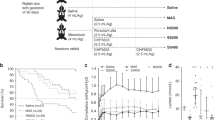
By using a meconium dose response assay, we focused more closely on differences between Curosurf plus CaCl2 and Curosurf plus PxB/CaCl2(Fig. 1). Addition of meconium increased γmin and γmax of surfactant preparations in a dose-dependent manner. If meconium concentration was ≥0.31 mg/mL, γmin of Curosurf/CaCl2 exceeded 10 mN/m and values for γmin and γmax were significantly increased, compared with the control without meconium (p < 0.01). This indicates surfactant inactivation.
Effects of increasing meconium concentrations on minimum (γmin) and maximum surface tension (γmax) of Curosurf 2.5 mg/mL and Curosurf 2.5 mg/mL plus 1% (wt/wt) PxB both in presence of 1.5 mM CaCl2. Values were recorded after 5 min of pulsation in the PBS and are expressed as mean + SD of γmin and γmax. **p < 0.01 vs 0 mg/mL meconium; n = 5 experiments.
Addition of 1% PxB to Curosurf/CaCl2 shifted the dose response curves to the right, i.e. a higher meconium concentration was needed for surfactant inactivation. In mixtures containing Curosurf and PxB, a 4-fold increase of the meconium concentration was needed to increase γmin and γmax significantly compared with control samples with only surfactant. Thus, addition of PxB to Curosurf/CaCl2 further increases resistance to meconium-induced inactivation.
Bacterial growth.
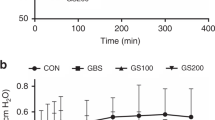
Concentrations of E. coli (Fig. 2 A), GBS (Fig. 2B), and S. aureus (data not shown) in samples containing only saline showed no significant change during 5 h of incubation.
Growth of (A) E. coli and (B) GBS in saline, meconium, meconium plus Curosurf, meconium plus PxB, and meconium plus Curosurf and PxB. Values are expressed as mean + SD of five experiments. *p < 0.05; **p < 0.01 vs time = 0 h. To enable comparison, growth of GBS is shown on two logarithmic scales, of which the left side is identical to panel A.
In meconium-containing samples, the concentration of Gram-positive GBS increased over the incubation time, whereas we did not observe any change in growth for S. aureus. After 5 h of incubation, a significant proliferation of GBS in samples containing meconium (p < 0.05) and meconium/Curosurf/PxB (p < 0.01) compared with the controls at 0 h was found (Fig. 2B).
The concentration of E. coli significantly increased by a factor of 100 in meconium after 3 and 5 h (p < 0.05) and in meconium/Curosurf mixtures after 5 h of incubation (p < 0.01). In contrast, addition of PxB or Curosurf plus PxB to meconium led to a significant decrease of the proliferation of E. coli already after 1 h (p < 0.01). After 5 h, the bacterial count was reduced at least by a factor of 104 (Fig. 2 B). The growth of E. coli in Curosurf plus PxB was slightly but not significantly higher than in PxB only.
DISCUSSION
In MAS, meconium-stained amniotic fluid enters the alveolar space, where meconium may interfere with surfactant function. For better understanding of the interaction between meconium and surfactant it would be useful to have an estimate of the intrapulmonary amounts of meconium and surfactant in babies with MAS. In term neonates, the surfactant pool size is 50–100 mg/kg (15) and the alveolar volume defined as the functional residual capacity is 25–30 mL/kg (16). Assuming uniform distribution of the surfactant pool in the still fluid-filled alveolar compartment, the surfactant concentration is at least 2.5–3 mg/mL, which is similar to the concentrations used in this study.
No information about the amount of meconium in lungs of neonates with MAS is available. In our study, we used lyophilized meconium at concentrations up to 5 mg/mL equivalent to approximately 20 mg/mL native meconium corresponding to 6–40 g in 0.3–2 L total amniotic fluid at term (17). Because the daily excretion of meconium is approximately 5 g (18), the concentrations used in our studies are within the expected range of MAS.
