Abstract
Hypoxia-inducible factor 1 (HIF-1) is a transcriptional activator that mediates changes in gene expression in response to changes in cellular oxygen concentrations. HIF-1 is a heterodimer consisting of an oxygen-regulated HIF-1α subunit and a constitutively expressed HIF-1β subunit. In mice, complete HIF-1α deficiency results in embryonic lethality at midgestation because of cardiac and vascular malformations. Analyses of animal and cell culture models as well as human tissue have provided evidence that HIF-1 plays important roles in the pathophysiology of preeclampsia, intrauterine growth retardation, hypoxia-mediated pulmonary hypertension, and cancer. HIF-1 promotes neovascularization in response to myocardial or retinal ischemia by activating transcription of the gene encoding vascular endothelial growth factor. HIF-1 may also mediate the protective response to cerebral ischemia known as late-phase preconditioning.
Similar content being viewed by others
Main
In humans, complex cardiovascular, hematopoietic, and respiratory systems develop to maintain oxygen homeostasis. Heart disease, cancer, cerebrovascular disease, and chronic obstructive lung disease are the most common causes of mortality in the United States, accounting for two thirds of all deaths annually. In these disorders, disruption of oxygen homeostasis represents a major aspect of disease pathophysiology. HIF-1 is a transcriptional activator that mediates changes in gene expression in response to changes in oxygen concentration. HIF-1 plays important roles in normal development, physiologic responses to hypoxia, and the pathophysiology of common human diseases.
MOLECULAR BIOLOGY
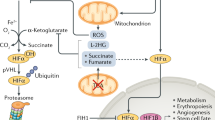
HIF-1 is a dimeric transcription factor composed of HIF-1α and HIF-1β subunits(1, 2). Under nonhypoxic conditions the HIF-1α subunit is subjected to ubiquitination and proteasomal degradation(3). In response to hypoxia, ubiquitination and degradation of HIF-1α are inhibited, resulting in rapid accumulation of the protein(4). In addition, the activity of the HIF-1α transactivation domains is induced by hypoxia(5, 6). HIF-1α dimerizes with HIF-1β, which is constitutively expressed, resulting in the formation of active HIF-1 protein that binds to the core sequence 5′-RCGTG-3′ present in target genes, several dozen of which have been identified thus far (Table 1). New target genes continue to be identified and it is likely that the total number of HIF-1-regulated genes in the human genome is an order of magnitude greater than what is presently known.
DEVELOPMENTAL BIOLOGY AND PHYSIOLOGY
Analysis of knockout mice has demonstrated that HIF-1α is required for embryonic development and survival. HIF-1α-deficient mouse embryos arrest in their development by d 9 of gestation (E9.0) and die by E10.5 with severe cardiovascular and neural tube defects and massive cell death, especially in the branchial and cephalic regions(7–9). Mice that are heterozygous for the knockout allele and thus partially HIF-1α deficient develop normally. However, when these mice are subjected to long-term hypoxia (10% O2 for 3 wk), the development of erythrocytosis and pulmonary vascular remodeling is significantly impaired(10). The impaired development of medial wall hypertrophy in small pulmonary arterioles, which is the hallmark of hypoxia-induced pulmonary hypertension, indicates that HIF-1α is essential for this process. These results suggest that partial pharmacologic inhibition of HIF-1 activity might provide a means to prevent pulmonary vascular remodeling without causing untoward side effects in at-risk patients with chronic obstructive lung disease.
MYOCARDIAL ISCHEMIA-INDUCED VASCULARIZATION
HIF-1α is also essential for angiogenesis in ischemic tissue. When HIF-1α-deficient embryonic stem cells are subjected to hypoxia, expression of mRNA encoding VEGF is not induced(7, 9, 11). In near-term fetal sheep, myocardial hypoxia-ischemia results in the induction of HIF-1α protein, VEGF mRNA and protein, and increased myocardial vascularization(12). The impaired angiogenic response to ischemia in older animals is caused in part by decreased VEGF production as a result of impaired HIF-1 DNA-binding activity (13–15). Preclinical trials of HIF-1α gene therapy for ischemia indicate that this strategy for therapeutic angiogenesis is at least as effective as VEGF gene therapy(16).
