Abstract
Among preterm infants there is a relationship between skin blood flow and transepidermal water loss (TEWL). The aim of this study was to assess whether halogen spotlight phototherapy without significant heat stress increases TEWL and affects maintenance fluid requirements in preterm infants. TEWL was measured noninvasively before the start and after 1 h of halogen spotlight phototherapy in a group of preterm infants, nursed in double-walled incubators with moderately high relative humidity. Relative humidity and ambient temperature in the incubator were tightly controlled. Mean ± SD birth weight of the 18 infants was 1412 ± 256 g, gestational age 30.6 ± 1.6 wk, and age at measurement 5 ± 3 d. Nine infants received ventilatory assistance. Relative humidity was 40–80% (mean 52%). Average TEWL increased from 13.6 to 16.5 g/m2/h during phototherapy. These data show that TEWL increases by approximately 20% during phototherapy despite constant skin temperature and relative humidity. Maintenance fluids of preterm infants should be increased by 0.35 mL/kg/h during exposure to halogen spotlight phototherapy.
Similar content being viewed by others
Main
Phototherapy is a standard therapy for hyperbilirubinemia in the newborn. Although it has proven to be an effective and relatively safe treatment (1), uncertainty still exists about whether phototherapy influences the fluid balance of the infant. Previously, studies on the effect of phototherapy upon insensible water loss reported a range of extra water loss between 0 and well over 100%(2–4). All of these studies were using bank lights.
The present study is designed to assess the amount of extra water loss through evaporation during phototherapy, given by the currently used halogen spotlights instead of the older bank lights. Various studies have demonstrated that the radiative energy of the phototherapy lamp increases skin blood flow (5–8). We therefore expect that the heat balance of a newborn infant during phototherapy is affected by heat loss through evaporation. Extra fluid intake will then be necessary. We measured TEWL in 18 preterm infants receiving halogen spotlight phototherapy to examine this hypothesis.
METHODS
Subjects.
All subjects were admitted to the neonatal intensive care unit of the Leiden University Medical Center/the Juliana Children's Hospital for treatment of prematurity and developed nonhemolytic hyperbilirubinemia during their nursery course for which they received phototherapy. Infants selected for this study were born after a gestational age of <34 wk, appropriate for gestational age, and nursed in a double-walled incubator (type 8000, Dräger, Lübeck, Germany) in which the RH was at least 40% at the time of the measurements. The infants were nursed naked on a white surface. Exclusion criteria included metabolic disorders and serious skin lesions. The Medical Ethics Committees of the hospitals approved the study and informed consent was obtained from the parents of all infants.
TEWL measurements were done on the skin of 18 preterm infants. The infants were born after a (mean ± SD) gestational age of 30.6 ± 1.6 wk (range 27–34 wk) and had a weight at birth of 1412 ± 256 g (range 966–1880 g). The Apgar score at 5 min had a median of 8 (range 3–9). At the time of the measurements, the infants had a postnatal age of 5 ± 3 d (range 1–11 d) and their weight had slightly diminished to 1367 ± 308 g (range 1010–2044 g). Nine infants received ventilatory assistance: eight with mechanical ventilation and one with nasal continuous positive airway pressure. RH at the time of the measurements was at least 40% (mean 52%, range 40–80%).
Instruments.
The infants in this study all received phototherapy for neonatal jaundice after nursery standards for unconjugated hyperbilirubinemia were met (9). Phototherapy was provided by a spotlight single-quartz lamp (Bililight Ohmeda, Ohmeda Medical, Columbia, MD, U.S.A.) at a distance of 55 cm from the skin of the infant. Thus, an irradiance of approximately 12.5 μW/cm2/nm was achieved, as measured by a phototherapy light energy meter (BiliBlanket Meter, Ohmeda). The wavelength of the radiation was mainly between 420 and 480 nm. Tamb in the incubator was measured by the temperature sensor of the incubator; Tskin and Troof were measured by YSI telethermometer probes (YSI Inc., Yellow Springs, OH, U.S.A.). We used the Tewameter TM210 (Courage&Khazaka electronic, Cologne, Germany), calibrated by the manufacturer, to measure TEWL. This device measures the evaporation rate noninvasively by determining the water vapor pressure gradient close to the skin surface. This instrument has been used in various studies and proved to be accurate (10, 11).