In our study, we used surfactant phospholipid concentrations of 2.5 or 5 mg/mL to investigate differences in resistance to meconium-induced inactivation. In the absence of meconium, PxB significantly reduced static surface tension only at 2.5 mg/mL Curosurf in the Wilhelmy balance, whereas no improvement was seen at 5 mg/mL or when identical samples were tested for static or dynamic surface tension in the PBS. Calkovska et al. (19) found improved dynamic surface activity after addition of 2% PxB to Curosurf 2 mg/mL. We used slightly higher Curosurf concentrations and found, even without addition of PxB, γmin <3 mN/m. Kobayashi et al. (20) found concentrations of porcine surfactant, as used in our and the study of Calkovska, as critical to obtain optimal γmin <3 mN/m in the PBS. After addition of 0.3 mg/mL meconium, Curosurf/CaCl2 was inactivated, whereas 1.3 mg/mL meconium were needed to inactivate Curosurf/CaCl2/PxB. In a pilot study, we found similar improvement of resistance to meconium-induced inactivation after addition of 1% PxB to Curosurf/CaCl2 (unpublished data).
Several strategies have been used to increase the resistance of exogenous surfactant to inactivation (21). Moses et al. (1) showed that an increase of the concentration resulted in increased surfactant resistance in a dose-dependent, but not stoichiometric manner. Sun et al. (22) studied the effects of increasing doses of human meconium on Curosurf at 10 mg/mL, i.e. a four times higher phospholipid concentration compared with our present investigation, and found inactivation at meconium concentrations of 2.5 mg/mL (wt/wt). These observations confirmed that modified natural surfactants at concentrations <5 mg/mL are more sensitive to inactivation in vitro than at concentrations ≥10 mg/mL.
Another strategy for improving the resistance of surfactant to inactivation is to add nonionic or ionic polymers. These polymers reversed meconium-induced surfactant inactivation in vitro and improved lung function of animals with meconium-induced lung injury (23,24). Recently, Lu et al. (25) showed that the anionic polymer hyaluronic acid at 1–1.25 mg/mL in vitro prevented serum induced inactivation of different surfactants.
Furthermore, the surfactant protein content may improve the resistance to inactivation. Curosurf enriched with 5% SP-A (normally absent in the product) had an increased resistance to inactivation by meconium, fibrinogen, albumin, and serum in vitro and improved pulmonary compliance in immature newborn rabbits after intratracheal instillation of fibrinogen compared with controls treated with only Curosurf (26). In vitro, natural rabbit surfactant (containing about 5% SP-A) was more resistant to inactivation by human meconium than the modified natural surfactants Curosurf, Alveofact, or Survanta (4).
PxB may connect lipid vesicles thus enlarging the “surface-associated surfactant reservoir” (27) in the hypophase and making phospholipids readily available for surface adsorption in the pulsating bubble. Moreover, PxB promoted bidirectional transfer of monoionic phospholipids like phosphatidylglycerol, but not of zwitterionic molecules such as dipalmitoylphosphatidylcholine (DPPC) (28). Enrichment of DPPC, the main component of surfactant, at the air liquid interface of the lung is believed to be a significant function of surfactant proteins (29). We speculate that PxB might contribute to this refinement by sorting the phospholipids.
In the absence of meconium, Curosurf at concentrations 1–20 mg/mL shows a bactericidal dose-dependent effect on GBS, but not on E. coli or S. aureus in absence of meconium (30). In this study, addition of meconium or meconium/Curosurf enhanced the growth of E. coli and GBS, but not of S. aureus. Furthermore, none of the bacterial species showed growth in nutrient-free saline. In rats, intratracheal instillation of E. coli combined with meconium increased mortality caused by bronchopneumonia, compared with controls treated with E. coli and saline (31). Meconium at concentrations ≥3 mg/mL in amniotic fluid promoted growth of E. coli and Listeria monocytogenes but reduced growth of S. aureus (32). Data from a recent study suggested that meconium counteracts the bacteriostatic effects of amniotic fluid and enhances the growth of GBS more than that of E. coli. Furthermore, the proliferation of both bacteria increased with meconium concentration and incubation time (33). Meconium diluted in saline to 20 mg/mL but not saline amplified growth of E. coli, GBS, S. aureus, and several other Gram-positive and Gram-negative bacterial pathogens (34).
