Abstract
Preterm human infants are often treated with volume expansion during their initial stabilization. There are limited data regarding the cerebral vascular effects of this therapeutic approach. The effects of blood volume expansion on cerebral vascular reactivity and oxygen metabolism in very immature animals have not been determined. We examined the effects of volume expansion, with and without hypoxia, on cerebral blood flow and metabolism in unanesthetized, chronically catheterized, preterm fetal sheep. Rapid volume expansion with i.v. dextran increased circulating blood volume. Arterial blood pressure did not increase, nor did cerebral blood flow. However, volume expansion resulted in lower arterial Hb concentration and, consequently, oxygen content without a compensatory increase in cerebral blood flow. Cerebral oxygen delivery fell significantly. Induction of severe hypoxia after volume expansion resulted in an increase in cerebral blood flow, as expected, but the increase in flow was not enough to maintain cerebral oxygen delivery. Rapid volume expansion in normovolemic preterm fetal sheep is associated with decreased cerebral oxygen delivery, and this is further compromised when oxygen content is decreased.
Similar content being viewed by others
Main
Very preterm infants are often treated with boluses of normal saline during their initial stabilization (1–3). The clinical indications are usually low blood pressure and/or poor perfusion, often without other signs of, or risk factors for, hypovolemia. There are limited data regarding the cerebral vascular effects of this therapeutic approach. In piglets, volume expansion after hypotension and asphyxia results in increased CBF (4). In adult dogs, CBF decreases when volume expansion is given with closed-chest cardiopulmonary resuscitation (5). Developmental or species differences in systemic responses, including baroreceptor activation and left ventricular responses, may account for these conflicting results (6).
Hypoxic cerebral vasodilatation occurs in the late-term fetal (7, 8), neonatal (9), and adult sheep (9). In preterm fetal sheep, this response is blunted, and severe hypoxia results in a limitation in cerebral OD when compared with the near-term fetus (10). Cerebral oxygen consumption is maintained, in part, by an increase in fractional oxygen extraction (10). Volume expansion in the face of hypoxemia could further impair cerebral OD by limiting oxygen carrying capacity, particularly in very preterm fetuses.
We hypothesized that acute volume expansion in normovolemic developing animals could result in abnormalities in CBF regulation, possibly associated with changes in cerebral venous pressure. We further hypothesized that these changes in cerebral venous pressure could limit hypoxic cerebral vasodilatation. We chose to study the preterm fetal sheep because Brace et al.(11) had previously demonstrated that i.v. dextran infusion could significantly expand fetal blood volume and that this effect could be maintained for several hours. However, Brace and colleagues did not examine the effects of volume expansion on arterial blood pressure nor CBF in the fetal sheep. Whether volume expansion will augment CBF is not known.
MATERIAL AND METHODS
All experimental protocols were approved by the University of Washington Animal Care Committee. Mixed-breed fetal sheep were obtained from time-dated pregnancies. Full-term gestation is 150 d. We examined responses to volume expansion in eight very preterm fetuses (95 ± 3 d gestation) and responses to volume expansion with severe hypoxia in a separate group of eight additional very preterm fetuses (95 ± 1 d gestation).
Surgical preparation.
For 24 h before fetal surgery, food was withheld from the ewe, but she was allowed free access to water. Fetal surgery was performed under sterile conditions. The ewe was premedicated with atropine (0.2 mg/kg i.m.) and xylaxine (0.1 mg/kg i.m.), then anesthetized with inhaled isoflurane (0.5–3%). The trachea was intubated and the ewe was mechanically ventilated. A 16-gauge venous catheter was placed percutaneously in a maternal jugular vein for fluid administration during surgery. After the abdominal skin was prepared, the uterus was exposed through a midline incision. The fetal head and limbs were exposed one at a time through small uterine incisions for placement of catheters into the superior sagittal sinus, the brachiocephalic trunk (via axillary arteries), and the IVC via pedal veins. A catheter (Tygon tubing) was sewn to a fetal hoof to measure amniotic fluid pressure. The fetal weight was estimated by visual inspection. Vascular catheters were filled with heparinized saline (10 units/mL). All incisions were sutured closed. The catheters were exteriorized to the ewe's flank and secured there in a pouch. The ewe received Bicillin 1,200,000 units i.m. upon completion of surgery. Ampicillin (500 mg) was administered into the amniotic fluid via the amniotic fluid catheter and the estimated volume of lost amniotic fluid was replaced with warmed saline. Fetuses were studied 24–48 h after surgery (12). Maternal analgesia was maintained with buprenorphine (0.005 mg/kg i.m. every 12 h) as needed.
