Abstract
Birth asphyxia is a serious problem worldwide, resulting in 1 million deaths and an equal number of neurologic sequelae annually. It is therefore important to develop new and better ways to treat asphyxia. In the present study we tested the effects of reoxygenation with room air or with 100% oxygen (O2) after experimental pneumothorax-induced asphyxia on the blood oxidative stress indicators, early neurologic outcome, and cerebral histopathology of newborn piglets. Twenty-six animals were studied in three experimental groups:1) sham-operated animals (SHAM, n = 6), 2) animals reoxygenated with room air after pneumothorax (R21, n = 10), and 3) animals reoxygenated with 100% O2 after pneumothorax (R100, n = 10). In groups R21 and R100, asphyxia was induced under anesthesia with bilateral intrapleural room air insufflation. Gasping, bradyarrhythmia, arterial hypotension, hypoxemia, hypercarbia, and combined acidosis occurred 62 ± 6 min (R21) or 65 ± 7 min (R100; mean ± SD) after the start of the experiments; then pneumothorax was relieved, and a 10-min reoxygenation period was started with mechanical ventilation with room air (R21) or with 100% O2 (R100). The newborn piglets then breathed room air spontaneously during the next 3 h. Blood oxidative stress indicators (oxidized and reduced glutathione, plasma Hb, and malondialdehyde concentrations) were measured at different stages of the experiments. Early neurologic outcome examinations (neurologic score of 20 indicates normal, 5 indicates brain-dead) were performed at the end of the study. The brains were next fixed, and various regions were stained for cerebral histopathology. In the SHAM group, the blood gas and acid-base status differed significantly from those measured in groups R21 and R100. In group R100, arterial Po2 was significantly higher after 5 (13.8 ± 5.6 kPa) and 10 min (13.2 ± 6.3 kPa) of reoxygenation than in group R21 (8.7 ± 2.8 kPa and 9.2 ± 3.1 kPa). The levels of all oxidative stress indicators remained unchanged in the study groups (SHAM, R21, and R100). The neurologic examination score in the SHAM group was 18 ± 0, in group R21 it was 13.5 ± 3.1, and in group R100 it was 9.5 ± 4.1 (significant differences between SHAM and R21 or R100, and between R21 and R100). Cerebral histopathology revealed marked damage of similar severity in both asphyxiated groups. We conclude that the blood oxidative stress indicators and cerebral histopathology did not differ significantly after a 10-min period of reoxygenation with room air or with 100% O2 after pneumothorax-induced asphyxia, but reoxygenation with 100% O2 might impair the early neurologic outcome of newborn piglets.
Similar content being viewed by others
Main
Birth asphyxia is a serious clinical problem worldwide. Each year approximately 4 million babies are born asphyxiated, which results in 1 million deaths and an equal number of serious neurologic sequelae, such as cerebral palsy, mental retardation, and epilepsy (1). It is therefore clinically important to develop new and better ways to treat asphyxia. The human infants affected are usually resuscitated with a high concentration of oxygen (2). The guidelines of the American Heart Association from 1992 for the resuscitation of newborns recommend ventilation via bags that can give as close to 100% O2 as possible (3). The possible toxic effects of hyperoxia have been known for many years (4). The sudden reintroduction of a high concentration of oxygen to hypoxic tissues may result in a burst of oxygen free radical formation (5–7), which may increase the hypoxic tissue damage. Reoxygenation and reperfusion after severe hypoxia and ischemia may contribute substantially to birth asphyxia-related brain injury (8). In a number of animal studies, resuscitation with room air has been shown to be as efficient as (9, 10), or even superior to (11–13), resuscitation with 100% O2. Finally, the gradual reintroduction of oxygen after ischemia has been shown to improve the functional and metabolic recovery of the CNS (14, 15).
A recently published clinical study indicates no major differences in outcome when resuscitation is performed with room air as compared with 100% O2(16). However, the time to the first breath and the time to the first cry were significantly shorter in the room air group than in the oxygen group. Studies on newborn animals have shown that room air is as effective as 100% O2 in normalizing both regional and cerebral blood flows and also the evoked potentials after hypoxemia (10, 17).
In the present study we tested the hypothesis that asphyxiated newborn piglets can be successfully resuscitated with room air. We additionally tested the effects of reoxygenation after pneumothorax-evoked asphyxia with 100% O2 on blood oxidative stress indicators, early neurologic outcome, and cerebral histopathology by using our newborn piglet asphyxia model (18–20). We further hypothesized that reoxygenation with 100% O2 after asphyxia would affect the blood oxidative stress indicators, early neurologic outcome, and cerebral histopathology of the animals, and the data were therefore compared with those obtained on SHAM and R21 newborn piglets.
METHODS
Surgical preparation and experimental protocol.