RETINAL VASCULARIZATION AND ISCHEMIC RETINOPATHY
Expression of HIF-1α protein and VEGF mRNA are spatially and temporally correlated during normal retinal development(17). These data are consistent with other studies indicating that hypoxia is an essential stimulus for retinal vascularization(18). In a mouse model of oxygen-induced ischemic retinopathy similar to retinopathy of prematurity, 1-wk-old (P7) mice are exposed to hyperoxia from P7 to P12, which blocks VEGF expression in the retina(19). When the mice are returned to normoxic conditions, retinal ischemia develops, which induces VEGF expression. Analysis of HIF-1α expression revealed a temporal and spatial correlation with VEGF mRNA expression, both with regard to the hyperoxic repression and ischemic induction(17), indicating that HIF-1-mediated VEGF expression may play a major role in the development of retinopathy of prematurity and other ischemic retinal disorders such as diabetic retinopathy. Retinal neovascularization can be prevented by blocking VEGF(20), suggesting that inhibition of HIF-1 activity may be of therapeutic utility in these conditions.
CEREBRAL ISCHEMIA AND DELAYED PRECONDITIONING
HIF-1 may also play a role in cerebral ischemia. Cerebral infarction can be induced in P7 rat pups by permanent ligation of the left common carotid artery and exposure to 8% O2 for 3 h. Seven days later the pups are killed and analyzed, revealing an approximately 40% reduction in hemispheric weight ipsilateral to the carotid occlusion. In contrast, P7 rats that are subjected to 8% O2 for 3 h and then 24 h later are subjected to carotid occlusion and hypoxia are dramatically protected against cerebral infarction(21), a phenomenon known as delayed (late-phase) preconditioning. The 3-h hypoxic preconditioning exposure was shown to induce HIF-1α protein expression throughout the brain(22). Analysis of HIF-1α expression in the brains of P7 rats subjected to carotid occlusion and hypoxia for 3 h and then killed immediately revealed induction of HIF-1α protein expression throughout the hemisphere contralateral to the occlusion, whereas in the ipsilateral hemisphere HIF-1α expression was decreased in the brain parenchyma and dramatically up-regulated in the cerebral microvasculature(22).
HIF-1α protein expression, HIF-1 DNA-binding and transcriptional activity, and expression of target genes can also be induced by exposing cultured cells to cobalt chloride or iron chelators such as desferrioxamine(1, 5, 23). A single injection of cobalt chloride or desferrioxamine induced HIF-1α expression in the brain and protected against the development of cerebral infarction after carotid occlusion and hypoxia(22). The ability of these agents to induce HIF-1α expression was correlated with their ability to induce protection (hypoxia > CoCl2 > desferrioxamine). The basis for this protective effect is unknown. HIF-1 has been shown to induce the expression of erythropoietin and VEGF (Table 1), each of which has been shown to function as a neuronal survival factor(24, 25). In addition, HIF-1 coordinately regulates the expression of genes encoding at least 13 different glucose transporters and glycolytic enzymes(7). After middle cerebral artery occlusion, there is a spatial and temporal correlation between induction of HIF-1α mRNA and of mRNAs encoding aldolase A, glucose transporter 1, lactate dehydrogenase A, phosphofructokinase L, and pyruvate kinase M (Table 1) in the penumbra, which is the viable tissue surrounding the infarction(26). The induction of glycolytic metabolism by HIF-1 may contribute to the protective effect of preconditioning with cobalt, desferrioxamine, or hypoxia. Whether the net effect of HIF-1 expression in the ischemic state is to protect against or promote infarction is unclear, as cell-based studies suggest that HIF-1 mediates hypoxia-induced apoptosis(27)via induction of p53(11, 28, 29).
