Abstract
Unprecedented demands are now placed on clinicians for early diagnosis as we enter into an era of advancing treatment opportunities for the mucopolysaccharidoses (MPS). Biochemical monitoring of any therapeutic avenue will also be prerequisite. To this end, we aimed to identify a range of urinary oligosaccharides that could be used to identify and characterize patients with MPS. We analyzed 94 urine samples from 68 patients with MPS and 26 control individuals for oligosaccharides derived from glycosaminoglycans using electrospray ionization-tandem mass spectrometry. The oligosaccharide profile for each patient group was compared with that of the control group. The Mann-Whitney U test was used to measure the difference between each patient group and the controls for each analyte. Urine samples from patients before and at successive times after bone marrow transplantation were also evaluated. A number of oligosaccharides were identified in the urine of each MPS subtype, and for each of these, specific oligosaccharide profiles were formulated. These profiles enabled the identification of all 68 patients and their subtypes with the exception of MPS IIIB and IIIC. Selected oligosaccharides were used to assess three individuals after a bone marrow transplant, and, in each case, a substantial reduction in the level of diagnostic oligosaccharides, posttransplantation, was observed. The identification and measurement of glycosaminoglycan-derived oligosaccharides in urine provides a sensitive and specific screen for the early identification of individuals with MPS. The resulting oligosaccharide profiles not only characterize subtype but also provide a disease-specific fingerprint for the biochemical monitoring of current and proposed therapies.
Similar content being viewed by others
Main
The mucopolysaccharidoses (MPS) are a group of inherited lysosomal storage disorders characterized by a deficiency in one of the lysosomal enzymes required to degrade glycosaminoglycans (GAG). There are 11 known enzyme deficiencies that give rise to seven distinct types of MPS, with a combined incidence of ∼1 in 16,000 (1). In all MPS subtypes, partially degraded GAG accumulate in the lysosomes of affected cells and/or are excreted in the urine. The lysosomal storage of GAG leads to the chronic and progressive deterioration of cells, tissues, and organs (2). The MPS share many clinical manifestations, including organomegaly, abnormal facial features, and dysostosis multiplex. Impaired hearing, vision, and joint mobility, as well as abnormal airway and cardiovascular function, are common, although there is wide clinical heterogeneity within each enzyme deficiency. Profound mental retardation is characteristic of the severe forms of MPS I, II, and VII and all subtypes of MPS III. Similar and severe skeletal and joint abnormalities are present in MPS I, II, VI, and VII, whereas MPS IV develops different skeletal pathology.
The clinical management for MPS is changing, as new treatment options, such as enzyme replacement therapy, that will complement and replace bone marrow transplantation undergo trials. Enzyme replacement therapy is currently available for MPS I (3,4), and clinical trials are in progress for MPS II and VI. Bone marrow transplantation has been used successfully for severe MPS I patients for >20 y. However, for therapy to achieve a good long-term outcome, particularly for MPS involving central nervous system (CNS) and bone pathology, treatment before the onset of irreversible pathology will be an important benchmark. In addition, prediction of disease severity will play a pivotal role in deciding on therapeutic alternatives. Enzyme replacement therapy is likely to treat only somatic pathology, whereas bone marrow transplantation, if performed early enough, will have an impact on CNS pathology (5). Similarly, the efficacy gained from enzyme replacement therapy in MPS VI cats has been shown to benefit by commencing therapy at birth (6). Earlier identification and provision of a prognosis for MPS patients, paralleled with the availability of multiple therapy options, should enable the commencement of therapy before the onset of serious and irreversible clinical symptoms. When therapy is commenced before the onset of clinical symptoms and in the absence of comprehensive natural history studies, biochemical markers of disease activity will be mandatory to evaluate the efficacy of therapy and will be useful for the determination of dosage. To address the need for early diagnosis, prognosis, and an ability to monitor therapy for MPS, we aimed to characterize a biochemical picture of disease activity. Using electrospray ionization-tandem mass spectrometry (ESI-MS/MS), we identified a range of sulfated oligosaccharides, derived from the partial degradation of GAG, in urine from MPS patients. For the most part, these oligosaccharides have a nonreducing terminus that consists of the specific substrate for the deficient enzyme in that particular MPS disorder. It is thought that they arise from endolytic cleavage of larger GAG followed by exolytic trimming to the resistant structures observed.