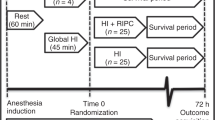
The present study was performed on 26 piglets from the Agriculturist Cooperative, Szeged, Hungary, which were between 3 and 6 h old at the beginning of the experiments and weighed 1.12 to 1.42 kg. The animals were anesthetized with ketamine hydrochloride (Ketanest, Parke-Davis, Morris Plains, NJ, U.S.A.; 10 mg/kg body weight intramuscularly). Figure 1 provides an outline of the experimental protocol. The animals were immobilized in the supine position, a tracheotomy was performed (local anesthesia, lidocaine hydrochloride, EGIS Ltd., Budapest, Hungary; 1.0 mL, 1.0 vol% s.c.), the animals were intubated (2.5–3.0 tubes, Portex Ltd, Hythe, Kent, U.K.), and one of the umbilical arteries was cannulated. The newborn piglets were divided into three experimental groups. SHAM newborn piglets (n = 6, three males and three females) served as controls: only Ketanest and local chest skin anesthesia (lidocaine hydrochloride, EGIS Ltd, to both sides) were applied, and no intrapleural air was insufflated. In 20 newborn piglets, after skin anesthesia, room air was insufflated through chest tubes inserted into the pleural cavities, and pneumothorax was produced by a constant air flow from a high-pressure room air cylinder (21). The clinical status of the animals worsened continuously, and by the end of 1 h after the induction of pneumothorax, gasping, bradyarrhythmia, arterial hypotension, hypoxemia, and severe combined acidosis had developed. The pneumothorax was then relieved, and the response to therapy was controlled by transillumination (Wild M4000 fiberscope, Heerburgg, Switzerland) of the thorax (18, 22, 23). Asphyxiated animals were randomly divided into two groups. Ten piglets (five males and five females) were artificially ventilated and reoxygenated with room air (Fio2 0.21) for 10 min (group R21), whereas the others (n = 10, five males and five females) were ventilated with 100% O2 (Fio2 1.0) for 10 min (group R100). A conventional, constant-volume, pressure-limited infant respirator (MTA-KUTESZ, Budapest, Hungary) was used to ventilate the involved animals on the basis of our previous laboratory practice (24). Ventilator settings were as follows: tidal volume, 10–16 mL; frequency, 40 breaths/min; inspiratory time, 0.75 s; peak inspiratory pressure, 1.18–1.48 kPa (12–15 cm H2O); and, with the avoidance of adverse effects (e.g. increased intracranial pressure and production of antidiuretic hormone), end-expiratory pressure, 0 kPa. The spontaneously breathing animals subsequently remained in room air (recovery phase) and were followed up to the end (240 min from the start) of the experiments. Rectal temperature was kept between 38 and 39°C; heart rate and mean arterial blood pressure were monitored continuously.
Experimental protocol of pneumothorax-evoked asphyxia with reoxygenation either with room air—R21 group—or 100% O2—R100 group—and SHAM group animals. A, bilateral pneumothorax, spontaneous breathing in room air;B1, mechanical ventilation with 21% O2;B2, mechanical ventilation with 100% O2;C, recovery, spontaneous breathing in room air;D, spontaneous breathing in room air;E, neurologic and cerebral histopathologic examination;BA, baseline;AS, asphyxia;MV5, 5 min of mechanical ventilation;MV10, 10 min of mechanical ventilation;RC1, 120 min after BA;RC2, 240 min after BA; * indicates time points of blood sampling.
Blood samples and measurements of blood oxidative stress indicators.
Arterial blood samples for gas, acid-base, and oxidative stress indicator analyses were drawn at different times (baseline, asphyxial, during mechanical ventilation, and recovery) as specified in Figure 1, according to standard methods. The withdrawn blood was replaced with a double volume of 0.9 vol% NaCl (maximal saline vol, 12 mL/animal). The laboratory methods used (briefly) were as follows. Glutathione assay: A highly sensitive and specific method was used (25). Twenty-five microliters of whole blood anticoagulated with EDTA was immediately hemolyzed in 2.5 mL of cold (4°C) 0.01 M potassium phosphate buffer containing 5 mM EDTA (pH 7.5) and stored at −70°C until further use. For analysis, 25 μL of hemolysate was added to the standard glutathione assay mixture (final volume = 1.0 mL) in the following sequence: 5,5′-dithio-bis-2-nitrobenzoic acid (0.6 μM), glutathione reductase (10 μg), and NADPH (0.2 μM). The combined action of 5,5′-dithio-bis-2-nitrobenzoic acid and NADPH in the presence of glutathione reductase results in a reaction cycle, the rate of which depends on the total concentration of reduced glutathione and oxidized glutathione recorded spectrophotometrically at 412 nm during the first 6 min. The concentrations of thiols were expressed as the oxidized glutathione to reduced glutathione ratio with reference to Hb determined by the cyanmethemoglobin method. Plasma Hb assay: Heparinized plasma samples were diluted 1:40 (vol/vol) with 5 mM PBS (pH 7.4) and measured spectrophotometrically (26). Plasma malondialdehyde assay: Heparinized plasma samples were hydrolyzed in dilute phosphoric acid (0.22 M). Malondialdehyde was reacted with thiobarbituric acid (7 mM) to form malondialdehyde-thiobarbituric acid adduct. After methanol precipitation of proteins, the amount of adduct formed was quantified by HPLC (Waters-Millipore Corp., Milford, MA, U.S.A.) and by spectrophotometric detection at 532 nm (Variable Wavelength Monitor, Pharmacia LKB, Uppsala, Sweden) (27).