CANCER
In contrast to the potentially protective effect of HIF-1 expression in the context of cerebral and myocardial ischemia, HIF-1 plays an important role in promoting tumor progression(30). Mutations that inactivate tumor suppressor genes or activate oncogenes have, as one of their consequences, up-regulation of HIF-1 activity, either through an increase in HIF-1α protein expression, HIF-1 transcriptional activity, or both (Table 2). Increased HIF-1 activity results in increased expression of target genes with important roles in tumor progression such as induction of tumor vascularization by VEGF (the angiogenic switch) and metabolic adaptation to hypoxia via increased glucose transporter and glycolytic enzyme activity (the Warburg effect). Immunohistochemical analysis of 40 human brain tumors revealed a significant correlation between HIF-1α protein expression, tumor grade, and tumor vascularization(31). HIF-1α is overexpressed in the majority of common human cancers, including breast, colon, lung, and prostate carcinoma(32).
The relationship between HIF-1α and the tumor suppressor p53 is of particular significance. Tumor cells subjected to hypoxia undergo p53-mediated apoptosis, which represents a powerful selection for cells that have sustained mutations that result in p53 loss of function(33). In unstimulated cells p53 is bound by MDM2, a ubiquitin-protein ligase that targets p53 for degradation by the proteasome(34, 35). In response to hypoxia, HIF-1α is induced and binds to p53, an interaction that protects p53 from degradation(28). Instead, MDM2 targets HIF-1α for degradation(36). Thus, two major consequences of p53 loss-of-function are the prevention of hypoxia-induced apoptosis and increased expression of HIF-1α. Increased HIF-1-mediated VEGF gene transcription results in increased vascularization of p53-nonexpressing as opposed to p53-expressing tumors(36). The von Hippel-Lindau tumor suppressor is also a ubiquitin-protein ligase that specifically targets HIF-1α for degradation under nonhypoxic conditions(37, 38). Von Hippel-Lindau loss-of-function in renal cell carcinomas and cerebellar hemangioblastomas results in constitutive overexpression of HIF-1α protein and VEGF mRNA, resulting in tumors that are among the most highly vascularized human cancers(31, 32, 37, 38).
CONCLUSION
HIF-1 is a master regulator of oxygen homeostasis, which is a fundamental requirement for survival. It orchestrates a multitude of biologic processes starting in early embryonic development and extending into adult life. Recent data suggest that HIF-1 may play an important role in the pathophysiology of preeclampsia(39) and intrauterine fetal growth retardation(40). In diseases that represent the most common causes of mortality in western societies (ischemic cardiovascular disease, cancer, and chronic lung disease), there is growing evidence suggesting that modulation of HIF-1 activity, using a pharmacologic or DNA-based approach, may have therapeutic effects.41–65
Abbreviations
- HIF-1:
-
hypoxia-inducible factor 1
- VEGF:
-
vascular endothelial growth factor
REFERENCES
Wang GL, Jiang B-H, Rue EA, Semenza GL 1995 Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA 92: 5510–5514
Wang GL, Semenza GL 1995 Purification and characterization of hypoxia-inducible factor 1. J Biol Chem 270: 1230–1237
Salceda S, Caro J 1997 Hypoxia-inducible factor 1α (HIF-1α) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions: its stabilization by hypoxia depends upon redox-induced changes. J Biol Chem 272: 22642–22647