Our data demonstrate that PxB with and without Curosurf reduces the growth of E. coli but not of GBS or S. aureus in meconium suspensions. PxB has potent bactericidal activity against Gram-negative bacteria (11) but no significant activity against Gram-positive bacteria or fungi (35).
Local pulmonary administration of aerosolized PxB has been used in treatment of chronic Gram-negative infections, as in cystic fibrosis (11). To increase local antimicrobial effects, tobramycin has been incorporated in liposomes, which is currently under preclinical investigation (36). In rats with experimental pulmonary Pseudomonas aeruginosa infection, treatment with PxB incorporated in liposomes, composed of DPPC and cholesterol, resulted in lower bacterial counts per lung compared with treatment with free PxB or liposomes without PxB (37,38). The efficiency of PxB incorporation depended on liposome composition (38). The amounts of PxB entering surfactant lipid vesicles after addition to the surfactant suspension, as used in this study, are not known.
PxB and SP-C bind in a stoichiometric manner to the lipid A moiety of LPS, which is part of the Gram-negative outer membrane (39,40). In Figure 2A, the curve of PxB alone shows a more rapid reduction of E. coli during the first hour, indicating a faster reduction of bacterial growth compared with Curosurf/PxB. However, a major binding of SP-C to LPS in Curosurf/PxB samples seems unlikely, since this difference of bacterial killing was not statistically significant.
The recommended dose level for intratracheal administration of exogenous modified natural pulmonary surfactant is 100–200 mg/kg body weight. Calkovska et al. (19) suggested an increased resistance of Curosurf to albumin-induced inactivation in vitro after addition of PxB 2% (wt/wt), corresponding with 2–4 mg/kg body weight. For aerosolized free PxB, a maximum dose of 2.5 mg/kg/d divided in four doses is recommended for adults with normal renal clearance (35). Therefore, we used PxB at a concentration of 1%, corresponding to 1–2 mg PxB/kg body weight, to approach clinically usable doses.
In summary, PxB-containing pulmonary surfactant is more resistant to inactivation by human meconium than modified natural surfactant in vitro. Antimicrobial activity of PxB against E. coli is maintained when PxB is added to pulmonary surfactant, and it is not significantly different from PxB alone. Thus, PxB combined with exogenous surfactant may be a useful adjunct in the therapy of neonatal pneumonia and/or MAS and should be further evaluated in animal models.
Abbreviations
- CFU:
-
colony-forming units
- DPPC:
-
dipalmitoylphosphatidylcholine
- LPS:
-
lipopolysaccharide
- MAS:
-
meconium aspiration syndrome
- PBS:
-
pulsating bubble surfactometer
- PxB:
-
polymyxin B
- SP:
-
surfactant protein
- γ:
-
surface tension
References
Moses D, Holm BA, Spitale P, Liu M, Enhorning G 1991 Inhibition of pulmonary surfactant by meconium. Am J Obstet Gynecol 164: 477–481
Sun B, Curstedt T, Song GW, Robertson B 1993 Surfactant improves lung function and morphology in newborn rabbits with meconium aspiration. Biol Neonate 63: 96–104
Findlay RD, Taeusch HW, Walther FJ 1996 Surfactant replacement therapy for meconium aspiration syndrome. Pediatrics 97: 48–52
Herting E, Rauprich P, Stichtenoth G, Walter G, Johansson J, Robertson B 2001 Resistance of different surfactant preparations to inactivation by meconium. Pediatr Res 50: 44–49
Johansson J, Curstedt T, Robertson B 2001 Artificial surfactants based on analogues of SP-B and SP-C. Pediatr Pathol Mol Med 20: 501–518