Piglet neurologic examination.
Neurologic examinations were performed by a blinded observer at 240 min from the start of the experiments (Fig. 1). The results were recorded and scored from 5 to 20, with 20 corresponding to normal and 5 to brain-dead, according to a standard scoring system (28) (Table 1).
Cerebral histopathology.
Newborn piglets were again anesthetized with Ketanest (20 mg/kg body weight, intramuscularly), the right atrium was punctured with a 12-gauge needle, and brains were perfused and fixed through the arterial catheter with 0.9 vol% NaCl (0.25 L/kg body weight), followed by phosphate-buffered formaldehyde (4.0 vol%, 0.15 L/kg body weight). The brain was next removed and preserved for later pathologic examination. Different paraffin sections were taken, stained with hematoxylin and eosin, and examined by light microscopy. The following regions were examined by one of the authors (I.B.), who was not informed of the mode of treatment or of the clinical information: frontal and temporal cortex, cerebellum, hippocampus, basal ganglia, and pons. The extent of damage for each region was graded as described previously (28), with minor modifications (Table 2). The hypoxic cellular change primarily involved largely shrunken hyperchromatic neurons with pyknotic nuclei; the scores were 5 to 1, depending on the proportions of damaged to spared cells.
Statistics.
All values are given as mean ± SD. The results of the neurologic and cerebral histopathologic examinations are also expressed as median values (25th and 75th percentiles). The examinations on the SHAM group were performed to investigate the stability of the preparation, and the results were included in the statistical analysis. The nonparametric Kruskal-Wallis analysis was followed by the Mann-Whitney U test to determine significant differences among groups. All analyses were performed with a statistical computer program (Statgraphics, Statistical Graphics Co., Englewood Cliffs, NJ, U.S.A.); and p values <0.05 were considered to be significant.
Approval.
The experimental procedures were performed in accordance with the prescriptions of the National Institutes of Health for the care and use of laboratory animals, and were approved by the Ethical Committee on Animal Investigation, Medical University, Szeged, Hungary (ATB 90).
RESULTS
There were no significant differences in age (4.5 ± 0.3, 4.2 ± 0.3, and 4.6 ± 0.2 h) or in body weight (1283 ± 56, 1326 ± 46, and 1329 ± 37 g) among the SHAM and the asphyxiated groups (R21 and R100, respectively), and no difference in pneumothorax time (62 ± 6 and 65 ± 7 min) between R21 and R100 groups, respectively. The three groups were also comparable with regard to the measures at baseline.
Cardiovascular, blood gas, and acid-base measurements.
Mean arterial blood pressure was decreased significantly in both pneumothorax groups (R21 and R100) at asphyxia, but was then significantly elevated both at 5 min and at 10 min during mechanical ventilation. Later, during the 180-min recovery phase (120 and 240 min after baseline), significant hypotension was observed (R21 and R100) as compared with both the baseline values and the data measured in the SHAM group (Fig. 2). Heart rate was decreased significantly at asphyxia in both pneumothorax groups (R21 and R100); it increased temporarily during mechanical ventilation, but remained significantly decreased during recovery as compared with both the baseline values and the data from the SHAM group (Fig. 3). Arterial pH was decreased significantly at asphyxia in both pneumothorax groups (R21 and R100); it then increased continuously during recovery, but it remained significantly decreased as compared with both the baseline values and those of the SHAM group (Fig. 4). Arterial Pco2 was increased significantly at asphyxia in both pneumothorax groups (R21 and R100), decreased significantly during mechanical ventilation, but remained significantly higher as compared with both the baseline values and those of the SHAM group during recovery (Fig. 5). Base deficit was increased significantly at asphyxia in both pneumothorax groups (R21 and R100), decreased significantly during mechanical ventilation and recovery, but remained significantly higher as compared with both the baseline values and the data from the SHAM group (Fig. 6). Arterial Po2 was decreased significantly at asphyxia in both pneumothorax groups (R21 and R100), and then increased significantly during mechanical ventilation. The values measured in the R100 group were significantly higher after both 5 and 10 min of mechanical ventilation as compared with the data from the R21 group. During recovery, arterial Po2 decreased significantly again as compared with both the baseline values and those during mechanical ventilation and the data from the SHAM group (Fig. 7). There were no significant differences between the two pneumothorax groups (R21 and R100) as regards any of the measurements followed during the study (except arterial Po2 during mechanical ventilation).
Mean arterial blood pressure (mm Hg) of the experimental animals. a, p < 0.05 vs baseline within the group;b, p < 0.05 vs R21 and SHAM;c, p < 0.05 vs R100 and SHAM. Values are mean ± SD. Abbreviations as in Figure 1.