Sutter CH, Laughner E, Semenza GL 2000 Hypoxia-inducible factor 1α protein expression is controlled by oxygen-regulated ubiquitination that is disrupted by deletions and missense mutations. Proc Natl Acad Sci USA 97: 4748–4753
Jiang B-H, Zheng JZ, Leung SW, Roe R, Semenza GL 1997 Transactivation and inhibitory domains of hypoxia-inducible factor 1α: modulation of transcriptional activity by oxygen tension. J Biol Chem 272: 19253–19260
Pugh CW, O’Rourke JF, Nagao M, Gleadle JM, Ratcliffe PJ 1997 Activation of hypoxia-inducible factor-1; definition of regulatory domains within the α subunit. J Biol Chem 272: 11205–11214
Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, Semenza GL 1998 Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1α. Genes Dev 12: 149–162
Kotch LE, Iyer NV, Laughner E, Semenza GL 1999 Defective vascularization of HIF-1α-null embryos is not associated with VEGF deficiency but with mesenchymal cell death. Dev Biol 209: 254–267
Ryan HE, Lo J, Johnson RS 1998 HIF-1α is required for solid tumor formation and embryonic vascularization. EMBO J 17: 3005–3015
Yu AY, Shimoda LA, Iyer NV, Huso DL, Sun X, McWilliams R, Beaty T, Sham JSK, Wiener CM, Sylvester JT, Semenza GL 1999 Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1α. J Clin Invest 103: 691–696
Carmeliet P, Dor Y, Herbert J-M, Fukumura D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R, Maxwell P, Koch CJ, Ratcliffe P, Moons L, Jain RK, Collen D, Keshet E 1998 Role of HIF-1α in hypoxia-mediated apoptosis, cell proliferation, and tumour angiogenesis. Nature 394: 485–490
Martin C, Yu AY, Jiang BH, Davis L, Kimberly D, Hohimer AR, Semenza GL 1998 Cardiac hypertrophy in chronically anemic fetal sheep: increased vascularization is associated with increased myocardial expression of vascular endothelial growth factor and hypoxia-inducible factor 1. Am J Obstet Gynecol 178: 527–534
Frenkel-Denkberg G, Gershon D, Levy AP 1999 The function of hypoxia-inducible factor 1 (HIF-1) is impaired in senescent mice. FEBS Lett 462: 341–344
Rivard A, Fabre JE, Silver M, Chen D, Murohara T, Kearney M, Magner M, Asahara T, Isner JM 1999 Age-dependent impairment of angiogenesis. Circulation 99: 111–120
Rivard A, Berthou-Soulie L, Principe N, Kearney M, Curry C, Branellec D, Semenza GL, Isner JM 2000 ge-dependent defect in VEGF expression is associated with reduced HIF-1 activity. J Biol Chem 275: 29643–29647
Vincent KA, Shyu K-G, Luo Y, Magner M, Tio RA, Jiang C, Goldberg MA, Akita GY, Gregory RJ, Isner JM 2000 Angiogenesis is induced in a rabbit model of hindlimb ischemia by naked DNA encoding a HIF-1α/VP-16 hybrid transcription factor. Circulation 102: 2255–2261
Ozaki H, Yu AY, Della N, Ozaki K, Luna JD, Yamada H, Hackett SF, Okamoto N, Zack DJ, Semenza GL, Campochiaro PA 1999 Hypoxia-inducible factor 1α is increased in ischemic retina: temporal and spatial correlation with VEGF expression. Invest Ophthalmol Vis Sci 40: 182–189
Stone J, Itin A, Alon T, Pe’er J, Gnessin H, Chan-Ling T, Keshet E 1995 Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J Neurosci 15: 4738–4747
Pierce EA, Avery RL, Foley ED, Aiello LP, Smith LE 1995 Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci USA 92: 905–909
Aiello LP, Pierce EA, Foley ED, Takagi H, Chen H, Riddle L, Ferrara N, King GL, Smith LE 1995 Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci USA 92: 10457–10461
Gidday JM, Fitzgibbons JC, Shah AR, Park TS 1994 Neuroprotection from ischemic brain injury by hypoxic preconditioning in the neonatal rat. Neurosci Lett 168: 221–224
