Abstract
In the present study, we compared brain development and metabolism of small-for-gestational-age (SGA) and appropriate-for-gestational-age (AGA) infants using proton magnetic resonance spectroscopy (1H-MRS). We tested the hypothesis that intrauterine growth retardation caused by placental insufficiency is associated with changes in cerebral metabolism and is followed by an adverse neurodevelopmental outcome at the age of 2 y. Twenty-six AGA and 14 SGA (birth weight <P 2.3) preterm infants with no major ultrasound abnormalities were enrolled prospectively. At 32 and 41 wk postmenstrual age, 1H-MRS and magnetic resonance imaging were performed. For 1H-MRS, a volume of interest was placed in the basal ganglia and in the periventricular white matter. Using echo times of 31 and 144 ms N-acetylaspartate/choline (NAA/Cho), lactate/Cho, myo-inositol/Cho (mI/Cho), and glutamate-glutamine-γ-aminobutyric acid/Cho (Glx/Cho) ratios were compared between AGA and SGA groups. Griffiths' developmental quotient (DQ) values were assessed at 24 mo corrected age. Griffiths' DQ (AGA, 104 ± 10; SGA, 99 ± 9) and brain development assessed using magnetic resonance imaging showed no significant differences between both AGA and SGA groups, and NAA/Cho, Lac/Cho, mI/ Cho, and Glx/Cho ratios were not significantly different between the groups. NAA/Cho ratios increased from 32 to 41 wk, whereas mI/Cho ratios decreased in both groups. No differences in cerebral metabolism, brain development, and DQ values between AGA and severely SGA infants could be demonstrated.
Similar content being viewed by others
Main
Preterm infants who are small for gestational age (SGA) as a result of placental insufficiency are at increased risk for adverse neurodevelopmental outcome in comparison with age-matched appropriate-for-gestational-age (AGA) neonates (1–4). Placental insufficiency can be demonstrated in these pregnancies using Doppler ultrasonography of the umbilical artery (5). Most of these pregnancies are terminated by cesarean section because of severe fetal compromise with impending hypoxia-ischemia (6).
During the past decade, it was demonstrated that perinatal hypoxia-ischemia may have long-lasting effects on cerebral metabolism as has been demonstrated using in vivo cerebral proton magnetic resonance spectroscopy (1H-MRS) (7,8). A decrease of the N-acetylaspartate/choline (NAA/Cho) ratio in asphyxiated full-term neonates predicts an adverse neurodevelopmental outcome (7,9–11). Elevated levels of lactate (Lac) in the brain of asphyxiated neonates, even months after the hypoxic-ischemic insult, have been shown to be predictive of neurodevelopmental delay (7,8,10,12). Myo-inositol (mI), which is one of the osmoregulators of the brain, can be found in astrocytes and is considered a glial cell marker (13). It increases in the early phase after hypoxia-ischemia and decreases during the perinatal period (14,15). Related metabolites such as glutamate, glutamine, and γ-aminobutyric acid are collectively known as Glx, the peak patterns of which are difficult to separate at 1.5 T. Glutamate, which is the main constituent of the Glx peak, is an excitatory amino acid, and its concentration increases after hypoxia-ischemia (16,17). Moreover, glutamate may play a role in brain development, by influencing neurite sprouting, synaptogenesis, and dendrite pruning (18).
The aim of the present study was to compare brain metabolism and imaging in a group of SGA and AGA preterm infants at 32 wk postmenstrual age and at term. We hypothesized that SGA and AGA infants have different 1H-MRS findings at both time points and that abnormal metabolite ratios in SGA pre-term infants precede an abnormal neurodevelopmental outcome at the age of 2 y.
METHODS
Patients.