Physiologic measurements.
Fetal arterial blood pressure, heart rate, amniotic fluid pressure, and superior SSP were continuously monitored. Arterial and sagittal sinus pressure were referenced to amniotic pressure. We used SSP as an estimate of ICP. CPP was calculated as the difference between MAP and SSP. Regional fetal brain blood flow was determined using the radiolabeled microsphere technique (13). Approximately 1,000,000 (0.4 mL) microspheres labeled with cobalt 57, chromium 51, ruthenium 103, niobium 95, tin 113, or scandium 46 (PerkinElmer Life Science, Boston, MA, U.S.A.) were injected into the fetal IVC. Reference samples were withdrawn from a brachiocephalic artery (1.5 mL/min) beginning 30 s before the microsphere injection and continuing for 1 min after the injection was completed. After completion of the study protocols, the ewe and fetus were killed with an overdose of pentobarbital followed by saturated potassium chloride solution. Fetal catheter positions were confirmed and the brain was removed for blood flow determination. All supratentorial brain tissue was counted to determine CBF. Radioactivity in tissue and blood samples was determined using a multichannel gamma counter (Minaxi gamma counting system, model 5550, PerkinElmer Life Science). Each sample was corrected for decay time, background counts, and spillover by use of a matrix inversion method (14).
Blood samples for pH, respiratory blood gases, Hb concentration, and oxygen saturation were drawn anaerobically into heparinized Natelson glass pipettes. Respiratory blood gases and pH were measured at ewe core body temperature using a Radiometer ABL 5 (Radiometer, Copenhagen, Denmark). Oxygen saturation and Hb concentration were measured using a Radiometer OSM-3 Hemoximeter (Radiometer).
Fetal blood volume was determined using the technetium 99 method (15). Red blood cells from approximately 1–2 mL fetal blood were labeled with freshly generated pertechnetate using a commercially available kit (UltraTag RBC Kit, Mallinckrodt Medical, St. Louis, MO, U.S.A). Tagged red blood cells were washed with saline, centrifuged, and resuspended in saline. A known quantity of tagged cells was injected into the fetal IVC and into control standards. Precisely measured samples of fetal blood (approximately 1 mL) were withdrawn at given intervals and counted with a multichannel gamma counter to determine blood volume at various times during the experimental protocol.
Severe fetal hypoxemia was induced in protocol 2 by having the ewe breathe lowered inspired oxygen concentration from a plastic bag placed around her head. Carbon dioxide gas was blended into this hypoxic gas mixture to maintain stable fetal Paco2 levels.
Fetal blood drawn during the experiments was replaced with fetal blood obtained via a partial exchange transfusion completed at least 1 h before study. In this procedure, fetal blood (10–12 mL) was withdrawn and replaced with maternal blood. The fetal blood obtained was refrigerated until needed and then placed in a warm water bath until used. This procedure avoided significant changes in fetal Hb-oxygen dissociation during the experimental protocol (10).
Measurements.
Blood samples were obtained from the brachiocephalic artery and superior sagittal sinus for determination of pH, respiratory blood gases, Hb concentration, and oxygen saturation. Additionally, 1 mL was withdrawn from the brachiocephalic artery for blood volume determination. After blood sampling and replacement, radiolabeled microspheres were injected into the fetal IVC while a reference blood sample was withdrawn from the brachiocephalic artery.
Protocol 1: volume expansion.
After two baseline measurements were obtained, 20 mL 6% dextran-70 (B. Braun Medical, Inc., Irvine, CA, U.S.A.) per kg estimated fetal weight (11) was infused into the fetal IVC over 15 min with continuous monitoring of fetal pressure responses. All measurements obtained at baseline were repeated 15 min after completion of volume infusion. A second volume infusion (identical to the first infusion) was then completed. All measurements obtained at baseline were repeated again 15 min after completion of the second volume infusion.
Protocol 2: volume expansion followed by severe hypoxia.
In the second protocol, severe fetal hypoxemia was induced after the initial two volume expansion steps. Severe fetal hypoxemia was defined as a 50% decrease from baseline in fetal arterial oxygen saturation. This is predicted to cause an approximate 50% increase in CBF in preterm fetal sheep (10). Measurements during severe hypoxemia were obtained after at least 20 min of fetal stability and again 20 min later. Blood volume measurements were not obtained in this group of animals because the initial group data (protocol 1) were quite consistent.
Data analysis/calculations.
