Abstract
Increased expression of CD38 on CD8+ T cells is associated with activation of the immune system, progression of HIV disease, and death in adults. The prognostic significance of these cells in HIV-infected children, where the picture is complicated by age-related differences in CD38 expression, remains controversial. Measuring the unimodal expression of CD38 on CD8+ T cells in adults and children by flow cytometry is best accomplished by quantitating the antigen on the cell surface. To our knowledge, this technique has not previously been reported in a pediatric population. Vertically HIV-infected children were age matched for mild (n = 26) and severe (n = 23) clinical disease. Eleven age-matched HIV-negative controls were included for comparison. Quantitation of CD38 on CD8+ T cells was performed at baseline and 1 y later. The ages of the children in the three clinical groups did not differ significantly (p = 0.6004). HIV-infected children had significantly increased CD38 measurements in comparison with the HIV-negative controls (p = 0.0131), and the severe disease group tended to have higher measurements than the mild disease group. Increased CD38+CD8+ T cells were significant predictors of death within the first year (p = 0.043). These findings support the view that increased CD38 expression on CD8+ T cells has the same prognostic significance in pediatric as in adult HIV disease.
Similar content being viewed by others
Main
Elevated CD38 expression on CD8+ T cells has been found to be a strong prognostic marker of disease progression to AIDS and death among HIV-infected male adults (1–3). In the pediatric setting, the situation appears unresolved. In infants and older children, increased CD8+ T cells expressing CD38 (%CD8+CD38+) have been documented to be positive predictors of survival in HIV infection (4–6) but have also been associated with advancing disease (7–9).
The immaturity of the immune system in childhood complicates assessment of the significance of CD38 expression in HIV-infected children. CD38, a glycoprotein expressed on early hemopoietic cells, is lost during cell maturation and re-expressed during cell activation (7, 10, 11). Newborns express high levels of CD38 on their T cells that decrease with age (2, 8, 11). CD38 in this patient population is therefore a marker of immaturity as well as of the immune activation that occurs in HIV infection (9).
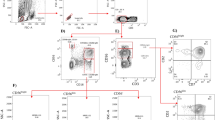
CD38 is ubiquitously expressed on lymphocytes in a unimodal distribution (9, 12). Therefore, in HIV-uninfected or -infected patients, cells that express CD38 are difficult to clearly distinguish from those that do not (Fig. 1) (12). Quantitating the mean intensity of CD38 expression on CD8+ T cells rather than just the proportion of positive cells (%CD8+ CD38+) is preferable and provides a better reflection of the continuous distribution of CD38 on CD8+ T cells (2, 6, 13).
An illustration of the CD38 measurements on CD8+ T cells on age-matched patients in the three clinical categories: (A) HIV-uninfected control, (B) HIV-infected child with mild clinical disease, and (C) HIV-infected child with severe clinical disease. Because no clear CD38-positive and -negative CD8+ T-cell population is discernible, an arbitrary cut-off was set for all cases (marker M). The percentage of CD8+ T cells expressing CD38 (%CD8+CD38+) in A of 31.06% is significantly different from B and C, with 76.53% and 87.76%, respectively. The quantitation of CD38 on CD8+ T cells [CD8+(CD38+)] in the three clinical groups shows an increasing geometric mean and CD38 ABC per CD8+ T cell in cases A to C.
To our knowledge, CD38 expression on CD8+ T cells in HIV-infected children has not been quantitated. The aim of this study was to quantitate CD38 expression on CD8+ T cells [CD8+ (CD38+)] in a vertically HIV-infected pediatric population naive to antiretroviral therapy and to assess its usefulness as a surrogate marker for disease progression and death.
METHODS
Subjects.
Perinatally HIV-infected patients at Chris Hani Baragwanath Hospital, Soweto, South Africa were age-matched for mild (n = 26) and severe (n = 23) clinical disease. Mild clinical disease was defined as no more than two hospital admissions for non-life-threatening events or diseases unrelated to HIV infection, no evidence of severe malnutrition, and no previous prolonged oxygen requirements. Severe clinical disease was defined as at least one admission for a severe or life-threatening event associated with HIV infection and/or more than two hospital admissions with HIV-associated conditions and/or severe failure to thrive (defined as an expected weight for age of <60%) and/or previous oxygen requirement of longer than 48 h. This clinical definition was preferred to the Centers for Disease Control clinical categories because the diagnosis of category-specific conditions often requires invasive procedures and expensive laboratory tests that are unattainable in a setting of limited resources. Eleven age-matched HIV negative controls were enrolled. These were well children returning to outpatient clinics for routine checkups after a hospital admission for an acute illness. All children were clinically free of infection at the time of enrollment into the study. The HIV-infected children had received no antiretroviral therapy but had access to prophylaxis for opportunistic infections and treatment for intercurrent infections. Ethics approval for this study was obtained from the Committee for Research on Human Subjects of the University of the Witwatersrand. Written informed consent was obtained from the parent or legal guardian of all children enrolled in the study.