All neonates in this study were born between March 1, 1999, and December 31, 2001, and were admitted to the neonatal unit of the Wilhelmina Children's Hospital/ University Medical Center Utrecht because of a gestational age of <32 wk and/or a birth weight of <1500 g. The study was approved by the Medical Ethical Committee of the Wilhelmina Children's Hospital, and informed parental consent was obtained in all cases. Inclusion criteria were 1) absence of fetal infections, congenital malformations, and metabolic and chromosomal disorders; 2) intraventricular hemorrhage grade ≤IIa according to the criteria of Levene et al. (19); 3) periventricular leukomalacia ≤grade I according to the criteria of de Vries et al. (20); and 4) stable clinical condition.
Twenty-six AGA and 14 SGA infants were eligible for this study. SGA was defined as a birth weight <p 2.3 according to Dutch national birth weight centiles (21). Placental insufficiency was diagnosed by abnormal Doppler flow patterns of the umbilical artery as described previously (5,6). Patients were examined at a postmenstrual age of 32 wk [early magnetic resonance imaging (MRI)] and/or 41 wk (late MRI). Of the 26 AGA neonates examined at 32 wk, 24 also had a second scan at term, and MRS data could be obtained in 21 of them. Of the 14 SGA newborns included, 7 had both an early and a late MRI, 2 had only an early MRI, and 5 had only a late MRI examination. In 2 AGA infants, parental permission was not obtained for the second MRI. Of the SGA infants, one infant was not examined at term because of episodes of hypoxia-ischemia at 39 wk. Another infant was not examined because of referral to another hospital at the time of the second MRI. Of the five SGA neonates who had a scan at term only, two were born at 33 and 34 wk, respectively, and were included for the examination at term because of their extreme growth retardation, and three others were studied at term only because they were not clinically stable at the time of the early MRI, i.e. at 32 wk gestational age. Patient characteristics are listed in Table 1.
Neurodevelopmental follow-up was performed at regular intervals up to the age of 24 mo as reported previously (7). Assessment of outcome was made using items from Amiel-Tison and from Grenier and Touwen and the Griffiths' mental developmental scale. The developmental quotient (DQ) of the Griffiths' test at 24 mo was calculated (22).
The MRI examinations were performed in unsedated neonates, using vacuum pillows (Med-Tec, Orange City, IA, U.S.A.) to avoid movements of the patient's head. Patients with a weight of <1500 g at the time of the MRI studies were examined using an MRI-compatible incubator (Dräger, Lübeck, Germany) (23). Heart rate and transcutaneous oxygen saturation were monitored using pulse oxymetry (Nonin, Minneapolis, MN, U.S.A.), and respiratory rate was monitored using an abdominal transducer (Philips Gyroscan ACS-NT, Best, the Netherlands).
MRI.
Standard MRI was performed for localization of the volume of interest (VOI) for 1H-MRS, using a 1.5 Tesla Philips ACS-NT system. MRI included sagittal T1 [repetition time(TR)/echo time (TE) 512/15 ms], axial turbo spin-echo T2 (TR/TE 5912/90 ms), and inversion recovery [IR; TR/ inversion time (TI)/TE 3500/950/32 ms for preterm infants or 4021/600/30 ms for term infants]; the slice thickness was 4 mm with a gap of 0.4 mm (T1- and T2-weighted images) or 0.8 mm (IR images). The MRIs were observed as described by Battin and Rutherford (24) for white matter abnormalities, cortical folding, and germinal matrix at both time points and in addition for signs of abnormal myelination of the internal capsule at term age.
1H-MRS.
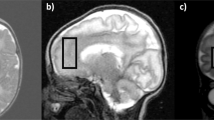
For the 1H-MRS examination, a VOI of ∼4 cm3 was placed in the basal ganglia (BG) of both hemispheres for the early examination. Both BG were included to optimize signal to noise. At term, the VOI was placed in the left BG as in our previous studies (11). A second VOI included the left periventricular white matter (PVWM;Fig. 1). Contact with the lateral ventricle and the cortex was avoided, including as much PVWM as possible to optimize signal to noise. A PRESS sequence with a TR of 2000 ms was used. The TEs were 31 and 144 ms. For the BG, 64 measurements were averaged, and for the PVWM, 128 measurements at the early examination and 64 measurements at the late examination were averaged. Total 1H-MRS examination time was ∼20 min for the early and 15 min for the late measurement.