Bergeron M, Gidday JM, Yu AY, Semenza GL, Ferriero DM, Sharp FR 2000 Role of hypoxia-inducible factor 1 (HIF-1) in hypoxia-induced ischemic tolerance in neonatal rat brain. Ann Neurol 48: 285–296
Wang GL, Semenza GL 1993 Desferrioxamine induces erythropoietin gene expression and hypoxia-inducible factor 1 DNA binding activity: implications for models of hypoxia signal transduction. Blood 82: 3610–3615
Hayashi T, Abe K, Itoyama Y 1998 Reduction of ischemic damage by application of vascular endothelial growth factor in rat brain after transient ischemia. J Cereb Blood Flow Metab 18: 887–895
Sakanaka M, Wen TC, Matsuda S, Masuda S, Morishita E, Nagao M, Sasaki R 1998 In vivo evidence that erythropoietin protects neurons from ischemic damage. Proc Natl Acad Sci USA 95: 4635–4640
Bergeron M, Yu AY, Solway K, Semenza GL, Sharp FR 1999 Induction of hypoxia-inducible factor-1 (HIF-1) and its target genes following focal ischemia in rat brain. Eur J Neurosci 11: 1–12
Halterman MW, Miller CC, Federoff HJ 1999 Hypoxia-inducible factor-1α mediates hypoxia-induced delayed neuronal death that involves p53. J Neurosci 19: 6818–6824
An WG, Kanekal M, Simon MC, Maltepe E, Blagosklonny MV, Neckers LM 1998 Stabilization of wild-type p53 by hypoxia-inducible factor 1α. Nature 392: 405–408
Banasiak KJ, Haddad GG 1998 Hypoxia-induced apoptosis: effect of hypoxic severity and role of p53 in neuronal cell death. Brain Res 797: 295–304
Semenza GL 2000 Hypoxia, clonal selection, and the role of HIF-1 in tumor progression. Crit Rev Biochem Mol Biol 35: 71–103
Zagzag D, Zhong H, Scalzitti JM, Laughner E, Simons JW, Semenza GL 2000 Expression of hypoxia-inducible factor 1α in human brain tumors: association with angiogenesis, invasion, and progression. Cancer 88: 2606–2618
Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL, Simons JW 1999 Overexpression of hypoxia-inducible factor 1α in common human cancers and their metastases. Cancer Res 59: 5830–5835
Graeber TG, Osmanian C, Jacks T, Housman DE, Koch CJ, Lowe SW, Giaccia AJ 1996 Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumors. Nature 379: 88–91
Haupt Y, Maya R, Kazaz A, Oren M 1997 Mdm2 promotes the rapid degradation of p53. Nature 387: 296–299
Kubbutat MHG, Jones SN, Vousden KH 1997 Regulation of p53 stability by Mdm2. Nature 387: 299–303
Ravi R, Mookerjee B, Bhujwalla ZM, Sutter CH, Artemov D, Zeng Q, Dillehay LE, Madan A, Semenza GL, Bedi A 2000 Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1α. Genes Dev 14: 34–44
Cockman ME, Masson N, Mole DR, Jaakkola P, Chang GW, Clifford SC, Maher ER, Pugh CW, Ratcliffe PJ, Maxwell PH 2000 Hypoxia inducible factor-α binding and ubiquitylation by the von Hippel-Lindau tumor suppressor protein. J Biol Chem 275: 25733–25741
Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ 1999 The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399: 271–275
Caniggia I, Mostachfi H, Winter J, Gassmann M, Lye SJ, Kuliszewski M, Post M 2000 Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGFβ3. J Clin Invest 105: 577–587
Tazuke SI, Mazure NM, Sugawara J, Carland G, Faessen GH, Suen L-F, Irwin JC, Powell DR, Giaccia AJ, Giudice LC 1998 Hypoxia stimulates insulin-like growth factor binding protein 1 (IGFBP-1) gene expression in HepG2 cells: a possible model for IGFBP-1 expression in fetal hypoxia. Proc Natl Acad Sci USA 95: 10188–10193
Wood SM, Wiesener MS, Yeates KM, Okada N, Pugh CW, Maxwell PH, Ratcliffe PJ 1998 Selection and analysis of a mutant cell line defective in the hypoxia-inducible factor-1a-subunit (HIF-1a): characterization of HIF-1α-dependent and independent hypoxia-inducible gene expression. J Biol Chem 273: 8360–8368