Measurements.
The study commenced in September 1998, and 1-y follow-up of the HIV-infected children was completed in April 2000. Clinical data were recorded at 6-mo intervals and blood samples were taken at baseline and 1 y later. Peripheral blood was sampled in EDTA. Venesection was delayed if the child was acutely ill to avoid the possibility of transient increases in CD38 expression (2). A white cell count and lymphocyte percentage was obtained using the H3 Technicon (Bayer AG, Wuppertal, Germany). A manual differential count was performed only when the automated analyzer failed to generate a result. Blood for flow cytometry was prepared within 4 h of sampling. CD4+ T cells, CD8+ T cells, and B cells were enumerated according to standard flow cytometry protocols (14–16) on an Epics XL flow cytometer (Beckman Coulter, Inc., Fullerton, CA, U.S.A.) with a multiloader and the Coulter Multi-Q-Prep system. The antibody reagents were all purchased from Beckman Coulter and included Tritest (CD3 energy-coupled dye, CD4-RD1, and CD8-FITC), CD19-FITC, and CD45-RD1.
CD38 measurements on CD8+ T cells were performed by whole blood analysis using Immunoprep (Beckman Coulter, Inc.) on a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, U.S.A.). The antibody reagents were purchased from BD Biosciences and included CD38 (Leu 17)-PE, chosen because the PE fluorochrome:MAb ratio was the closest to a 1:1 conjugate, CD8-peridinin chlorophyll protein, and CD3-FITC.
T cells, defined as CD3+ cells with low side scatter, were gated. Five thousand CD8+ T cells, defined as CD3 and CD8 co-expressing cells, were acquired from within the T-cell gate. CD38 measurements were performed on the CD8+ T-cell population.
Because of the continuous spectrum of CD38 expression on CD8+ T cells, %CD8+ CD38+ was measured by setting an arbitrary cut-off point for all cases. This cut-off point was predetermined using a PE-conjugated anti-mouse IgG1 isotypic control on CD8+ T cells in 10 cases. The average of the mean channel number at which the signal became negative was 325 and was designated as the cut-off point.
The quantitation of CD38 expression on CD8+ T cells [CD8+(CD38+)] was done using commercially available QuantiBRITE PE beads (BD Biosciences) to determine number of ABC as described previously (13). The same PE voltage settings were used for the QuantiBRITE beads and the patient samples. The voltage was determined by placing the lowest and highest fluorescent peaks of the beads within the second and fourth logs of PE fluorescence to ensure linearity of the fluorescence intensity (17). The linear regression curve obtained using the QuantiBRITE PE beads was used to convert the geometric mean of the logarithmic FL2 fluorescence of a cell population into the number of ABC.
Quality control measures for both flow cytometry instruments were performed as described elsewhere (15, 16). Both instruments participate in an external quality assurance program viz National External Quality Assurance Scheme.
The Quantiplex HIV RNA bDNA system (Chiron, Emeryville, CA, U.S.A.) was used to measure viral loads, which are reported in RNA copies per milliliter of plasma.
Data analysis.
Because many of the variables were correlated with age, comparisons of groups were done using an analysis of covariance (ANCOVA), adjusting for age. Data were transformed where necessary, to obtain approximate normality of the residuals. However, as age did not differ significantly between the clinical groups, the nonparametric Kruskal-Wallis test was used to confirm results where there was skewness in the data that could not be corrected by transformation. Because results using the two tests were very similar, the p values reported in this paper are from the Kruskal-Wallis tests. Changes between baseline and y 1 were tested using a Wilcoxon matched pairs test. In view of the skewness of many of the variables, the correlations reported are Spearman rank correlations, and medians with minimum and maximum values are reported rather than means and SD. The Kruskal-Wallis test was used to test for differences between the three clinical groups. Where significant differences were found, the Wilcoxon-Mann-Whitney test determined which pairs differed significantly. Linear discriminant analysis was used to examine the predictiveness of various variables.