NAA, creatinine, Cho, and Lac peaks were identified in the spectra obtained with a TE of 144 ms at, respectively, 2.02, 3.02, 3.24, and 1.33 ppm, whereas Glx and mI were identified at 2.10–2.25 and 3.56 ppm, respectively, using a TE of 31 ms. Lac was identified as an inverted doublet with a TE of 144 ms. Curve fitting was performed using MRUI software, including VARPRO/AMARES (EC Human Capital & Mobility/ Networks program) (25). Previous knowledge included the position of the inverted doublet of Lac at 1.33 ppm, with an interpeak distance of 7 Hz. Line width was set at a maximum of 7 Hz. Previous knowledge of the Glx peak was a line width of 7 Hz. No further previous knowledge was entered. NAA/ Cho, Lac/Cho (TE 144 ms) and Glx/Cho, and mI/Cho (TE 31 ms) were calculated. In case of coupled resonances (Lac), the combined area under both peaks was used for calculations. Children were examined as close as possible to 32 and 41 wk of postmenstrual age.
STATISTICAL ANALYSIS
Statistical analysis was performed using SPSS software, version 9.0 (SPSS, Chicago, IL, U.S.A.). Mann-Whitney U tests or t tests were used to compare the ratios between SGA and AGA groups at 32 and 41 wk, depending on the distribution of the data. Mann-Whitney U tests were performed to compare the ratios within each group at the two time points. P < 0.05 was considered significant. Power analysis demonstrated that with an expected 15% difference between the AGA and SGA groups in NAA/Cho or Lac/Cho ratios and an expected variation coefficient of 10%, a group size of 11 would be sufficient to demonstrate differences with P = 0.05 and a power of 0.90.
RESULTS
There were no significant differences between the AGA and SGA infants in clinical variables (Table 1), except for birth weight and a postmenstrual age difference of 5 d at the time of the first examination. Eleven SGA infants had a head circumference <2.3rd centile, two between the 2.3rd and 10th centiles, and one at the 25th centile. All infants were normoglycemic during examinations, and blood gas values, including base deficit, were within the normal range.
MRI.
MRI was in concordance with cranial ultrasound findings and did not show major brain pathology. No differences in cerebral development (cortical folding, germinal matrix, white matter, and myelination) were observed between the SGA and AGA groups at both time points.
1H-MRS.
An example of spectra of the BG using TEs of both 31 and 144 ms at 32 and 41 wk is presented in Fig. 2. The NAA/Cho, Lac/Cho, mI/Cho, and Glx/Cho ratios (mean ± SD) of the BG and PVWM at 32 and 41 wk are presented in Fig. 3. No significant differences were found between the AGA and SGA groups at both time points. Effects of age were demonstrated on NAA/Cho and mI/Cho ratios: NAA/Cho increased between 32 and 41 wk, whereas mI/Cho decreased. Changes in Lac/Cho and Glx/Cho were not significant. Three of the SGA infants showed an increase in Lac/Cho, compared with none of the AGA infants. No differences were demonstrated between SGA infants with a head circumference above or below the 2.3rd centile (data not shown).
Neurodevelopmental outcome.
No significant differences in DQ between the AGA (104 ± 10) and SGA (99 ± 9) infants were found at the age of 2 y. One infant in each group had a DQ below 85.
DISCUSSION
The present study compared brain metabolism of the BG and PVWM in 26 preterm AGA infants with 14 preterm SGA infants, studied at 32 and 41 wk postmenstrual age using 1H-MRS. In contrast with previous observations that SGA infants, as a result of placental insufficiency, carry a high risk of an abnormal neuromotor development (1–4), Griffiths' DQ values in the present study at 24 mo (corrected age) did not differ between the SGA and AGA infants. Although differences in neurodevelopment might be demonstrated at a later age, the present SGA group performed just as well as the AGA group at the corrected age of 2 y. It could be that subtle changes in medical care over the last 5 y may have improved outcome of these extremely SGA neonates, which explains the difference with the aforementioned older studies (1,2). Furthermore, the neonates selected for the study had no abnormalities in the PVWM on MRI and had a gestational age of at least 25 wk and therefore may be considered a selected group of patients. Cerebral development assessed with MRI according to Battin and Rutherford (24) showed no differences between the two groups.