Mifsud AJ, Efstratiou A, Charlett A, McCartney AC . Health Protection Agency Group B Streptococcus Working Group. 2004 Early-onset neonatal group B streptococcal infection in London: 1990–1999. BJOG 111: 1006–1011
Healy CM, Palazzi DL, Edwards MS, Campbell JR, Baker CJ 2004 Features of invasive staphylococcal disease in neonates. Pediatrics 114: 953–961
Cordero L, Rau R, Taylor D, Ayers LW 2004 Enteric Gram-negative bacilli bloodstream infections: 17 years' experience in a neonatal intensive care unit. Am J Infect Control 32: 189–195
Herting E, Sun B, Jarstrand C, Curstedt T, Robertson B 1997 Surfactant improves lung function and mitigates bacterial growth in immature ventilated rabbits with experimentally induced neonatal group B streptococcal pneumonia. Arch Dis Child Fetal Neonatal Ed 76: 3–8
Herting E, Gefeller O, Land M, van Sonderen L, Harms K, Robertson B 2000 Surfactant treatment of neonates with respiratory failure and group B streptococcal infection. Members of the Collaborative European Multicenter Study Group. Pediatrics 106: 957–964
Evans ME, Feola DJ, Rapp RP 1999 Polymyxin B sulfate and colistin: old antibiotics for emerging multiresistant Gram-negative bacteria. Ann Pharmacother 33: 960–967
Zaltash S, Palmblad M, Curstedt T, Johansson J, Persson B 2000 Pulmonary surfactant protein B: a structural model and a functional analogue. Biochim Biophys Acta 1466: 179–186
Enhorning G 1977 Pulsating bubble technique for evaluating pulmonary surfactant. J Appl Physiol 43: 198–203
Notter RH, Tabak SA, Mavis RD 1980 Surface properties of binary mixtures of some pulmonary surfactant components. J Lipid Res 21: 10–22
Rebello CM, Jobe AH, Eisele JW, Ikegami M 1996 Alveolar and tissue surfactant pool sizes in humans. Am J Respir Crit Care Med 154: 625–628
Schmalisch G, Wauer RR 1993 [Percentile curves of functional residual capacity of newborn infants.]. Monatsschr Kinderheilkd 141: 714–720
Brace RA, Wolf EJ 1989 Normal amniotic fluid volume changes throughout pregnancy. Am J Obstet Gynecol 161: 382–388
Aziz S, Leroy P, Servaes R, Eggermont E, Fevery J 2001 Bilirubin-IXbeta is a marker of meconium, like zinc coproporphyrin. J Pediatr Gastroenterol Nutr 32: 287–292
Calkovska A, Some M, Linderholm B, Johansson J, Curstedt T, Robertson B 2005 Biophysical and physiological properties of porcine surfactant enriched with polymyxin B. Biol Neonate 88: 101–108
Kobayashi T, Shido A, Nitta K, Inui S, Ganzuka M, Robertson B 1990 The critical concentration of surfactant in fetal lung liquid at birth. Respir Physiol 80: 181–192
Taeusch HW 2000 Treatment of acute (adult) respiratory distress syndrome. The holy grail of surfactant therapy. Biol Neonate 77: 2–8
Sun B, Curstedt T, Robertson B 1993 Surfactant inhibition in experimental meconium aspiration. Acta Paediatr 82: 182–189
Tashiro K, Cui XG, Kobayashi T, Curstedt T, Robertson B 2003 Modified protocols for surfactant therapy in experimental meconium aspiration syndrome. Biol Neonate 83: 49–56
William Taeusch H, Lu KW, Goerke J, Clements JA 1999 Nonionic polymers reverse inactivation of surfactant by meconium and other substances. Am J Respir Crit Care Med 159: 1391–1395
Lu KW, Goerke J, Clements JA, Taeusch HW 2005 Hyaluronan decreases surfactant inactivation in vitro. Pediatr Res 57: 237–241
Sun B, Curstedt T, Lindgren G, Franzen B, Alaiya AA, Calkovska A, Robertson B 1997 Biophysical and physiological properties of a modified porcine surfactant enriched with surfactant protein A. Eur Respir J 10: 1967–1974