Heart rate (beats/min) of the experimental animals. a, p < 0.05 vs baseline within the group;b, p < 0.05 vs R21 and SHAM;c, p < 0.05 vs R100 and SHAM. Values are mean ± SD. Abbreviations as in Figure 1.
Arterial blood pH of the experimental animals. a, p < 0.05 vs baseline within the group;b, p < 0.05 vs R21 and SHAM;c, p < 0.05 vs R100 and SHAM. Values are mean ± SD. Abbreviations as in Figure 1.
Arterial blood Pco2 (kPa) of the experimental animals. a, p < 0.05 vs baseline within the group;b, p < 0.05 vs R21 and SHAM;c, p < 0.05 vs R100 and SHAM. Values are mean ± SD. Abbreviations as in Figure 1.
Arterial blood base deficit (mM) of the experimental animals. a, p < 0.05 vs baseline within the group;b, p < 0.05 vs R21 and SHAM;c, p < 0.05 vs R100 and SHAM. Values are mean ± SD. Abbreviations as in Figure 1.
Arterial blood Po2 (kPa) of the experimental animals. a, p < 0.05 vs baseline within the group;b, p < 0.05 vs R21 and SHAM;c, p < 0.05 vs R100 and SHAM. Values are mean ± SD. Abbreviations as in Figure 1.
Blood oxidative stress measurements.
There were no significant differences either within or among the three groups during the study (Table 3).
Piglet neurologic examination.
Results of the neurologic examinations are shown in Table 4. Neurologic examination scores were significantly lower in both pneumothorax groups (R21 and R100) as compared with the score for the SHAM group. Moreover, the score for the R100 group was significantly lower than that for the R21 group.
Cerebral histopathology.
Histopathologic examination scores are shown in Table 5. The results on the SHAM piglets (not subjected to pneumothorax) were significantly higher (except for the region of the pons) than those on the asphyxiated groups (R21 and R100). Marked microscopic neuronal damage (Fig. 8) was seen in the newborn piglets with asphyxia, without any significant differences between the two groups (R21 and R100). No significant damage was observed in the pons without any significant differences between the two groups (R21 and R100). No significant damage was found in the pons.
High power view of Grade 2 damage (ratio of damaged to spared neurons = 33–66%) in the temporal cortex of an asphyxiated animal reoxygenated with 100% O2 (R100 group). The damaged neurons appear as shrunken, hyperchromatic and eosinophilic-staining cells in contrast to the lightly stained normal neurons.
DISCUSSION
The present study on a clinically relevant animal model confirms previous findings relating to piglets (9) and human newborns (16, 29) that room air and 100% O2 are equally effective in normalizing cardiorespiratory, blood gas, and acid-base abnormalities during early recovery in newborns with asphyxia. The measured cardiovascular, blood gas, and acid-base values revealed the well-known characteristics (bradyarrhythmia, arterial hypotension followed by significant hypertension, then mild hypotension, hypoxemia, and combined acidosis) routinely seen in clinical practice during or after asphyxia in human newborns. Animals treated with 100% O2 had significantly higher, although clinically still normoxic arterial Po2 values during postasphyxial mechanical ventilation as compared with the data from the room air-treated, relatively hypoxemic group. Furthermore, it emerged that a brief period (10 min) of reoxygenation with 100% O2 might have some influence on cerebral damage, as piglets treated with room air had a significantly better neurologic score, predicting a more favorable early neurologic outcome, as compared with animals receiving 100% O2 ventilation. It seems that normoxia after cerebral hypoxia augments brain injury in the present model, whereas a lower arterial Po2 is neuroprotective. Meanwhile, higher arterial Po2 values during mechanical ventilation had no significant effects on the blood oxidative stress indicator values measured during the study and led to microscopic cerebral histopathologic alterations of similar severity in both asphyxiated groups at the end point of the experiments.
The experimental model of pneumothorax was chosen to evoke severe asphyxia because both laboratory (7, 18–20) and clinical (30, 31) data published previously confirmed a strong association between the neonatal air-leak syndrome and cerebral damage. Therefore, it is not surprising that we observed significant cerebral damage in both groups of asphyxiated animals. Moreover, pneumothorax is far more frequent in newborns than in any other period of life (23, 32, 33), and the brain of the newborn piglet is comparable both histologically (34) and electrophysiologically (35) with that of human infants at 36–38 wk of gestation.