METHODS
Derivatization of urine samples.
Urine samples were submitted to the Women's and Children's Hospital for diagnosis, used with consent, and the hospital ethics committee approved this study. Urine samples (0.5-μmol creatinine equivalents) were lyophilized and then resuspended in 100 μL of 250 mM of 1-phenyl-3-methyl-5-pyrazolone, 400 mM of NH4OH containing 1 nmol of deuterated N-acetylglucosamine-6-sulphate (GlcNAc6S-d3) as described previously (7), and 2 nmol of 4-deoxy-l-threo-hex-4-enopyranosyluronic (1→3) N-acetylgalactosamine-4-sulphate (ΔUA-GalNAc4S) as internal standards. The derivatization was performed at 70°C for 90 min and then acidified with a 2-fold molar excess of HCO2H. Samples were made up to 500 μL with H2O and then extracted with an equal volume of CHCl3 to remove excess 1-phenyl-3-methyl-5-pyrazolone and centrifuged at 13,000 × g for 5 min. Solid-phase extraction cartridges (25 mg of C18) were primed with 1 mL each of CH3OH and then H2O, after which the sample was applied and allowed to enter the solid phase. Samples were desalted with three consecutive 1 mL H2O washes and dried on a Supelco Visiprep24 vacuum manifold, and any remaining 1-phenyl-3-methyl-5-pyrazolone was removed with two CH3Cl washes. The columns were again dried, and derivatized oligosaccharides were eluted in an aqueous solution of 50% (vol/vol) CH3CN/0.025% (vol/vol) HCO2H.
Mass spectrometry.
Mass spectrometry was performed on a PE Sciex API 3000 triple-quadruple mass spectrometer with a turbo-ionspray source (200°C) and Analyst 1.1 data system. Samples (20 μL) were injected into the electrospray source with a Gilson 233 autosampler using a carrying solvent of 50% CH3CN/0.025% HCO2H in H2O at a flow rate of 80 μL/min. N2 was used as the collision gas at a pressure of 2 × 10−5 Torr and for the nebulizing process. Quantification of 1-phenyl-3-methyl-5-pyrazolone-derivatized oligosaccharides was performed using multiple-reaction monitoring in negative ion mode. Each ion pair was monitored for 100 ms with a resolution of 1 amu at half-peak height and averaged from continuous scans over the injection period. Relative oligosaccharide levels were calculated by relating the peak heights of the 1-phenyl-3-methyl-5-pyrazolone-oligosaccharides to the peak height of the internal standard. The GlcNAc6S-d3 was used as the internal standard for the monosaccharides; di- and larger oligosaccharides were related to ΔUA-GalNAc4S.
Statistical analysis.
The Mann-Whitney U test was used to measure the difference between each patient group and the control for each analyte.
RESULTS
A total of 26 oligosaccharides was identified in the urine from a number of MPS and control individuals. They ranged in size from mono- to hexasaccharides consisting of alternating uronic acid (UA) and hexosamine (HN) residues with varying degrees of sulfation and N-acetylation. Proposed structures for these oligosaccharides were suggested on the basis of mass-to-charge ratios and fragmentation patterns from ESI-MS/MS. Of these 26 oligosaccharides, 10 were shown to be required for the diagnosis of an MPS (Table 1). For every MPS subtype, at least one analyte—but in the majority of instances, a number of analytes–is significantly elevated or, in one case, decreased relative to the control population. This enabled complete discrimination of each MPS subtype from unaffected individuals.