RESULTS
The ages of the children ranged from 4 mo to 11 y and did not differ significantly (p = 0.6004) between the three clinical groups (Table 1). A total of seven patients, three with mild and four with severe clinical disease, had no follow-up CD38 measurements performed (Table 2). Four of these seven patients were excluded from follow up when they became eligible for enrollment in an antiretroviral therapy trial. Three patients were lost to follow-up within the first year. Six patients, three with mild disease and three with severe disease, who had CD38 measurements at 1 y had no baseline measurements performed. Nine children, eight of whom were classified as having severe disease, died during the first year. The CD4+ T-cell percentages, viral loads, and CD38 measurements were all significantly negatively correlated with age (Table 3). Viral load was positively correlated with CD8+ (CD38+) and %CD8+ CD38+ measurements at baseline and at 1 y (r = 0.4539, p = 0.002 and r = 0.4665, p = 0.005 for CD8+(CD38+); and r = 0.3839, p = 0.011 and r = 0.4435, p = 0.009, for %CD8+CD38+, respectively). The sample size was too small to definitively establish whether the correlation between the viral load and the CD38 measurements was related to age, however, a separate examination of older versus younger children indicated that the correlation was not the result of the joint correlation with age.
At baseline, HIV-infected children classified as having severe disease had significantly higher CD8+ (CD38+) and %CD8+CD38+ than the HIV-uninfected children (Table 4). Hb levels in HIV-infected children were significantly lower than in HIV-uninfected children. The children with clinically mild disease had higher CD4+ T cell percentages (p ≤ 0.0005) and lower viral loads (p = 0.0034) and CD8+ T-cell percentages (p ≤ 0.0047) than those with severe disease. These findings, in conjunction with eight deaths occurring in the group with severe clinical disease and only one death in the mild clinical disease group (Table 2), suggest that the clinical categorization used in this study was justifiable.
Figure 1 illustrates the differences in CD38 expression on CD8+ T cells measured in three children between 22 and 24 mo of age, representing the three different clinical categories.
Combining the severely and mildly affected patient groups, the HIV-infected group had higher CD8+(CD38+) (p = 0.0131) and %CD8+CD38+ (p = 0.0277) in comparison with the control group at baseline (Fig. 2). The children with severe clinical disease had higher CD8+(CD38+) (p = 0.0514) and %CD8+CD38+ than those with mild disease (p = 0.1886) (Table 4 and Fig. 2). The preferable method for measurement of CD38 expression on CD8+ T cells viz the CD8+(CD38+) approached statistical significance. As expected, the CD8+ (CD38+) and %CD8+CD38+ were strongly positively correlated (r = 0.9569 at baseline, 0.9853 at y 1, both p < 0.0005) (1).
Box and whisker plots illustrating (A) the %CD8+CD38+ and (B) the CD8+(CD38+) in ABC according to clinical status at baseline and 1 y of follow-up. The box plot represents the median values with the 25th and 75th percentiles. Maximum and minimum values, including outliers, are indicated. The number of patients in each group is shown on the x axis
Bottled CD38 PE, estimated to have an average of 79% of the intensity of purified CD38 PE that has a PE fluorochrome:MAb ratio of 1:1, was used in this study (13). The values reported for CD38 ABC on CD8+ T cells in this population of children are therefore likely to be underestimated.
The clinical category to which the children were assigned at baseline remained constant at the 1-y follow-up except for three children who progressed from the mild to the severe clinical disease group, one of whom died (Table 2). The CD4+ T-cell absolute numbers (p = 0.0001) and percentages (p = 0.0347) decreased over the 1-y follow-up period but the viral loads (p = 0.8042) and CD8+(CD38+) (p = 0.6164) did not change significantly over that time. The %CD8+CD38+ decreased significantly (p = 0.0139) over the year with the decrease occurring in the group with mild disease (p = 0.0218) but not the group with severe disease (p = 0.4413). This may reflect the decrease in %CD8+CD38+ expected with age in the group of patients with mild, nonprogressive disease (Fig. 2).
Increased CD8+ (CD38+) was a stronger predictor of death than the viral load measurement, although less significant than the CD4+ T cell percentage and Hb levels (Table 5).
Discriminant analysis confirms CD8+ (CD38+), CD4+ T-cell percentage, and Hb level at baseline as statistically significant predictors of progression to death within the first year (Table 6). The cut-off values obtained may be useful as a guide in clinical practice, however, the error rates should be taken into consideration.