The experimental protocol followed in this study was set up on the basis of our extensive laboratory practice during a period of 20 y, documenting evidence that pneumothorax-asphyxiated piglets are able to survive the asphyxiation load (for up to 4 h) without further mechanical respiratory support (7, 36). Newborns should be ventilated as long as necessary to obtain reasonable blood gas values, avoiding lung and other organ injury as much as possible. As the arterial gas values measured 10 min postasphyxially were very close to those reported to be clinically acceptable (37), the piglets were weaned from the respirator and reventilation was not introduced. The study substance oxygen was not further supplemented. As the blood glucose levels proved to be sufficiently maintained postasphyxially (38) and as most routinely used drugs, e.g. sodium bicarbonate, colloids, vasopressors, or atropine, could have potential deleterious effects on the CNS in newborns (39, 40), no parenteral medication was given, except normal saline. Similarly, as in another study published recently (41), no additional attempt was made to correct the systemic hypotension or the metabolic acidosis in postasphyxic piglets. In spite of the random allocation, mean arterial blood pressure measured at the start of mechanical ventilation in piglets reoxygenated with 100% O2 was slightly lower than that in animals treated with room air; however, all of them had values higher than 35 mm Hg, which is the critical level for well-maintained cerebral perfusion in newborn piglets (42, 43). It is worth mentioning that there was no correlation between mean arterial blood pressure and neurologic score in any of the groups. This is not surprising, as cerebral blood flow was found to be independent of mean arterial blood pressure in preterm infants undergoing intensive care, when they were able to maintain adequate cerebral perfusion at a mean arterial blood pressure in the range 23.7–39.3 mm Hg (39). As a widely accepted drug (44–46) with more favorable cardiovascular effects than those of inhalation drugs in piglets (35), ketamine was used in our experiments as part of the anesthesia. Although it is known from the literature that ketamine could offer some neuroprotective effects as a noncompetitive antagonist of the N-methyl-d-aspartate receptor, all animals received the same anesthetic, which makes all the results comparable.
As the major finding of a better early neurologic outcome after room air reoxygenation is of important clinical relevance, this observation requires further explanation. Increasing Fio2 levels facilitates posthypoxic cerebral cortical hyperoxia (47) and results not only in cerebral, but also in systemic, oxidative damage (48). Although the blood oxidative stress indicator levels in our study did not reveal any significant liberation of potentially highly damaging mediators, on the basis of previously published observations (12, 49) (as there were no other significant differences between the two asphyxiated groups apart from the arterial Po2 values) we presume the deleterious effects of hyperoxia on the neuronal cell membranes were caused either by increasing cerebral dopamine concentration (50, 51) or by other lipid peroxidation products (52–55) not measured here. It also seems likely that the oxidative stress remained localized within the CNS, and the elevations in the biochemical markers become lost on dilution in the plasma (56). These substances might disturb the most complex neuronal membrane transport processes, which are regulated by the very sensitive enzyme Na+/K+ ATPase. The function of the enzyme is critical in maintaining intra- and extracellular ion concentrations in the brain. A significant decrease in its activity, leading to an influx of sodium and calcium into the cell, accompanied by water, resulting in cytotoxic cellular edema, was experienced in both the cortex (49) and the striatum (12) in piglets treated with 100% O2. Moreover, a highly significant production of reactive oxygen species was recently demonstrated intravitally, in asphyxiated newborn piglets likewise resuscitated with 100% O2 for 10 min (57), and concentrations of both conjugated dienes and fluorescent compounds were significantly elevated in the cerebral cortex of asphyxiated piglets (52, 58) with almost identical arterial Po2 values (approximately 13.0 kPa) to those we measured in animals ventilated with 100% O2. Although the arterial Po2 values in our R100 group did not significantly exceed the values measured in the SHAM group, both systemic xanthine oxidoreductase conversion (54) and the accumulation of purine metabolites (5–7, 9, 13, 19, 54) must have taken place in the R100 animals during the asphyxic period with global hypoxia-ischemia, which was not present in the SHAM animals without asphyxia. It is also worth emphasizing that both the cardiac morphology (59) and the cerebral circulation (60) are almost identical in human newborns and piglets. The preductal (cerebral) arterial Po2 might therefore have been significantly higher, which we were able to measure in blood taken from the umbilical artery, owing to a functional right-to-left shunt through the patent ductus arteriosus. The observed phenomenon that the Po2 values measured in R100 animals were not different from those for the SHAM animals could have resulted from both intrapulmonary and intracardiac right-to-left shunts caused by severe pneumothorax-evoked pulmonary hypertension (61). Microscopic neuronal lesions might therefore have developed during the profound hypoxemia-ischemia, which were very similar in severity in both groups with asphyxia, whereas functional neurologic disturbances might have originated from postasphyxial events, with less serious handicaps in R21 piglets. The lower degree of damage observed in the pons in the asphyxiated animals might have originated from the better-maintained blood flow and oxygenation of the brain stem structures as compared with those of other brain regions during asphyxia. Moreover, a cerebral intraparenchymal sodium accumulation was demonstrated earlier in our neonatal pneumothorax model both in vitro(18, 62) and in vivo(19, 63).
As an exceptional laboratory finding, pial-arachnoidal arterial micro-air embolization was previously demonstrated in our piglet model. Because this cerebral vascular damage was in each case accompanied by rapidly occurring visible morphologic alterations, i.e. diffuse cortical bleeding (64), it can be excluded with certainty from taking part in the present set of experiments, as the cerebral histopathology in numerous regions of the perfused and fixed piglet brains did not reveal any concomitant alterations anywhere within the CNS.