1H-MRS findings showed that NAA/Cho ratios were not significantly different between SGA and AGA infants. The NAA signal reflects tissue concentrations of both NAA and N-acetylaspartylglutamate (NAAG). NAA has been reported to reflect the presence of neurons, oligodendroglial lineage cells, and axons in the CNS (26–28). It has been suggested that NAA(G) may be an acetyl-group carrier between mitochondria and cytoplasm in neuronal cells (29,30). A decrease of the NAA signal is usually interpreted as a reduction in the number of neurons, but it may also reflect altered function of neuronal mitochondria. The finding that no differences in NAA/Cho could be demonstrated between SGA and AGA infants seems to indicate that no differences in neuronal density or function are present between the two groups, despite fetal compromise in SGA infants. The increase of NAA/Cho ratios in cerebral tissue as a result of maturation was previously described in detail and is confirmed in the present study (14,31–34).
mI is a crucial constituent of living cells and participates in several physiologic functions. It is a major osmolyte and also serves as the precursor to phosphatidylinositol. mI has been used as a glial cell marker (13). The decrease in mI/Cho ratios between 32 and 41 wk found in the present study was described before by Kreis et al. (14), who suggested that the concentration of mI was associated with postnatal life rather than with gestational age. Because we did not find an effect of postnatal age on mI/Cho (data not shown), our study does not support this suggestion. The decline of mI/Cho between 32 and 41 wk might be due to the decrease in water content of the human brain during this time period with accompanying changes in osmoregulation of the brain (13). No significant differences in mI/Cho ratios were found between the groups, indicating that there was no significant difference in cerebral osmoregulation.
Glx plays a major role in the cascade resulting in cellular brain injury after hypoxia-ischemia, and concentrations may be elevated if measured within the first week of postnatal life after perinatal asphyxia (17,35). Glx/Cho ratios were not elevated in the SGA infants in the present study. It is possible that Glx normalized in the 2.5 wk between birth and the first MRI examination, a period during which the infants were normoxic.
Of the 7 SGA infants who were tested longitudinally, 3 had an increase in Lac/Cho ratios between 32 and 41 wk, compared with none of the 21 AGA infants tested longitudinally. It should be realized that the relatively low Lac resonance may be a potential source of inaccurate measurements of Lac/Cho ratios. Further studies are necessary to confirm these findings. The role of Lac as a source of high-energy substrate for the preterm brain has been suggested before. Leth et al. (36) described the presence of Lac in 10 of 10 preterm SGA neonates and in 10 of 13 preterm AGA infants, whereas it was not detected in 7 term infants. They suggested that Lac might be produced locally or in peripheral tissues and that it may play a role in brain metabolism. Cady et al. (37) found a decreasing Lac concentration with increasing gestational age in the thalamus and occipital-parietal region, indicating a changing glycolytic activity in the immature brain of AGA infants. Lac may be used as fuel for the brain but also for the synthesis of myelin (38). No differences in the degree of myelination of the internal capsule between AGA and SGA groups were found in the present study, supporting the similarity of the AGA and SGA groups. Because the present study focused only on differences between the AGA and SGA groups, detailed discussion of differences in 1H-MRS between the BG and PVWM, previously also reported by others (32), are beyond the scope of this article.
There are a few limitations of this study. First, the group of SGA infants is small. On the basis of the findings of the present study, a new power analysis reveals that group sizes of at least 110 would be necessary to demonstrate differences in NAA/ Cho. Second, metabolite ratios were calculated, not absolute concentrations. Because measurements of absolute concentrations are often based on assumptions of the concentration of water in the brain (32), we decided to study ratios of metabolites in these neonates, who show a very rapid brain development and possibly a rapidly changing water content of the brain.