CBF was calculated as CBF = CPMbrain/CPMref × reference withdrawal rate in milliliters per minute, where CPMbrain and CPMref represent radioactivity cpm in brain and reference samples, respectively. CMRO2 was calculated as CMRO2 = [Cao2 − Cvo2] × CBF, where Cao2 and Cvo2 represent cerebral arterial and venous oxygen content, respectively. Cerebral OD was calculated as OD = [Cao2] × CBF and cerebral oxygen extraction (E) as E = CMRO2/OD. CVR was calculated as CVR = (MAP − SSP)/CBF.
Measurements were calculated and data reported as mean ± SEM for all study fetuses. Differences between groups were analyzed by one-way repeated-measures ANOVA. If the F test was significant, specific differences were sought with the Student-Newman-Keuls test. Significance was considered at p < 0.05.
RESULTS
Protocol 1: effects of volume expansion.
Eight preterm fetuses [age 92–99 d gestation (mean 95 ± 3 d); weight 0.78 ± 0.14 kg; six females; five singletons, three from twin gestations] were studied.
Blood volume.
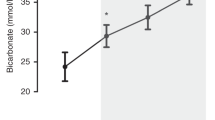
Estimation of fetal weight by visual inspection of the fetuses at the time of catheterization provided quite accurate approximations of actual weight. The actual volume of dextran infused (initially based on estimated fetal weight) was 19.88 ± 3.44 mL/kg. i.v. dextran boluses caused the predicted increase in fetal blood volume as measured by the technetium 99 method. Figure 1 demonstrates the significant increase in blood volume after both the first and second volume boluses. The initial dextran bolus resulted in an increase in measured blood volume exceeding the administered volume significantly, suggesting that dextran brought further fluid into the fetal circulation. After the first bolus, blood volume increased by 38.6 ± 6.2% (p < 0.05 versus predicted). After the second bolus, the blood volume increase was as predicted.
Vascular pressures.
Volume expansion had no effect on MAP (37.5 ± 1.8 versus 40.8 ± 3.0 versus 40.3 ± 2.0; NS; baseline versus bolus 1 versus bolus 2). SSP was not altered by either bolus. CPP did not change significantly from baseline with either bolus (32.0 ± 1.5 versus 34.5 ± 2.0 versus 33.5 ± 2.4; NS).
Hb and oxygen content.
Volume expansion decreased Hb concentration after both boluses (−14.5 ± 6.5% and −18.7 ± 9.8% after bolus 1 and 2, respectively) (Fig. 2, top). Consequently, arterial oxygen content also decreased (−21.7 ± 9.1% and −28.9 ± 12.6 after bolus 1 and 2, respectively) (Fig. 2, bottom).
(Top) Changes in arterial Hb concentration after rapid volume expansion. (Bottom) Changes in arterial oxygen content after rapid volume expansion. VE1, data 15 min after first volume bolus; VE2 data 15 min after second volume bolus. *Value is significantly different from baseline value; + value is significantly different from first volume bolus.
CBF and CVR.
Volume expansion had no effect on CBF or CVR (Fig. 3).
Cerebral OD and metabolism.
Volume expansion decreased cerebral OD in the preterm fetus (−15.1 ± 14.7% and −22.4 ± 17.0% after bolus 1 and 2, respectively) (Table 1). Cerebral fractional oxygen extraction and CMRO2 did not change from baseline after volume expansion (Table 1).
Protocol 2: effects of volume expansion plus severe hypoxemia.
Eight preterm fetuses (age 95 ± 1 d; weight 0.73 ± 0.04 kg; five females; two singletons, six from twin gestations) were studied.
CBF and oxygen content, delivery, and extraction responses to two volume boluses were similar to the responses we observed in Protocol 1. Therefore, responses to hypoxia after volume expansion will be presented in comparison to values after the second volume bolus.
Vascular pressures.
Hypoxia had no further effect on mean arterial blood pressure or CPP after volume expansion.
Hb and oxygen content.
Arterial Hb concentration was not affected further by severe hypoxia after volume expansion. Arterial oxygen content decreased significantly during severe hypoxia as predicted (5.46 ± 0.23 mM after second volume bolus to 3.58 ± 0.33 mM after 20 min hypoxia to 3.54 ± 0.25 mM after 40 min hypoxia, p < 0.05).
CBF and CVR.
Severe hypoxia was associated with a significant increase in CBF (35.4 ± 6.8% and 45.4 ± 14.2% after 20 and 40 min of hypoxia versus CBF after the second volume bolus, respectively) (Fig. 4, top). CVR decreased significantly during hypoxia (−14.3 ± 37.7% and 3.3 ± 10.0% after 20 and 40 min of hypoxia, respectively) (Fig. 4, bottom).