In conclusion, this study suggests that normalization of the neonatal cardiorespiratory status after pneumothorax-evoked asphyxia is just as efficient when reoxygenation is performed with room air as it is with the often-recommended 100% O2. Although there were no significant differences as regards blood oxidative stress measuremtns and cerebral histopathologic damage between the two reoxygenation modalities (room air or 100% O2), reoxygenation with 100% O2 seems to impair the early neurologic outcome, whereas relative hypoxemia is neuroprotective in asphyxiated newborns. Further research is necessary to reveal the causative mechanisms. We suggest that during the treatment of newborn infants, especially after an asphyxic episode, inspiratory oxygen levels should be minimized by using the lowest necessary increase in Fio2 above that in normal air.
Abbreviations
- R21:
-
reoxygenation with room air
- R100:
-
reoxygenation with 100% oxygen
- SHAM:
-
sham-operated
- Fio2:
-
fraction of O2 in the inspired gas
References
World Health Organization 1991 Child Health and Development: Health of the Newborn. Geneva, Switzerland, World Health Organization
Milner AD 1991 Resuscitation of the newborn. Arch Dis Child 66: 66–69
Emergency Cardiac Care Committee and Subcommittees, American Heart Association 1992 Guidelines for cardiopulmonary resuscitation and emergency cardiac care. Part VII. Neonatal resuscitation. JAMA 268: 2276–2281
Saugstad OD 1990 Oxygen toxicity in the neonatal period. Acta Paediatr Scand 79: 881–892
Saugstad OD, Aasen AO 1980 Plasma hypoxanthine concentration in pigs: a prognostic aid in hypoxia. Eur Surg Res 12: 123–129
Saugstad OD 1996 Role of xanthine oxidase and its inhibitor in hypoxia: reoxygenation study. Pediatrics 98: 103–107
Temesvári P, Ábrahám C, Kovács J, Baranyai ZS 1991 Experimental pneumothorax increases hypoxanthine concentration in vitreous humor of newborn piglets. Acta Paediatr Scand 80: 472–473
Shadid M, Moison R, Steendijk P, Hiltermann L, Berger HM, van Bel F 1998 The effect of antioxidative combination therapy on post hypoxic-ischemic perfusion, metabolism, and electrical activity of the newborn brain. Pediatr Res 44: 119–124
Rootwelt T, Loberg EM, Moen A, Oyasaeter S, Saugstad OD 1992 Hypoxemia and reoxygenation with 21% or 100% oxygen in newborn pigs: changes in blood pressure, base deficit, and hypoxanthine and brain morphology. Pediatr Res 32: 107–113
Rootwelt T, Odden JP, Hall C, Ganes T, Saugstad OD 1993 Cerebral blood flow and evoked potentials during reoxygenation with 21% or 100% O2 in newborn pigs. J Appl Physiol 75: 2054–2060
Zwemer CF, Whitesall SE, D Alecy LG 1994 Cardiopulmonary-cerebral resuscitation with 100% oxygen exacerbates neurological dysfunction following nine minutes of normothermic cardiac arrest in dogs. Resuscitation 27: 159–170
Goplerud JM, Kim S, Delivoria Papadopoulos M 1995 The effect of post-asphyxial reoxygenation with 21% or 100% O2 on Na,K ATPase activity in striatum of newborn piglets. Brain Res 696: 161–164
Feet BA, Yu X, Rootwelt T, Oyasaeter S, Saugstad OD 1997 Effects of hypoxemia and reoxygenation with 21% or 100% O2 in newborn piglets: extracellular hypoxanthine in cerebral cortex and femoral muscle. Crit Care Med 25: 1384–1391
Orandacova J, Marsala M, Marsala J 1991 The blood-brain barrier permeability in graded postischemic spinal cord reoxygenation in rabbits. Neurosci Lett 128: 143–146
Fercakova A, Marsala M, Marsala J 1994 Influence of graded postischemic reoxygenation on reperfusion alterations in rabbit dorsal root ganglion neurons. J Hirnforsch 35: 295–302
Saugstad OD, Rootwelt T, Aalen O 1998 Resuscitation of asphyxiated newborn infants with room air or oxygen: an international controlled trial, the Resair 2 Study. Pediatrics 102: e1–e7
Stahlman M, Gray J, Young WC, Shepard FM 1967 Cardiovascular response of the neonatal lamb to hypoxia and hypercapnia. Am J Physiol 213: 899–904
Temesvári P, Hencz P, Joó F, Eck E, Szerdahelyi P, Boda D 1984 Modulation of the blood-brain barrier permeability in neonatal cytotoxic brain edema: laboratory and morphological findings obtained on newborn piglets with experimental pneumothorax. Biol Neonate 46: 198–208
Temesvári P, Ábrahám CS, Joó F, Kovács J, Baranyai ZS, Rácz K 1990 Disturbed brain purine metabolism results in a gross opening of the blood-brain barrier in newborn piglets following experimental pneumothorax. Neurosci Lett 113: 163–168