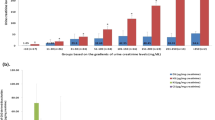
Figure 1A shows the relative levels of the 10 oligosaccharides and their variation in the 26 control samples analyzed. Figure 1B shows a characteristic profile of these 10 oligosaccharides in each of the MPS subtypes. The 15 MPS I patients all could be distinguished by the high levels of a monosulfated di- and trisaccharide [UA-HNAc(S) and UA-HNAc-UA(S)]. These di- and trisaccharides were also elevated in the 13 MPS II patients, but the increase of a hexasaccharide (unknown structure), at proportionately higher levels than the di- and trisaccharide in these patients, enabled discrimination of MPS II from MPS I. MPS IIIA and IIIB patients were easily identified by increased levels of a heparan sulphate–derived disaccharide (HNS-UA) and tetrasaccharide [HNAc-UA-HNAc-UA(S)], respectively. There were four MPS IIIC patients included in this study, but only two of these could be discriminated from the control population using the tetrasaccharide [(HNAc-UA)2(S)]. In the other two patients, all analytes were in the normal range; however, the ratio of the hexasaccharide (unknown structure) to the trisaccharide [UA-HN-UA(S)2] delineated all four patients from the control population. Nevertheless, we were unable to differentiate the MPS IIIB from the IIIC subtypes. MPS IIID, IVA, and VI all have previously been identified using a sulfated N-acetylhexosamine (HNAcS) (7,8). The MPS VI patients could be distinguished from IVA and IIID patients by an elevated ratio of the monosulfated to disulfated monosaccharide (HNAcS2). Only two MPS IIID patients were included in this study, and they could be distinguished from the IVA patients by an elevation in the HNS-UA with a Mann-Whitney U value of 0 (p < 0.05).
Relative levels of GAG-derived oligosaccharides in control and MPS individuals. Urine samples from 26 control samples (A) and 68 MPS individuals representing eight subtypes (B) were analyzed for 10 GAG-derived oligosaccharides. The results were normalized to the control population in which the average value for each analyte was assigned a value of 1. The box plot shows the median level of each analyte in the control group and each MPS subtype (center bar), the 25th and 75th centiles (boxes), and the upper and lower limits (upper and lower bars). The circles and asterisks represent outliers and extreme outliers, respectively.
Figure 2 shows the relative levels of selected markers in MPS I, IVA, and VI patients who had been treated with bone marrow transplantation. All patients showed a substantial reduction in the level of diagnostic oligosaccharides with time after transplantation. The urine samples from the MPS I patient who received a bone marrow transplant were also assayed for total urinary GAG (9). A concomitant decrease of the oligosaccharides measured in MPS I was observed with total urinary GAG levels.
Relative levels of GAG-derived oligosaccharides in urine from MPS-affected individuals before and after bone marrow transplant. Urine samples from MPS I, MPS IVA, and MPS VI patients were collected before transplant and at successive times after transplantation. Each sample was analyzed for GAG-derived oligosaccharides as described, and the results were normalized to the control population, in which each analyte was assigned a value of 1. The MPS I, IVA, and VI patients received bone marrow transplants at 1.2, 1.9, and 12.1 y of age, respectively. Oligosaccharides monitored in each patient were total urinary GAG (♦), HNAcS (⋄), HNAcS2 (□), UA-HNAcS (○), HNAcS-UA (▴), UA-HNAc-UA(S) (▪), (HNAc-UA)2(S) (▵), and hexasaccharide (•).
DISCUSSION
Cell death and/or exocytosis results in the appearance of high molecular weight GAG and GAG-derived oligosaccharides in the urine of MPS patients at increased levels over that seen in unaffected individuals. These oligosaccharides are probably a consequence of endolytic cleavage of the high molecular weight GAG chains. The nature of the oligosaccharides that are elevated in each MPS disorder is primarily a reflection of the block in catabolism as a result of the enzyme deficiency. The sulfated disaccharide [UA-HNAc(S)] and trisaccharide [UA-HNAc-UA(S)] seen in MPS I contain α-l-iduronic acid at the nonreducing end as determined by digestion with α-l-iduronidase (data not shown). The presence of these oligosaccharides is a direct consequence of the deficiency in α-l-iduronidase. In MPS II, oligosaccharides with the same composition are elevated, but they are likely to be structural isomers with the sulphate on the terminal nonreducing α-l-iduronic acid. The marker used to identify MPS IIIA patients was an HNS-UA, whereas the marker specific for MPS IIIB was a monosulfated tetrasaccharide [(HNAc-UA)2(S)], both showing a nonreducing end specific for the deficient enzyme. We were unable to differentiate the MPS IIIB from the IIIC patients, although it is noteworthy that the incidence of MPS IIIB is seven times higher than that for IIIC (1). Further work will be required to identify oligosaccharides specific for these two subtypes. MPS IIID, IVA, and VI store GAG with nonreducing end, β-linked, sulfated N-acetylhexosamine (HNAcS). The elevated level of HNAcS in these patients is due to an alternate degradation pathway in which β-N-acetylhexosaminidase cleaves the nonreducing end sulfated N-acetylhexosamine (8). The disulfated monosaccharide (HNAcS2) was also elevated in MPS VI patients, as the N-acetylgalactosamine-4-sulphatase action precedes N-acetylgalactosamine-6-sulphatase action in the hydrolysis of the HNAcS2 (7,8). No urine samples were available from MPS VII patients, but an unsulfated trisaccharide (UA-HNAc-UA) has been shown to be elevated in amniotic fluids from affected fetuses and as such should provide a diagnostic marker for MPS VII (unpublished observations). In addition, some oligosaccharides may result from secondary storage, in which lysosomal function is impaired to such a degree that oligosaccharides with nonreducing ends not reflective of the enzyme deficiency accumulate. Nonetheless, they seem to be a minority.