Protocol.
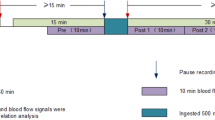
Measurements started when hyperbilirubinemia was diagnosed. The TEWL probe was placed on the chest or on the back, depending on the position of the infant, and performed its 9-min measurement. Approximately 60 min after the start of the phototherapy the second measurement took place. At both time points TEWL was in a steady state. The nursery staff adjusted Tamb if Tskin exceeded 37.5°C. The RH was kept constant during the entire procedure. Measurements included TEWL, RH, Tamb, Tskin, and Troof.
Data analysis.
Data are presented as mean ± SD. From the data obtained from Troof, Tskin, Tamb, and TEWL, heat exchange (H) through convection (Hconv), radiation (Hrad), and evaporation (Hevap) was calculated using the equations: MATH MATH MATH where c1 is the convection coefficient (2.7 W/m2/K), S0 the Stefan-Bolzman's constant (5.7 · 10−8 W/m2/K4), e1 the emissivity of the skin (1), e2 the emissivity of the surrounding walls (0.97), and c2 the latent heat of evaporation (2.4 · 103 J/g) (12). Statistical analysis was performed using the Student t test on paired observations. p Values <0.05 were considered statistically significant.



RESULTS
Before phototherapy, Troof was 32.2 ± 1.2°C (range 30.5–33.5°C) and after 1 h of phototherapy, it was 32.5 ± 1.1°C (range 30.4–33.2°C, p = 0.20). To prevent the skin temperature from exceeding 37.5°C, Tamb was manually adjusted from 32.3 ± 1.6°C (range 29.5–34.7°C) before to 32.0 ± 1.4°C (range 29.5–34.0°C, p = 0.02) during phototherapy. This resulted in a stable Tskin, with values of 36.7 ± 0.6°C (range 35.2–37.4°C) before and 36.9 ± 0.4°C (range 35.8–36.8°C, p = 0.40) during phototherapy. These changes led to a shift in heat exchange: Hconv augmented from 11.0 W/m2 to 12.3 W/m2 (p = 0.02), whereas Hrad seemed stable (27.8 W/m2 before phototherapy, 27.6 W/m2 after 1 h of phototherapy (p = 0.91). These observations agree with known industry standards (Dräger).
The mean TEWL before phototherapy was 13.6 ± 6.3 g/m2/h. After 1 h of phototherapy, TEWL had risen to 16.5 ± 6.2 g/m2/h (p = 0.003), an increase of 21.3%. Consequently, Hevap changed from 9.1 to 11.0 W/m2. There were no differences between TEWL measurements in the abdomen and back and TEWL had returned to prephototherapy values within 1 h after discontinuation of phototherapy.
Data obtained during a pilot study show that initiating phototherapy increases Troof by 2°C. Adjusting Tamb decreased Troof again, resulting in an unaffected Hrad and a slight increase of Hconv after 1 h of phototherapy. Total heat loss through convection, radiation, and evaporation was not influenced by starting phototherapy treatment [46.3 W/m2 before phototherapy, 50.7 W/m2 after 1 h (p = 0.10)].
DISCUSSION
Our data show that TEWL from the skin of a preterm infant increases by about 20% during phototherapy, even when ambient conditions are manipulated in such a way that heat balance and skin temperature remain virtually unaffected. Calculating the amount of extra fluid needed during phototherapy, we used the equation: MATH validated for low birth weight infants (13, 14). Using this equation, the body surface area of the preterm infants in this study was 0.12 ± 0.03 m2. Thus, 2.9 g/m2/h of extra fluid loss should be compensated by 0.35 mL/kg/h. Based on these observations, we recommend an extra fluid intake for preterm babies receiving phototherapy of about 10 mL/kg/d.