Eckhart AD, Yang N, Xin X, Faber JE 1997 Characterization of the α1B-adrenergic receptor gene promoter region and hypoxia regulatory elements in vascular smooth muscle. Proc Natl Acad Sci USA 94: 9487–9492
Cormier-Regard S, Nguyen SV, Claycomb WC 1998 Adrenomedullin gene expression is developmentally regulated and induced by hypoxia in rat ventricular cardiac myocytes. J Biol Chem 273: 17787–17792
Mukhopadhyay CK, Mazumder B, Fox PL 2000 Role of hypoxia-inducible factor 1 in transcriptional activation of ceruloplasmin by iron deficiency. J Biol Chem 275: 21048–21054
Hu J, Discher DJ, Bishopric NH, Webster KA 1998 Hypoxia regulates expression of the endothelin-1 gene through a proximal hypoxia-inducible factor-1 binding site on the antisense strand. Biochem Biophys Res Commun 245: 894–899
Jiang B-H, Rue E, Wang GL, Roe R, Semenza GL 1996 Dimerization, DNA binding, and transactivation properties of hypoxia-inducible factor 1. J Biol Chem 271: 17771–17778
Lee PJ, Jiang B-H, Chin BY, Iyer NV, Alam J, Semenza GL, Choi AMK 1997 Hypoxia-inducible factor 1 mediates transcriptional activation of the heme oxygenase-1 gene in response to hypoxia. J Biol Chem 272: 5375–5381
Feldser D, Agani F, Iyer NV, Pak B, Ferreira G, Semenza GL 1999 Reciprocal positive regulation of hypoxia-inducible factor 1a and insulin-like growth factor 2. Cancer Res 59: 3915–3918
Melillo G, Musso T, Sica A, Taylor LS, Cox GW, Varesio L 1995 A hypoxia-responsive element mediates a novel pathway of activation of the inducible nitric oxide synthase promoter. J Exp Med 182: 1683–1693
Palmer LA, Semenza GL, Stoler MH, Johns RA 1998 Hypoxia induces type II NOS gene expression in pulmonary artery endothelial cells via HIF-1. Am J Physiol 274: L212–L219
Bhattacharya S, Michels CL, Leung MK, Arany ZP, Kung AL, Livingston DM 1999 Functional role of p35srj, a novel p300/CBP binding protein, during transactivation by HIF-1. Genes Dev 13: 64–75
Kietzmann T, Roth U, Jungermann K 1999 Induction of the plasminogen activator inhibitor-1 gene expression by mild hypoxia via a hypoxia response element binding the hypoxia-inducible factor-1 in rat hepatocytes. Blood 94: 4177–4185
Takahashi Y, Takahashi S, Shiga Y, Yoshimi T, Miura T 2000 Hypoxic induction of prolyl 4-hydroxylase alpha (I) in cultured cells. J Biol Chem 275: 14139–14146
Rolfs A, Kvietikova I, Gassmann M, Wenger RH 1997 Oxygen-regulated transferrin expression is mediated by hypoxia-inducible factor-1. J Biol Chem 272: 20055–20562
Lok CN, Ponka P 1999 Identification of a hypoxia response element in the transferrin receptor gene. J Biol Chem 274: 24147–24152
Tacchini L, Bianchi L, Bernelli-Zazzera A, Cairo G 1999 Transferrin receptor induction by hypoxia: HIF-1-mediated transcriptional activation and cell-specific post-transcriptional regulation. J Biol Chem 274: 24142–24146
Gerber H-P, Condorelli F, Park J, Ferrara N 1997 Differential transcriptional regulation of the two vascular endothelial growth factor receptor genes: Flt-1, but not Flk-1/KDR, is upregulated by hypoxia. J Biol Chem 272: 23659–23667
Zhong H, Chiles K, Feldser D, Laughner E, Hanrahan C, Georgescu M-M, Simons JW, Semenza GL 2000 Modulation of HIF-1α expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res 60: 1541–1545
Zundel W, Schindler C, Haas-Kogan D, Koong A, Kaper F, Chen E, Gottschalk AR, Ryan HE, Johnson RS, Jefferson AB, Stokoe D, Giaccia AJ 2000 Loss of PTEN facilitates HIF-1-mediated gene expression. Genes Dev 14: 391–396
Minet E, Arnould T, Michel G, Roland D, Mottet D, Raes M, Remacle J, Michiels C 2000 ERK activation upon hypoxia: involvement in HIF-1 activation. FEBS Lett 484: 53–58