(Top) Changes in CBF after rapid volume expansion followed by severe hypoxia. (Bottom) Changes in CVR after rapid volume expansion followed by severe hypoxia. VEH1, data after 20 min of severe fetal hypoxia; VEH2, data after 35 min of severe fetal hypoxia. *Value is significantly different from VE2.
Cerebral OD and consumption.
Cerebral OD during hypoxia fell significantly in the preterm fetuses (−18.8 ± 3.1% and 13.4 ± 17.4% after 20 and 40 min of hypoxia, respectively) (Table 2). Cerebral oxygen extraction and CMRO2 did not change during severe hypoxia (Table 2).
DISCUSSION
The major findings of this study are as follows: 1) rapid volume expansion in the normoxic normovolemic preterm fetal sheep causes a dilutional anemia without a compensatory increase in CBF and, consequently, a decrease in fetal cerebral OD; and 2) severe fetal hypoxemia induced after volume expansion further compromises cerebral OD.
Little data exist regarding the effect of volume expansion on cerebral perfusion in developing animals. Laptook et al.(4) found that volume expansion in piglets with preceding hypotension and asphyxia resulted in increased CBF. Ditchey et al.(5) in adult dogs, found decreased CBF when closed-chest cardiopulmonary resuscitation was accompanied by volume expansion. Szymonowicz et al.(16) demonstrated that hemorrhagic hypotension in fetal sheep led to decreased CBF. This effect was more pronounced in the preterm versus the late-term fetus, suggesting that the less mature brain may be more vulnerable to ischemic insults. Developmental or species differences in systemic responses, including baroreceptor activation and left ventricular responses, may account for these conflicting results (6).
Our data show that volume expansion during fetal normoxia did not result in an increase in central venous pressure as we had hypothesized. This was likely secondary to the capacitance of the fetal-placental circulation.
The decreases in arterial Hb concentration and oxygen content were expected, as we anticipated that dextran would be retained in the fetal circulation. However, we did not observe any increase in CBF after volume expansion alone. We might have expected such an increase in response to this induction of mild anemic hypoxia. Although such an effect has been noted in adult dogs (17), cats (18), and rats (19), similar studies in very preterm fetal animals have not been completed. Our data suggest that anemic hypoxia of this mild degree does not alter CBF. Further study of this issue is warranted.
In previous studies of acute hypoxia in preterm fetal sheep, there is an approximately 50% increase in CBF when the oxygen content decreases by 50%(10). This increase in CBF is less than that observed in more mature fetuses or newborns and is not adequate to maintain OD. Maintenance of cerebral oxygen consumption in preterm fetuses is supported, in part, by an increase in cerebral oxygen extraction (10). Lack of changes in oxygen consumption and extraction might suggest that, despite the severity of the hypoxemic state, OD to the brain was still sufficient to meet demand. Indeed, when compared with the data from Gleason et al.(10), oxygen content was still at levels above those associated with an increase in oxygen extraction. Therefore, with more severe hypoxemia than that induced in the present study, one would anticipate changes first in extraction followed by a fall in consumption as the oxygen tension fell below a critical value.
The advantage of the chronically catheterized fetal sheep model is the wealth of previously published information regarding the cerebral circulation at various gestational ages. Moreover, the fetal sheep brain undergoes a known maturational pattern that closely approximates the human brain. For example, the fetal sheep brain at approximately 90 d of gestation is similar to the human brain at 26 wk gestation (20). The fetal sheep model allows one to manipulate various parameters without the complexities of a critically ill and unstable preparation. Finally, the fetus is studied without anesthesia, which may alter CBF and metabolism. The disadvantage of this model, particularly for volume expansion studies, is the presence of the high-capacitance placental circulation. Fetal responses to volume expansion may be altered in some way by unique placental vascular or metabolic responses. However, we were successful in establishing and maintaining a predictable expansion of fetal blood volume. Thus, although the results may not be easily extrapolated to neonates, our group comparisons should be valid.
This study demonstrates that volume expansion in the preterm fetal sheep causes no changes in CBF. Although volume expansion did not limit the expected increase in CBF with acute hypoxia, it does result in limitation of cerebral OD.