From the present study, we conclude that NAA/Cho, Lac/ Cho, mI/Cho, and Glx/Cho ratios were not significantly different in SGA infants compared with AGA infants at 32 and 41 wk postmenstrual age. Effects of postmenstrual age on NAA/ Cho (increase between 32 and 41 wk) and mI/Cho ratios (decrease) could be demonstrated. Griffiths' DQ at 24 mo and cerebral maturation as assessed with MRI were not different between the SGA and AGA infants selected for the present study.
Abbreviations
- AGA:
-
appropriate for gestational age
- BG:
-
basal ganglia
- Cho:
-
choline
- DQ:
-
developmental quotient
- Glx:
-
glutamate/glutamine/γ-aminobutyric acid
- 1H-MRS:
-
proton magnetic resonance spectroscopy
- Lac:
-
lactate
- mI:
-
myo-inositol
- MRI:
-
magnetic resonance imaging
- NAA:
-
N-acetylaspartate
- NAAG:
-
N-acetylaspartylglutamate
- PVWM:
-
periventricular white matter
- SGA:
-
small for gestational age
- TE:
-
echo time
- TI:
-
inversion time
- TR:
-
repetition time
- VOI:
-
volume of interest
References
Hutton JL, Pharoah PO, Cooke RW, Stevenson RC 1997 Differential effects of preterm birth and small gestational age on cognitive and motor development. Arch Dis Child Fetal Neonatal Ed 76: F75–F81.
Kok JH, den Ouden AL, Verloove-Vanhorick SP, Brand R 1998 Outcome of very preterm small for gestational age infants: the first nine years of life. Br J Obstet Gynaecol 105: 162–168.
Topp M, Langhoff-Roos J, Uldall P, Kristensen J 1996 Intrauterine growth and gestational age in preterm infants with cerebral palsy. Early Hum Dev 44: 27–36.
Wallace IF, McCarton CM 1997 Neurodevelopmental outcomes of the premature, small-for-gestational-age infant through age 6. Clin Obstet Gynecol 40: 843–852.
Reuwer PJ, Sijmons EA, Rietman GW, van Tiel MW, Bruinse HW 1987 Intrauterine growth retardation: prediction of perinatal distress by Doppler ultrasound. Lancet 2: 415–418.
GRIT Study Group, 2003 A randomised trial of timed delivery for the compromised preterm fetus: short term outcomes and Bayesian interpretation. BJOG 110: 27–32.
Groenendaal F, Veenhoven RH, van der Grond J, Jansen GH, Witkamp TD, de Vries LS 1994 Cerebral lactate and N-acetyl-aspartate/choline ratios in asphyxiated full-term neonates demonstrated in vivo using proton magnetic resonance spectroscopy. Pediatr Res 35: 148–151.
Robertson NJ, Cox IJ, Cowan FM, Counsell SJ, Azzopardi D, Edwards AD 1999 Cerebral intracellular lactic alkalosis persisting months after neonatal encephalopathy measured by magnetic resonance spectroscopy. Pediatr Res 46: 287–296.
Peden CJ, Rutherford MA, Sargentoni J, Cox IJ, Bryant DJ, Dubowitz LM 1993 Proton spectroscopy of the neonatal brain following hypoxic-ischemic injury. Dev Med Child Neurol 35: 502–510.
Barkovich AJ, Baranski K, Vigneron D, Partridge JC, Hallam DK, Hajnal BL, Ferriero DM 1999 Proton MR spectroscopy for the evaluation of brain injury in asphyxiated, term neonates. AJNR Am J Neuroradiol 20: 1399–1405.
Roelants-van Rijn AM, van der Grond J, de Vries LS, Groenendaal F 2001 Value of 1H-MRS using different echo times in neonates with cerebral hypoxia-ischemia. Pediatr Res 49: 356–362.