Temesvári P, Joó F, Kovács J, Ábrahám CS 1995 Ischemia-reperfusion-induced alteration of blood-brain barrier transport in newborn pigs. Am J Physiol 269: H750–H751
Vásárhelyi B, Dobos M, Temesvári P, Ábrahám CS, Pintér S, Tulassay T 1998 Postasphyxial reoxygenation reduces the activity of Na/K-ATPase in the erythrocytes of newborn piglets. Biol Neonate 74: 445–450
Kuhns LR, Bednarek FJ, Wyman ML, Roloff DW, Borer RC 1975 Diagnosis of pneumothorax or pneumomediastinum in the neonate by transillumination. Pediatrics 56: 355–360
McIntosh N, Becher JC, Cunningham S, Stenson B, Laing IA, Lyon AJ, Badger P 2000 Clinical diagnosis of pneumothorax is late: use of trend data and decision support might allow preclinical detection. Pediatr Res 48: 408–415
Temesvári P, Ábrahám CS, Speer CP, Kovács J, Megyeri P 1993 Escherichia coli O111 B4 lipopolysaccharide given intracisternally induces blood-brain barrier opening during experimental meningitis in piglets. Pediatr Res 34: 182–186
Németh I, Boda D 1994 Blood glutathione redox ratio as a parameter of oxidative stress in premature infants with IRDS. Free Radic Biol Med 16: 347–353
Winterbourn CC 1979 Oxidative reactions of hemoglobin. Method Enzymol 186: 265–272
Wong SHY, Knight JA, Hopfer SM, Zacaria O, Leach CN Jr Sunderman FW Jr 1987 Lipoperoxides in plasma as measured by liquid chromatographic separation of malondialdehyde-thiobarbituric acid adduct. Clin Chem 33: 214–220
LeBlanc MH, Li XQ, Huang M, Patel DM, Smith EE 1995 AMPA antagonist LY293558 does not affect the severity of hypoxic-ischemic injury in newborn pigs. Stroke 26: 1908–1915
Ramji S, Ahula S, Thirupuram S, Rootwelt T, Rooth G, Saugstad OD 1993 Resuscitation of asphyxic newborn infants with room air or 100% oxygen. Pediatr Res 34: 809–812
Hill A, Perlman JM, Volpe JJ 1982 Relationship of pneumothorax to occurrence of intraventricular hemorrhage in the premature newborn. Pediatrics 69: 144–149
Volpe JJ 1995 Neurology of the Newborn, 3rd Ed. WB Saunders, Philadelphia, 490–514.
Wood B, Dubik M 1995 A new device for pleural drainage in newborn infants. Pediatrics 96: 955–956
Avery GB, Fletcher MA, MacDonald MG 1999 Neonatology: Pathophysiology and Management of the Newborn. Lippincott Williams & Wilkins, Philadelphia, 506–508.
Laptook A, Stonestreet BS, Oh W 1982 The effects of different rates of plasmanate infusions upon brain blood flow after asphyxia and hypotension in newborn piglets. J Pediatr 100: 791–796
Thoresen M, Haaland K, Loberg EM, Whitelaw A, Apricena F, Hanko E, Steen PA 1996 A piglet survival model of posthypoxic encephalopathy. Pediatr Res 40: 738–748
Temesvári P, Joó F, Koltai M, Eck E, Ádám G, Siklós L, Boda D 1984 Cerebroprotective effect of dexamethasone by increasing the tolerance to hypoxia and preventing brain oedema in newborn piglets with experimental pneumothorax. Neurosci Lett 49: 87–92
Avery GB, Fletcher MA, MacDonald MG 1999 Neonatology: Pathophysiology and Management of the Newborn. Lippincott Williams & Wilkins, Philadelphia, 451–454.
Ábrahám CS, Temesvári P, Kovács J, Schulz K, Molnár D 1996 Plasma and cerebrospinal fluid hyperinsulinism in asphyxiated piglets. Biol Neonate 70: 296–303
Tyszszuk L, Meek J, Elwell C, Wyatt JS 1998 Cerebral blood flow is independent of mean arterial blood pressure in preterm infants undergoing intensive care. Pediatrics 102: 337–341
Leuthner SR, Jansen RD, Hageman JR 1994 Cardiopulmonary resuscitation of the newborn: an update. Pediatr Clin North Am 41: 893–907
Pourcyrous M, Parfenova H, Bada HS, Korenes SB, Leffler CW 1997 Changes in cerebral cyclic nucleotides and cerebral blood flow during prolonged asphyxia and recovery in newborn pigs. Pediatr Res 41: 617–623
Edison TH, Edrington JL, Alburquerque SL, Zuckerman SL, Leffler CW 1994 Light/dye microvascular injury eliminates pial arteriolar dilation in hypotensive piglets. Pediatr Res 37: 10–14
Yonetani M, Huang CH, McGowan J, Lajevardi N, Pastuszko A, Delivoria-Papadopoulos M, Wilson DF 1994 Effect of hemorrhagic hypotension on extracellular level of dopamine, cortical oxygen pressure and blood flow in brain of newborn piglets. Neurosci Lett 180: 247–252