Schurch S, Qanbar R, Bachofen H, Possmayer F 1995 The surface-associated surfactant reservoir in the alveolar lining. Biol Neonate 67: 61–76
Cajal Y, Ghanta J, Easwaran K, Surolia A, Jain MK 1996 Specificity for the exchange of phospholipids through polymyxin B mediated intermembrane molecular contacts. Biochemistry 35: 5684–5695
Veldhuizen EJ, Batenburg JJ, van Golde LM, Haagsman HP 2000 The role of surfactant proteins in DPPC enrichment of surface films. Biophys J 79: 3164–3171
Rauprich P, Moller O, Walter G, Herting E, Robertson B 2000 Influence of modified natural or synthetic surfactant preparations on growth of bacteria causing infections in the neonatal period. Clin Diagn Lab Immunol 7: 817–822
Bryan SC 1967 Enhancement of bacterial infection by meconium. Johns Hopkins Med J 121: 9–13
Florman AL, Teubner D 1969 Enhancement of bacterial growth in amniotic fluid by meconium. J Pediatr 74: 111–114
Eidelman AI, Nevet A, Rudensky B, Rabinowitz R, Hammerman C, Raveh D, Schimmel MS 2002 The effect of meconium staining of amniotic fluid on the growth of Escherichia coli and group B streptococcus. J Perinatol 22: 467–471
Lembet A, Gaddipati S, Holzman IR, Berkowitz RL, Bottone EJ 2003 Meconium enhances the growth of perinatal bacterial pathogens. Mt Sinai J Med 70: 126–129
Horton J, Pankey GA 1982 Polymyxin B, colistin, and sodium colistimethate. Med Clin North Am 66: 135–142
Marier JF, Brazier JL, Lavigne J, Ducharme MP 2003 Liposomal tobramycin against pulmonary infections of Pseudomonas aeruginosa: a pharmacokinetic and efficacy study following single and multiple intratracheal administrations in rats. J Antimicrob Chemother 52: 247–252
Omri A, Suntres ZE, Shek PN 2002 Enhanced activity of liposomal polymyxin B against Pseudomonas aeruginosa in a rat model of lung infection. Biochem Pharmacol 64: 1407–1413
McAllister SM, Alpar HO, Brown MR 1999 Antimicrobial properties of liposomal polymyxin B. J Antimicrob Chemother 43: 203–210
Morrison DC, Jacobs DM 1976 Binding of polymyxin B to the lipid A portion of bacterial lipopolysaccharides. Immunochemistry 13: 813–818
Augusto L, Le Blay K, Auger G, Blanot D, Chaby R 2001 Interaction of bacterial lipopolysaccharide with mouse surfactant protein C inserted into lipid vesicles. Am J Physiol Lung Cell Mol Physiol 281: 776–785
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the Swedish Research Council (Project No. 3351), King Oscar II's Jubilee Foundation, and the German Research Council (DFG He 2072/2–2).
Rights and permissions
About this article
Cite this article
Stichtenoth, G., Jung, P., Walter, G. et al. Polymyxin B/Pulmonary Surfactant Mixtures Have Increased Resistance to Inactivation by Meconium and Reduce Growth of Gram-Negative Bacteria In Vitro. Pediatr Res 59, 407–411 (2006). https://doi.org/10.1203/01.pdr.0000200806.32822.e6
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/01.pdr.0000200806.32822.e6
This article is cited by
-
Restoration of surfactant activity by polymyxin B in lipopolysaccharide-potentiated injury of immature rabbit lungs
Scientific Reports (2021)
-
Bronchoalveolar lavage with pulmonary surfactant/dextran mixture improves meconium clearance and lung functions in experimental meconium aspiration syndrome
European Journal of Pediatrics (2008)
-
Deleted in Malignant Brain Tumors 1 (DMBT1) is present in hyaline membranes and modulates surface tension of surfactant
Respiratory Research (2007)
-
What’s new in surfactant?
European Journal of Pediatrics (2007)
-
The respiratory distress syndrome (RDS) in preterm infants
Intensivmedizin und Notfallmedizin (2007)