DISCUSSION
Immune activation in HIV infection may differ in adults and children inasmuch as most children are infected perinatally and HIV is introduced into a naive and developing immune system (9). Information regarding immune activation in HIV-infected adults can therefore not be extrapolated to the pediatric population. There is conflicting evidence regarding CD38 expression as a prognostic marker in HIV-infected children (4–9). This evidence is based predominantly on measurements of %CD8+CD38+. The %CD8+CD38+ and CD8+(CD38+) measurements are highly correlated, but the latter is preferable because of the heterogeneous expression of CD38 on the cell surface (2, 6, 12, 13). This study confirmed that CD38 expression on CD8+ T cells is negatively correlated with age (2, 8, 11) and increased in HIV-infected children in comparison to HIV-uninfected age-matched controls (1, 4, 5, 8, 9). These findings are consistent with CD38 being a marker of immaturity and immune activation. Total CD4+ T-cell depletion and CD8+ T-cell expansion occurred in HIV-infected children when compared with age-matched controls (8).
It has previously been observed that HIV-infected children who were clinically symptomatic seemed to have brighter CD38 expression on CD8+ T cells than those that were asymptomatic (8, 9). This increased fluorescence suggests a greater density of CD38 on the CD8+ T cells of children with more advanced HIV disease. Formal quantitation measurements suggest that CD38 expression on CD8+ T cells is increased in severe compared with mild clinical disease. As is the situation in the adult HIV-infected population, increased CD38 expression on CD8+ T cells in HIV-infected children appears to be associated with disease progression.
The significance of increased %CD8+CD38+ is controversial as it has been associated with a favorable prognosis (4–6) and with progression to AIDS in HIV-infected infants and older children (7, 9). Quantitation of CD38 on CD8+ T cells supports the latter view by demonstrating that increased CD8+(CD38+) was a significant predictor of death in children.
A positive correlation between CD38 expression on CD8+ T cells and viral load has been documented in HIV-infected adults (1). The same correlation was suggested in children by this study. An opposing view in perinatally infected children who had survived for more than 5 y is that an inverse correlation between viral load and %CD8+CD38+ existed (5). These children were, however, all receiving antiretroviral therapy at the time of the study, which may have influenced the results. Further evidence to support the findings documented here is that children who fail to respond to HAART and maintain high viral loads also maintain high %CD8+CD38+, whereas those who have a good virological response to HAART have decreased %CD8+CD38+(10). This situation in response to HAART has also been documented in HIV-infected adults (2).
CD8+(CD38+) is a stronger predictor of progression to death in HIV-infected children than viral load. However, the %CD4+ T cells and Hb levels were the best predictors of progression to death in these children and are easier and less expensive to perform than CD38 quantitation. In the adult situation, CD38 on CD8+ T cells was a stronger predictor of outcome than the percentage or absolute number of CD4+ T cells (2). A tentative classification for using specific reference ranges of CD8+(CD38+) to predict the risk of disease progression to AIDS has been proposed for use in adults (2). In children, where age is negatively correlated with CD38 on CD8+ T cells, establishing such reference ranges would require a larger study to enable age-specific values to be related to the risk of disease progression. Age-specific ranges for CD8+(CD38+) may strengthen the prognostic value of this marker in children with regard to CD4+ T cells thus concurring with the situation seen in adults (2).
Increased CD38 expression on CD8+ T cells in HIV infection is associated with high viral loads, disease progression, and death in adults. The findings of this study would suggest that the same is true in pediatric HIV disease. The utility of CD8+(CD38+) as a prognostic marker in HIV-infected children in clinical practice is complicated by the variation of this marker with age.