Water balance in the preterm infant is of major importance (15). Various studies state that a slightly negative water balance is favorable, because excess fluid can result in patent ductus arteriosus, bronchopulmonary dysplasia, congestive heart failure, and necrotizing enterocolitis (16–21). If, on the other hand, compensation of the high water losses in the preterm infant fails, dehydration, hypernatremia, and hyperkalemia may result, and it even may contribute to the complications of intraventricular hemorrhage and arrhythmia (22).
Potential pitfalls when evaluating the results of this study include the position of the infants in the incubator (we measured TEWL from the skin of the upper abdomen or the upper back, depending on the position of the infant at the time of phototherapy). A recent study by Yosipovitch et al. (11) compared the skin barrier properties in different skin body areas in appropriate for gestational age infants. Although marked differences in TEWL were found depending on the anatomical site of measurement, water loss from the skin of the abdomen and upper back did not differ statistically. Another potential artifact of accurate measurements is the influence of direct light from the phototherapy unit (23). Therefore, the Tewameter was protected during phototherapy by a small shield, which did not influence the airflow (23) or the body area exposed to phototherapy.
Early studies have reported larger increases of insensible water loss than we found in this study (2, 3). Based on insensible weight loss, these studies report that insensible water loss during phototherapy may increase by as much as 110%. Although at the time of those studies no device for measuring evaporative heat loss was available, this increase was suggested to be partially due to increased respiratory water loss (24). The use of a Potter baby scale for measuring the weight loss may have overestimated the amount of insensible water loss as well (25).
Kjartansson et al. (4) reported no significant increase in water loss from the skin during phototherapy. This study used the Evaporimeter, a device for determining evaporative water loss based on the same method as the Tewameter. The average skin temperature of the preterm infants in their study (36.2°C, both before and during phototherapy) was significantly lower than the normal range in our hospital. To keep the Tskin constant, Tamb as well as RH were adjusted. Troof rose by as much as 7.5°C, probably as a result of the use of bank lights and the close proximity of the lights to the incubator roof. This, in combination with the constant (low) Tskin, changed heat loss through radiation during phototherapy from 33.6 W/m2 to a heat gain of 15.9 W/m2. When the three ways of losing heat (Hconv, Hrad, and Hevap) were added up, the infants in their study were losing 40.5 W/m2 before phototherapy, while with the lamp switched on, they heated up by 1.5 W/m2.
The use of the halogen spotlight phototherapy lamp instead of a bank light diminishes the increase of Troof during phototherapy by 2.1°C (unpublished comparative data). This influences heat loss through radiation enormously. In previous studies, servocontrol on skin temperature lowered the ambient temperature of the incubator when the bank light was switched on (4, 26). The use of halogen spotlights leads to smaller Tamb adjustments and thus to a stable heat balance. Other reasons for using spotlight phototherapy for nonhemolytic hyperbilirubinemia instead of bank lights are the higher irradiance levels obtained (27) and the easy positioning of the light over the incubator. Moreover, the skin color of the infant is less distorted in spotlight phototherapy (28).
The observed increase in evaporation rate in these preterm infants cannot be ascribed to a change in thermoregulation. Various studies suggest that phototherapy increases skin blood flow by as much as 70%(5–8). Although in full-term infants an increase in skin blood flow does not account for extra TEWL (29), we suggest that in preterm infants, who lack the ability to sweat, there is a relationship between skin blood flow and TEWL. Phototherapy increases skin blood flow by a mechanism known as photorelaxation (30). The pathway of this mechanism is still not completely solved, but there is evidence of involvement of so-called nitrosothiols. Whereas for a long time nitric oxide was thought to be the active compound in photorelaxation (31), various recent studies have pointed out that S-nitrosothiols account for the relaxation in vascular smooth muscle cells (32–35), both by nitric oxide release and by direct binding to nitrosothiol recognition sites (33). This is an attractive hypothesis for the increase of skin blood flow in infants receiving phototherapy, as nitrosothiol isomers can suppress baroreceptor reflexes in the aortic arch (33). This might be an explanation for the slight decrease in cardiac output during phototherapy despite the fall in vascular resistance (7).
We conclude from our findings that in preterm infants TEWL increases by approximately 20% during phototherapy with halogen spotlights despite constant skin temperature and relative humidity. Maintenance fluids of these infants should be increased by 0.35 mL/kg/h during exposure to phototherapy or about 10 mL/kg/d.