Amess PN, Penrice J, Wylezinska M, Lorek A, Townsend J, Wyatt JS, Amiel-Tison C, Cady EB, Stewart A 1999 Early brain proton magnetic resonance spectroscopy and neonatal neurology related to neurodevelopmental outcome at 1 year in term infants after presumed hypoxic-ischaemic brain injury. Dev Med Child Neurol 41: 436–445.
Isaacks RE, Bender AS, Kim CY, Prieto NM, Norenberg MD 1994 Osmotic regulation of myo-inositol uptake in primary astrocyte cultures. Neurochem Res 19: 331–338.
Kreis R, Ernst T, Ross BD 1993 Development of the human brain: in vivo quantification of metabolite and water content with proton magnetic resonance spectroscopy. Magn Reson Med 30: 424–437.
Robertson NJ, Lewis RH, Cowan FM, Allsop JM, Counsell SJ, Edwards AD, Cox IJ 2001 Early increases in brain myo-inositol measured by proton magnetic resonance spectroscopy in term infants with neonatal encephalopathy. Pediatr Res 50: 692–700.
Hagberg H, Thornberg E, Blennow M, Kjellmer I, Lagercrantz H, Thiringer K, Hamberger A, Sandberg M 1993 Excitatory amino acids in the cerebrospinal fluid of asphyxiated infants: relationship to hypoxic-ischemic encephalopathy. Acta Paediatr 82: 925–929.
Groenendaal F, Roelants-van Rijn AM, van der Grond J, Toet MC, de Vries LS 2001 Glutamate in cerebral tissue of asphyxiated neonates during the first week of life demonstrated in vivo using proton magnetic resonance spectroscopy. Biol Neonate 79: 254–257.
Nguyen L, Rigo JM, Rocher V, Belachew S, Malgrange B, Rogister B, Leprince P, Moonen G 2001 Neurotransmitters as early signals for central nervous system development. Cell Tissue Res 305: 187–202.
Levene MI, de Crespigny LC 1983 Classification of intraventricular hemorrhage. Lancet 1: 643
de Vries LS, Eken P, Dubowitz LM 1992 The spectrum of leukomalacia using cranial ultrasound. Behav Brain Res 49: 1–6.
Kloosterman GJ 1969 Intrauterine growth and intrauterine growth curves. Maandschrift voor Kindergeneeskunde 37: 209–225.
Griffiths R 1976 The Abilities of Babies: A Study in Mental Measurement. Amersham, Bucks
Groenendaal F, Leusink C, Nijenhuis M, Janssen MJ 2002 Neonatal life support during magnetic resonance imaging. J Med Eng Technol 26: 71–74.
Battin M, Rutherford MA 2002 Magnetic resonance imaging of the brain in preterm infants: 24 weeks' gestation to term. In: Rutherford MA (ed) MRI of the Neonatal Brain. Saunders, London, pp 25–49.
Naressi A, Couturier C, Castang I, de Beer R, Graveron-Demilly D 2001 Java-based graphical user interface for MRUI, a software package for quantitation of in vivo/ medical magnetic resonance spectroscopy signals. Comput Biol Med 31: 269–286.
Urenjak J, Williams SR, Gadian DG, Noble M 1993 Proton nuclear magnetic resonance spectroscopy unambiguously identifies different neural cell types. J Neurosci 13: 981–989.
Martin E, Capone A, Schneider J, Hennig J, Thiel T 2001 Absence of N-acetylaspartate in the human brain: impact on neurospectroscopy?. Ann Neurol 49: 518–521.
Bjartmar C, Battistuta J, Terada N, Dupree E, Trapp BD 2002 N-acetylaspartate is an axon-specific marker of mature white matter in vivo: a biochemical and immunohistochemical study on the rat optic nerve. Ann Neurol 51: 51–58.
Patel TB, Clark JB 1979 Synthesis of N-acetyl-L-aspartate by rat brain mitochondria and its involvement in mitochondrial/cytosolic carbon transport. Biochem J 184: 539–546.