Abbreviations
- Cao2:
-
arterial oxygen content
- CBF:
-
cerebral blood flow
- CMRO2:
-
cerebral metabolic rate of oxygen or, cerebral oxygen consumption
- CPP:
-
cerebral perfusion pressure
- Cvo2:
-
venous oxygen content
- CVR:
-
cerebral vascular resistance
- ICP:
-
intracranial pressure
- i.m.:
-
intramuscular
- IVC:
-
inferior vena cava
- MAP:
-
mean arterial pressure
- OD:
-
oxygen delivery
- Paco2:
-
arterial CO2
- SSP:
-
sagittal sinus pressure
References
Bada HS, Korones SB, Perry EH, Arheart KL, Ray JD, Pourcyrous M, Magill HL, Runyan W, Somes GW, Clark FC 1990 Mean arterial blood pressure changes in premature infants and those at risk for intraventricular hemorrhage. J Pediatr 117: 607–614
Baumgart S, Costarino AT 2000 Water and electrolyte metabolism of the micropremie. Clin Perinatol 27: 131–146
Miall-Allen VM, de Vries LS, Whitelaw AG 1987 Mean arterial blood pressure and neonatal cerebral lesions. Arch Dis Child 62: 1068–1069
Laptook A, Stonestreet BS, Oh W 1982 The effects of different rates of plasmanate infusions upon brain blood flow after asphyxia and hypotension in newborn piglets. J Pediatr 100: 791–796
Ditchey RV, Lindenfeld J 1984 Potential adverse effects of volume loading on perfusion of vital organs during closed-chest resuscitation. Circulation 69: 181–189
Romero TE, Friedman WF 1979 Limited left ventricular response to volume overload in the neonatal period: a comparative study with the adult animal. Pediatr Res 13: 910–915
Jones MD, Sheldon RE, Peeters LL, Makowski EL, Meschia G 1978 Regulation of cerebral blood flow in the ovine fetus. Am J Physiol 235: H162–H166
Rosenberg AA, Harris AP, Koehler RC, Hudak ML, Traystman RJ, Jones MD 1986 Role of O2-hemoglobin affinity in the regulation of cerebral blood flow in fetal sheep. Am J Physiol 251: H56–H62
Jones MD, Traystman RJ 1984 Cerebral oxygenation of the fetus, newborn, and adult. Semin Perinatol 8: 205–216
Gleason CA, Hamm C, Jones MD 1990 Effect of acute hypoxemia on brain blood flow and oxygen metabolism in immature fetal sheep. Am J Physiol 258: H1064–H1069
Brace RA 1983 Fetal blood volume responses to intravenous saline solution and dextran. Am J Obstet Gynecol 147: 777–781
Harris AP, Koehler RC, Gleason CA, Jones MD, Traystman RJ 1989 Cerebral and peripheral circulatory responses to intracranial hypertension in fetal sheep. Circ Res 64: 991–1000
Heymann MA, Payne BD, Hoffman JI, Rudolph AM 1977 Blood flow measurements with radionuclide-labeled particles. Prog Cardiovasc Dis 20: 55–79
Schosser R, Arfors KE, Messmer K 1979 MIC-II—a program for the determination of cardiac output, arterio-venous shunt and regional blood flow using the radioactive microsphere method. Comput Programs Biomed 9: 19–38
International Committee for Standardization in Haematology 1980 Recommended methods for measurement of red-cell and plasma volume. J Nucl Med 21: 793–800
Szymonowicz W, Walker AM, Yu VYH, Stewart ML, Cannata J, Cussen L 1990 Regional cerebral blood flow after hemorrhagic hypotension in the preterm, near-term, and newborn lamb. Pediatr Res 28: 361–366
Murray JF, Gold P, Johnson BL 1963 The circulatory effects of hematocrit variations in normovolemic and hypervolemia dogs. J Clin Invest 42: 1150–1159
Ulatowski JA, Bucci E, Nishikawa T, Razynska A, Williams MA, Takeshima R, Traystman RJ, Keohler RC 1996 Cerebral O2 transport with hematocrit reduced by cross-linked hemoglobin transfusion. Am J Physiol 270: H466–H475
Todd MM, Weeks JB, Warner DS 1992 Cerebral blood flow, blood volume, and brain tissue hematocrit during isovolemic hemodilution with hetastarch in rats. Am J Physiol 263: H75–H82
Astrom KE 1967 On the early development of the isocortex in fetal sheep. Prog Brain Res 26: 1–59
Acknowledgements
The authors thank Robin Mondares, Richard Tuck, Dana Ness, and Sandra Guidotti for their expert technical assistance. We also thank Dr. David Woodrum for his thoughtful review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by Grant R01-NS34057-01 from the National Institutes of Health–National Institute of Neurological Disorders and Stroke.
Rights and permissions
About this article
Cite this article
Mayock, D., Gleason, C. Cerebrovascular Effects of Rapid Volume Expansion in Preterm Fetal Sheep. Pediatr Res 55, 395–399 (2004). https://doi.org/10.1203/01.PDR.0000111284.29388.E7
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/01.PDR.0000111284.29388.E7