Abbreviations
- %CD8+CD38+:
-
percentage of CD8+ T cells expressing CD38
- CD8+(CD38+):
-
quantitation of CD38 on CD8+ T cells
- ABC:
-
antibodies bound per cell
- PE:
-
phycoerythrin
- HAART:
-
highly active antiretroviral therapy
References
Liu Z, Hultin LE, Cumberland WG, Hultin P, Schmid I, Matud JL, Detels R, Giorgi JV 1996 Elevated relative fluorescence intensity of CD38 antigen expression on CD8+ T cells is a marker of poor prognosis in HIV infection: results of 6 years of follow-up. Cytometry 26: 1–7
Liu Z, Cumberland WG, Hultin LE, Prince HE, Detels R, Giorgi JV 1997 Elevated CD38 antigen expression on CD8+ T cells is a stronger marker for the risk of chronic HIV disease progression to AIDS death in the Multicenter AIDS Cohort study than CD4 cell count, soluble immune activation markers, or combinations of HLA-DR CD38 expression. J Acquir Immune Defic Syndr 16: 83–92
Giorgi JV, Liu Z, Hultin LE, Cumberland WG, Hennessey K, Detels R 1993 Elevated levels of CD38+CD8+ T cells in HIV infection add to the prognostic value of low CD4+ T cell levels: results of 6 years of follow-up. J Acquir Immune Defic Syndr 6: 904–912
Schlesinger M, Peters V, Jlang JD, Roboz JP, Bekesi JG 1995 Increased expression of activation markers on CD8 lymphocytes in children with human immunodeficiency virus-1 infection. Pediatr Res 38: 390–396
de Martino M, Rossi ME, Azzari C, Gelli MG, Galli L, Vierucci A 1998 Different meaning of CD38 molecule expression on CD4+ and CD8+ cells of children perinatally infected with human immunodeficiency virus type 1 infection surviving longer than five years. Pediatr Res 43: 752–758
Savarino A, Bottarel F, Malavasi F, Dianzani U 2000 Role of CD38 in HIV-1 infection: an epiphenomenon of T-cell activation or an active player in virus/host interactions?. AIDS 14: 1079–1089
Sirera R, Bayona A, Carbonell F, Perez-Tamarit D, Otero MC, Asensi F, Gonzalez-Molina A. 1998 The expression of CD38 and DR are markers of immune activation and disease progression in HIV+ children. XII International Conference on AIDS. Geneva. [abstract 213/31166],June 28–July 8, 1998
Plaeger-Marshall S, Hultin P, Bertolli J, O'Rourke S, Kobayashi R, Kobayashi AI, Giorgi JV, Bryson Y, Stiehm ER 1993 Activation differentiation antigens on T cells of healthy, at-risk, HIV-infected children. J Acquir Immune Defic Syndr 6: 984–993
Plaeger-Marshall S, Isacescu V, O'Rourke S, Bertolli J, Bryson YJ, Stiehm ER 1994 T cell activation in pediatric AIDS pathogenesis: three-color immunophenotyping. Clin Immunol Immunopathol 1: 19–26
Saresella AV, Rusconi S, Ferrante P, Clerici M 1998 Expression of CD38 on CD8 T cells predicts maintenance of high viraemia in HAART-treated HIV-1-infected children. Lancet 352: 1905–1910
McCloskey TW, Cavaliere T, Bakshi S, Harper R, Fagin J, Kohn N, Pahwa S 1997 Immunophenotyping of T lymphocytes by three-color flow cytometry in healthy newborns, children adults. Clin Immunol Immunopathol 84: 46–55
Schmitz JL, Czerniewski MA, Edinger M, Plaeger S, Gelman R, Wilkening CL, Zawadzki JA, Wormsley SB, and the ACTG Advanced Flow Cytometry Focus Group 2000 Multisite comparison of methods for the quantitation of the surface expression of CD38 on CD8+ lymphocytes. Cytometry 42: 174–179
Iyer SB, Hultin LE, Zawadzki JA, Davis KA, Giorgi JV 1998 Quantitation of CD38 expression using QuantiBRITETMbeads. Cytometry 33: 206–212
Sherman GG, Galpin JS, Patel JM, Mendelow BV, Glencross DK 1999 CD4+ T cell enumeration in HIV infection with limited resources. J Immunol Methods 222: 209–217
1994 revised guidelines for the performance of CD4+ T-cell determinations in persons with human immunodeficiency virus (HIV) infections.Centers for Disease Control Prevention. MMWR Recomm Rep 1994 43( RR-3): 1–21
1997 revised guidelines for performing CD4+ T-cell determinations in persons infected with human immunodeficiency virus (HIV). Centers for Disease Control Prevention. MMWR Recomm Rep 1997 46( RR-2): 1–29
Schmid I, Schmid P, Giorgi JV 1988 Conversion of logarithmic channel numbers into relative linear fluorescence intensity. Cytometry 9: 533–538
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the South African Institute for Medical Research, Johannesburg, South Africa, and the Medical Research Council, Johannesburg, South Africa.
Rights and permissions
About this article
Cite this article
Sherman, G., Scott, L., Galpin, J. et al. CD38 Expression on CD8+ T Cells as a Prognostic Marker in Vertically HIV-Infected Pediatric Patients. Pediatr Res 51, 740–745 (2002). https://doi.org/10.1203/00006450-200206000-00013
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-200206000-00013
This article is cited by
-
Association between HIV replication and serum leptin levels: an observational study of a cohort of HIV‐1‐infected South African women
Journal of the International AIDS Society (2010)