In the absence of a family history, diagnosis of MPS can be a long and drawn-out process. Presymptomatic diagnosis for MPS has been proposed through newborn screening programs but has been thwarted by a lack of appropriate technology (10). The use of ESI-MS/MS has enabled the measurement of GAG-derived oligosaccharides from small amounts (∼100 μL) of urine to definitively diagnose each MPS type. This type of urine screen is amenable to high throughput and reduces the need for expensive, time-consuming enzymology. Confirmation of the subtype would be required by enzymology but would involve only one enzyme assay. The only exception would be in the instance of MPS IIIB and IIIC, which cannot be delineated and therefore would require two enzyme assays. Extension of this approach into dried blood spots may have application in newborn screening programs for these disorders as either a primary screen or a confirmation test subsequent to a screen.
The potential applicability of these oligosaccharides to monitor effectiveness of therapy regimens was demonstrated by analyzing urine samples from three patients who had been treated by bone marrow transplantation. Both the MPS IVA and VI patients showed a concomitant decrease in HNAcS with time after transplant, with a similar reduction in HNAcS2 in the MPS VI patient. A number of other primary and possibly secondarily stored oligosaccharides were also shown to decrease after bone marrow transplantation in these patients (Fig. 2), with all analytes approaching normal (a level of 1) within 2–3 y after transplantation. The MPS I patient, with the genotype W402X/Q70X, is predicted to develop a severe phenotype and showed reductions in the primary oligosaccharides [UA-HNAcS and UA-HNAc-UA(S)] over the first 2 y after transplant. Total GAG levels were also measured on the MPS I patient and shown to follow the same trend as the individual oligosaccharides (Fig. 2). Progression of the pathology in this MPS I patient has not continued as predicted from the patient's genotype. These are preliminary results, and follow-up of these and additional patients will be required to fully validate these oligosaccharide markers for the monitoring of therapy for MPS.
The continued development of therapies for MPS raises additional issues. A major hurdle facing clinical trials for enzyme replacement therapy resides in the inability to adequately monitor their efficacy. There is considerable heterogeneity within each MPS subtype even between patients with the same genotype (11), making not only prediction of disease progression arduous but also comparative reference points for clinical trials almost impossible. We observed a correlation in the amount of these oligosaccharides with genotype in a few instances. The levels of most of the oligosaccharides from MPS I patients with genotypes (W402X homozygous and W402X/Q70X) that are known to lead to severe clinical phenotypes were higher than the levels of the same oligosaccharides detected in urine from MPS I patients with mutations (e.g. R89Q) that are known to lead to milder phenotypes (12). Furthermore, a patient whose MPS IIIA was diagnosed in the fourth decade of life, with very mild symptoms (13), was shown to have very low levels of the heparan sulphate–derived disaccharide. Notwithstanding these initial observations, creatinine is derived from muscle metabolism and levels increase with age; therefore, for phenotype predictions using this approach, affected individuals should be compared with age-matched control subjects. We believe that with continued evaluation, GAG-derived oligosaccharides and the metabolic profiles generated by their determination will be instrumental in the clinical management of this diverse and complex group of disorders.