Truckenmiller ME, Namboodiri MA, Brownstein MJ, Neale JH 1985 N-Acetylation of L-aspartate in the nervous system: differential distribution of a specific enzyme. J Neurochem 45: 1658–1662.
van der Knaap MS, van der Grond J, van Rijen PC, Faber JA, Valk J, Willemse K 1990 Age-dependent changes in localized proton and phosphorus MR spectroscopy of the brain. Radiology 176: 509–515.
Kreis R, Hofmann L, Kuhlmann B, Boesch C, Bossi E, Huppi PS 2002 Brain metabolite composition during early human brain development as measured by quantitative in vivo 1H magnetic resonance spectroscopy. Magn Reson Med 48: 949–958.
Kimura H, Fujii Y, Itoh S, Matsuda T, Iwasaki T, Maeda M, Konishi Y, Ishii Y 1995 Metabolic alterations in the neonate and infant brain during development: evaluation with proton MR spectroscopy. Radiology 194: 483–489.
Hüppi PS, Fusch C, Boesch C, Burri R, Bossi E, Amato M, Herschkowitz N 1995 Regional metabolic assessment of human brain during development by proton magnetic resonance spectroscopy in vivo and by high-performance liquid chromatography/gas chromatography in autopsy tissue. Pediatr Res 37: 145–150.
Pu Y, Li QF, Zeng CM, Gao J, Qi J, Luo DX, Mahankali S, Fox PT, Gao JH 2000 Increased detectibility of alpha brain glutamate/glutamine in neonatal hypoxic-ischemic encephalopathy. AJNR Am J Neuroradiol 21: 203–212.
Leth H, Toft PB, Pryds O, Peitersen B, Lou HC, Henriksen O 1995 Brain lactate in preterm and growth-retarded neonates. Acta Paediatr 84: 495–499.
Cady EB, Penrice J, Amess PN, Lorek A, Wylezinska M, Aldridge RF, Franconi F, Wyatt JS, Reynolds EO 1996 Lactate, N-acetylaspartate, choline and creatine concentrations, and spin-spin relaxation in thalamic and occipito-parietal regions of developing human brain. Magn Reson Med 36: 878–886.
Sanchez-Abarca LI, Tabernero A, Medina JM 2001 Oligodendrocytes use lactate as a source of energy and as a precursor of lipids. Glia 36: 321–329.
Acknowledgements
We thank the technicians Cheraar Leusink and Marco Nijenhuis for testing the equipment and all other technicians of the MRI institute for their enthusiastic cooperation. The MRUI software package was kindly provided by the participants of the EU Network programs: Human Capital and Mobility (CHRX-CT94-0432) and Training and Mobility of Researchers (ERB-FMRX-CT970160). We thank our colleagues Corine Koopman-Esseboom and Inge-Lot van Haastert for help with the follow-up examinations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roelants-van Rijn, A., van der Grond, J., Stigter, R. et al. Cerebral Structure and Metabolism and Long-Term Outcome in Small-for-Gestational-Age Preterm Neonates. Pediatr Res 56, 285–290 (2004). https://doi.org/10.1203/01.PDR.0000132751.09067.3F
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/01.PDR.0000132751.09067.3F
This article is cited by
-
Brain proton magnetic resonance spectroscopy and neurodevelopment after preterm birth: a systematic review
Pediatric Research (2022)
-
The long-term effect of erythropoiesis stimulating agents given to preterm infants: a proton magnetic resonance spectroscopy study on neurometabolites in early childhood
Pediatric Radiology (2018)
-
Magnetic resonance spectroscopy in very preterm-born children at 4 years of age: developmental course from birth and outcomes
Neuroradiology (2018)
-
Brain metabolite concentrations are associated with illness severity scores and white matter abnormalities in very preterm infants
Pediatric Research (2013)
-
Neuroimaging biomarkers of preterm brain injury: toward developing the preterm connectome
Pediatric Radiology (2012)