Abbreviations
- Hconv:
-
heat exchange through convection
- Hevap:
-
heat exchange through evaporation
- Hrad:
-
heat exchange through radiation
- Htot:
-
total heat loss
- RH:
-
relative humidity
- TEWL:
-
transepidermal water loss
- Tamb:
-
ambient temperature in the incubator
- Troof:
-
temperature of the incubator roof
- Tskin:
-
skin temperature
References
Brown AK, Kim MH, Wu PYK, Bryla DA 1985 Efficacy of phototherapy in prevention and management of neonatal hyperbilirubinemia. Pediatrics 75: 393–400
Oh W, Karecki H 1972 Phototherapy and insensible water loss in the newborn infant. Am J Dis Child 124: 230–232
Wu PYK, Moosa A 1978 Effect of phototherapy on nitrogen and electrolyte levels and water balance in jaundiced preterm infants. Pediatrics 61: 193–198
Kjartansson S, Hammarlund K, Sedin G 1992 Insensible water loss from the skin during phototherapy in term and preterm infants. Acta Paediatr 81: 764–768
Oh W, Yao AC, Hanson JS, Lind J 1973 Peripheral circulatory response to phototherapy in newborn infants. Acta Paediatr Scand 62: 49–54
Wu PYK, Wong WH, Hodgman JE, Levan N 1974 Changes in blood flow in the skin and muscle with phototherapy. Pediatr Res 8: 257–262
Walther FJ, Wu PYK, Siassi B 1985 Cardiac output changes in newborns with hyperbilirubinemia treated with phototherapy. Pediatrics 76: 918–921
Jahnukainen T, Lindqvist A, Jalonen J, Kero P, Välimäki I 1999 Responsiveness of cutaneous vasculature to thermal stimulation during phototherapy in neonatal jaundice. Eur J Pediatr 158: 757–760
de Jaegere APMC, Van den Anker JN, Van Lingen RA, Sauer PJJ 2000 Neonatologie. In: Derksen-Lubsen G, Moll HA, Büller HA (eds) Compendiom kindergeneeskunde. Diagnostiek en behandeling, 2nd Ed. Bohn Stafleu Van Loghum, Houten, the Netherlands, 693–694
Barel AO, Clarys P 1995 Study of the stratum corneum barrier function by transepidermal water loss measurements: comparison between two commercial instruments: Evaporimeter and Tewameter. Skin Pharmacol 8: 186–195
Yosipovitch G, Maayan-Metzger A, Merlob P, Sirota L 2000 Skin barrier properties in different body areas in neonates. Pediatrics 106: 105–108
Hammarlund K, Strömberg B, Sedin G 1986 Heat loss from the skin of preterm and full-term newborn infants during the first weeks after birth. Biol Neonate 50: 1–10
Brion L, Fleischman AR, Schwartz GJ 1985 Evaluation of four length-weight formulas for estimating body surface area in newborn infants. J Pediatr 107: 801–803
Haycock GB, Schwartz GJ, Wisotsky DH 1978 Geometric method for measuring body surface area: a height-weight formula validated in infants, children, and adults. J Pediatr 93: 62–66
Bauer K, Bovermann G, Roithmaier A, Gotz M, Proiss A, Versmold HT 1991 Body composition, nutrition, and fluid balance during the first two weeks of life in preterm neonates weighing less than 1500 grams. J Pediatr 118: 615–620
Shaffer SG, Meade VM 1989 Sodium balance and extracellular volume regulation in very low birth weight infants. J Pediatr 115: 285–290
Stevenson JG 1977 Fluid administration in the association of patent ductus arteriosus complicating respiratory distress syndrome. J Pediatr 90: 257–261
Brown ER, Stark A, Sosenko I, Lawson EE, Avery ME 1978 Bronchopulmonary dysplasia: possible relationship to pulmonary edema. J Pediatr 92: 982–984
Bell EF, Warburton D, Stonestreet BS, Oh W 1980 Effect of fluid administration on the development of symptomatic patent ductus arteriosus and congestive heart failure in premature infants. N Engl J Med 302: 598–604
Bell EF, Warburton D, Stonestreet BS, Oh W 1979 High-volume fluid intake predisposes premature infants to necrotising enterocolitis. Lancet 2: 90