Richard DE, Berra E, Gothie E, Roux D, Pouyssegur J 1999 p42/p44 mitogen-activated protein kinases phosphorylate hypoxia-inducible factor 1α (HIF-1α) and enhance the transcriptional activity of HIF-1. J Biol Chem 274: 32631–32637
Mazure NM, Chen EY, Laderoute KR, Giaccia AJ 1997 Induction of vascular endothelial growth factor by hypoxia is modulated by a phosphatidylinositol 3-kinase/Akt signaling pathway in Ha-ras-transformed cells through a hypoxia inducible factor-1 transcriptional element. Blood 90: 3322–3331
Jiang B-H, Agani F, Passaniti A, Semenza GL 1997 V-SRC induces expression of hypoxia-inducible factor 1 (HIF-1) and transcription of genes encoding vascular endothelial growth factor and enolase 1: involvement of HIF-1 in tumor progression. Cancer Res 57: 5328–5335
Agani F, Semenza GL 1998 Mersalyl is a novel inducer of vascular endothelial growth factor gene expression and hypoxia-inducible factor 1 activity. Mol Pharmacol 54: 749–754
Zelzer E, Levy Y, Kahana C, Shilo B-Z, Rubinstein M, Cohen B 1998 Insulin induces transcription of target genes through the hypoxia-inducible factor HIF-1α/ARNT. EMBO J 17: 5085–5094
Acknowledgements
Work from my laboratory cited above was performed by a talented group of postdoctoral fellows (Guang Wang, Bing-Hua Jiang, Narayan Iyer, Faton Agani, Aimee Yu, Carrie Hayes Sutter, and Lori Kotch) and research technicians (Sandra Leung, Rick Roe, Erik Laughner, David Feldser, and Kelly Chiles) to whom I am enormously endebted. This work was also facilitated by collaborations with a group of outstanding investigators both at Johns Hopkins (Dmitri Artemov, Atul Bedi, Zaver Bhujwalla, Peter Campochiaro, Angelo De Marzo, John Gearhart, Ann Lawler, Rajani Ravi, Larissa Shimoda, Jonathan Simons, J.T. Sylvester, Charles Wiener, and Hua Zhong) and elsewhere (Marcelle Bergeron, Peter Buechler, Lowell Davis, Maria-Magdelena Georgescu, Jeff Gidday, David Hilton, Roger Hohimer, Jeff Isner, Rajiv Ratan, Joanne Scalzitti, Frank Sharp, and David Zagzag).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by grants from the American Heart Association National Center and Mid-Atlantic Affiliate, Children’s Brain Tumor Foundation, and the National Institutes of Health (R01-DK39869 and R01-HL55338).
Recipient of the Society for Pediatric Research 2000 E. Mead Johnson Award for Research in Pediatrics, presented at the 2000 Annual Meeting of the Pediatric Academic Societies, Boston, MA, U.S.A.
Rights and permissions
About this article
Cite this article
Semenza, G. Hypoxia-Inducible Factor 1: Control of Oxygen Homeostasis in Health and Disease. Pediatr Res 49, 614–617 (2001). https://doi.org/10.1203/00006450-200105000-00002
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-200105000-00002
This article is cited by
-
Hypoxia-Induced miR-101 Impairs Endothelial Barrier Integrity Through Altering VE-Cadherin and Claudin-5
Molecular Neurobiology (2024)
-
Hypoxia regulates adipose mesenchymal stem cells proliferation, migration, and nucleus pulposus-like differentiation by regulating endoplasmic reticulum stress via the HIF-1α pathway
Journal of Orthopaedic Surgery and Research (2023)
-
Cardiomyocyte Maturation–the Road is not Obstructed
Stem Cell Reviews and Reports (2022)
-
Hypoxia-inducible factor-1α promotes proliferation of airway smooth muscle cells through miRNA-103-mediated signaling pathway under hypoxia
In Vitro Cellular & Developmental Biology - Animal (2021)
-
On the correlation between material-induced cell shape and phenotypical response of human mesenchymal stem cells
Scientific Reports (2020)