Abbreviations
- ESI-MS/MS:
-
electrospray ionization-tandem mass spectrometry
- GAG:
-
glycosaminoglycans
- HN:
-
hexosamine
- MPS:
-
mucopolysaccharidoses
- UA:
-
uronic acid
References
Meikle PJ, Hopwood JJ, Clague AE, Carey WF 1999 Prevalence of lysosomal storage disorders. JAMA 281: 249–254
Neufeld EF, Muenzer J 2001 The mucopolysaccharidoses. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill, New York, pp 3421–3452
Kakkis ED, Muenzer J, Tiller GE, Waber L, Belmont J, Passage M, Izykowski B, Phillips J, Doroshow R, Walot I, Hoft R, Neufeld EF 2001 Enzyme-replacement therapy in mucopolysaccharidosis I. N Engl J Med 344: 182–188
2002 Laronidase. Biodrugs 16: 316–318
Whitley CB, Belani KG, Chang PN, Summers CG, Blazar BR, Tsai MY, Latchaw RE, Ramsay NK, Kersey JH 1993 Long-term outcome of Hurler syndrome following bone marrow transplantation. Am J Med Genet 46: 209–218
Crawley AC, Niedzielski KH, Isaac EL, Davey RC, Byers S, Hopwood JJ 1997 Enzyme replacement therapy from birth in a feline model of mucopolysaccharidosis type VI. J Clin Invest 99: 651–662
Ramsay SL, Meikle PJ, Hopwood JJ 2003 Determination of monosaccharides and disaccharides in mucopolysaccharidoses patients by electrospray ionisation mass spectrometry. Mol Genet Metab 78: 193–204
Hopwood JJ, Elliott H 1985 Urinary excretion of sulphated N-acetylhexosamines in patients with various mucopolysaccharidoses. Biochem J 229: 579–586
de Jong JGN, Wevers RA 1992 A modified DMB-Tris assay. Clin Chem 38: 803–807
Meikle PJ, Brooks DA, Ravenscroft EM, Yan M, Williams RE, Jaunzems AE, Chataway TK, Karageorgos LE, Davey RC, Boulter CD, Carlsson SR, Hopwood JJ 1997 Diagnosis of lysosomal storage disorders: evaluation of lysosome-associated membrane protein LAMP-1 as a diagnostic marker. Clin Chem 43: 1325–1335
Scott HS, Nelson PV, Litjens T, Hopwood JJ, Morris CP 1993 Multiple polymorphisms within the α-L-iduronidase gene (IDUA): implications for a role in the modification of MPS-I disease phenotype. Hum Mol Genet 2: 1471–1473
Scott HS, Bunge S, Gal A, Clarke LA, Morris CP, Hopwood JJ 1995 Molecular genetics of mucopolysaccharidosis type I: diagnostic, clinical and biological implications. Hum Mutat 6: 288–302
Van Hove JL, Wevers RA, Van Cleemput J, Moerman P, Sciot R, Matthijs G, Schollen E, de Jong JG, Carey WF, Muller V, Nicholls C, Perkins K, Hopwood JJ 2003 Late-onset visceral presentation with cardiomyopathy and without neurological symptoms of adult Sanfilippo A syndrome. Am J Med Genet 118A: 382–387
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by the National Health and Medical Research Council of Australia, The Wellcome Trust (UK) grant reference number 060104Z/00/Z and TLH Research.
Rights and permissions
About this article
Cite this article
Fuller, M., Rozaklis, T., Ramsay, S. et al. Disease-Specific Markers for the Mucopolysaccharidoses. Pediatr Res 56, 733–738 (2004). https://doi.org/10.1203/01.PDR.0000141987.69757.DD
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/01.PDR.0000141987.69757.DD
This article is cited by
-
Fast, sensitive method for trisaccharide biomarker detection in mucopolysaccharidosis type 1
Scientific Reports (2018)
-
Contribution of tandem mass spectrometry to the diagnosis of lysosomal storage disorders
Journal of Inherited Metabolic Disease (2018)
-
Lysosomal diseases: diagnostic update
Journal of Inherited Metabolic Disease (2014)
-
Plasmatic kinetics of dermatan sulfate during enzyme replacement therapy with iduronate-2-sulfatase in a mucopolysaccharidosis II Patient
Glycoconjugate Journal (2013)
-
Liver Production of Sulfamidase Reverses Peripheral and Ameliorates CNS Pathology in Mucopolysaccharidosis IIIA Mice
Molecular Therapy (2012)