Green TP, Thompson TR, Johnson DE, Lock JE 1983 Diuresis and pulmonary function in premature infants with respiratory distress syndrome. J Pediatr 103: 618–623
Omar SA, DeCristofaro JD, Agarwal BI, La Gamma EF 1999 Effects of prenatal steroids on water and sodium homeostasis in extremely low birth weight neonates. Pediatrics 104: 482–488
Pinnagoda J, Tupker RA, Agner T, Serup J 1990 Guidelines for transepidermal water loss (TEWL) measurement. A report from the Standardization Group of the European Society of Contact Dermatitis. Contact Dermatitis 22: 164–178
Kjartansson S, Hammarlund K, Riesenfels T, Sedin G 1992 Respiratory water loss and oxygen consumption in newborn infants during phototherapy. Acta Paediatr 81: 769–773
Darnall RA 1981 Insensible weight loss measurements in newborn infants: possible overestimation with the Potter baby scale. J Pediatr 99: 794–797
Bell EF, Neidich GA, Cashore WJ, Oh W 1979 Combined effect of radiant warmer and phototherapy on insensible water loss in low-birth-weight infants. J Pediatr 94: 810–813
Mosenkis R 1995 CITECH test report: Neonatal phototherapy lamps: comparison of heating effects. Neonatal Intensive Care 3: 18–21 ( http://www.citechtest.com/fomp.html)
Sarici SU, Alpay F, Unay B, Ozcan O, Gokcay E 1999 Comparison of the efficacy of conventional special blue light phototherapy and fiberoptic phototherapy in the management of neonatal hyperbilirubinaemia. Acta Paediatr 88: 1249–1253
Strömberg B, Hammarlund K, Öberg PÅ, Sedin G 1983 Transepidermal water loss in newborn infants IX. The relationship between skin blood flow and evaporation rate in full-term infants nursed in a warm environment. Acta Paediatr Scand 72: 729–733
Furchgott RF 1991 Endothelium-dependent relaxation, endothelium-derived relaxing factor and photorelaxation of blood vessels. Semin Perinatol 15: 11–15
Palmer RMJ, Ferrige AG, Moncada S 1987 Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 327: 524–526
Goud C, Watts SW, Webb RC 1996 Photorelaxation is not attenuated by inhibition of the nitric oxide-cGMP pathway. J Vasc Res 33: 299–307
Davisson RL, Travis MD, Bates JN, Lewis SJ 1996 Hemodynamic effects ofl- andd-S-nitrosocysteine in the rat. Stereoselective S-nitrosothiol recognition sites. Circ Res 79: 256–262
Lovren F, Triggle CR 1998 Involvement of nitrosothiols, nitric oxide and voltage-gated K+ channels in photorelaxation of vascular smooth muscle. Eur J Pharmacol 347: 215–221
Hussain AS, Crispino NH, McLaughlin BE, Brien JF, Marks GS, Nakatsu K 1999 Glyceryl trinitrate-induced vasodilatation is inhibited by ultraviolet irradiation despite enhanced nitric oxide generation: evidence for formation of a nitric oxide conjugate. J Pharmacol Exp Ther 289: 895–900
Acknowledgements
The authors thank the nursing staff of the neonatal intensive care units for their help with this study and Dick de Haas for excellent technical support. The Tewameter TM210 was kindly provided by Courage&Khazaka electronic, Cologne, Germany.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grünhagen, D., De Boer, M., De Beaufort, A. et al. Transepidermal Water Loss During Halogen Spotlight Phototherapy in Preterm Infants. Pediatr Res 51, 402–405 (2002). https://doi.org/10.1203/00006450-200203000-00022
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-200203000-00022
This article is cited by
-
Transepidermal water loss and cerebral hemodynamics in preterm infants: conventional versus LED phototherapy
European Journal of Pediatrics (2007)
-
Besonderheiten der Haut des Neugeborenen und jungen Säuglings
Der Hautarzt (2005)