Meadow WL, Rudinsky BF, Strates E, Komar KJ 1987 Oxygen delivery, oxygen consumption, and metabolic acidosis during group B streptococcal sepsis in piglets. Pediatr Res 22: 509–512
Armstead WM, Kurth CD 1994 The role of opioids in newborn pig fluid percussion brain injury. Brain Res 660: 19–26
Wilderman MJ, Armstead WM 1997 Role of neuronal NO synthase in relationship between NO and opioids in hypoxia-induced pial artery dilation. Am J Physiol 273: H1807–H815
Tammela OKT, Lajevardi N, Huang CC, Wilson DF, Delivoria-Papadopoulos M, Pastuszko A 1996 The effect of induced apneic episodes on cerebral cortical oxygenation in newborn piglets. Brain Res 741: 160–165
Matsumoto F, Sakai H, Yamaguchi M, Nakano H, Matsumiya A, Kumada K, Yoshida K, Shimura H, Machida H, Takeuchi S, Sasaya S, Midorikawa T, Sanada Y 1997 Allopurinol reduces hepatic ischemia-reperfusion injury exacerbated by inhalation of high-concentration oxygen in rats. Eur Surg Res 29: 429–437
Goplerud JM, Mishra OP, Delivoria-Papadopoulos M 1993 Brain cell membrane dysfunction following acute asphyxia in newborn piglets. Biol Neonate 61: 33–41
Huang CC, Yonetani M, Lajevardi N, Delivoria-Papadopoulos M, Wilson D, Pasztuszko A 1995 Comparison of postasphyxial resuscitation with 100% and 21% oxygen on cortical oxygen pressure and striatal dopamine metabolism in newborn piglets. J Neurochem 64: 292–298
Blennow M, Zeman J, Dahlin I, Lagercrantz H 1995 Monoamine neurotransmitters and metabolites in the cerebrospinal fluid following perinatal asphyxia. Biol Neonate 67: 407–413
Schneiderman R, Kubin J, Mishra OP, Delivoria-Papadopoulos M 1994 Brain cell membrane modification following hypercapnia and recovery in newborn piglets. Pediatr Pulmonol 18: 81–88
Pourcyrous M, Leffler CW, Busija DW 1990 Role of prostanoids in cerebrovascular response to asphyxia and reventilation in newborn pigs. Am J Physiol 259: H662–H667
Fellman V, Raivio KO 1997 Reperfusion injury as the mechanism of brain damage after perinatal asphyxia. Pediatr Res 41: 599–606
Groenendaal F, Mishra OP, McGowan JE, Hoffman DJ, Delivoria-Papadopoulos M 1997 Function of cell membranes in cerebral cortical tissue of newborn piglets after hypoxia and inhibition of nitric oxide synthase. Pediatr Res 42: 174–179
Winterbourn CC, Chan T, Buss IH, Inder TE, Mogridge N, Darlow BA 2000 Protein carbonyls and lipid peroxidation products as oxidation markers in preterm infant plasma: associations with chronic lung disease and retinopathy and effects of selenium supplementation. Pediatr Res 48: 84–90
Kondo M, Itoh S, Isobe K, Kondo M, Kunikata T, Imai T, Onishi S 2000 Chemiluminescence because of the production of reactive oxygen species in the lungs of newborn piglets during resuscitation periods after asphyxiation load. Pediatr Res 47: 524–527
Goplerud JM, Mishra OP, Delivoria-Papadopoulos M 1992 Brain cell membrane dysfunction following acute asphyxia in newborn piglets. Biol Neonate 61: 33–41
Haworth SG, Hislop AA 1981 Adaptation of the pulmonary circulation to extrauterine life in the pig and its relevance to the human infant. Cardiovasc Res 15: 108–119
Haaland K, Orderud WJ, Thoresen M 1995 The piglet as a model for cerebral circulation: an angiographic study. Biol Neonate 68: 75–80
Medbo S, Yu XQ, Asberg A, Saugstad OD 1998 Pulmonary hemodynamics and plasma endothelin-1 during hypoxemia and reoxygenation with room air or 100% oxygen in a piglet model. Pediatr Res 44: 843–849
Ábrahám CS, Deli M, Temesvári P, Kovács J, Szerdahelyi P, Joó F, Torpier G 1999 Regional differences in asphyxia and reperfusion-induced cytotoxic and vasogenic brain edema formation in newborn pigs. Neurosci Res Commun 25: 173–182
Temesvári P, Kovács J 1988 Selective opening of the blood-brain barrier in newborn piglets with experimental pneumothorax. Neurosci Lett 93: 38–43
Temesvári P, Kovács J, Ábrahám CS 1996 Pneumothorax and neonatal stroke. Neuropediatrics 27: 167–168
Acknowledgements
The authors thank Ágota Fábián Nagy, Ilona Szécsi, and Ildikó Wellinger for their skillful technical assistance and for the analysis of blood samples, and Dr. Róbert Zánthó for revising the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the Hungarian Ministry of Health (Budapest, Hungary, grant ETT-80-4/1998), the Hungarian Research Council for Science (Budapest, Hungary, grant T 017111 and T 026295), and the Alexander von Humboldt Foundation (Bonn, Germany, grant V-8100).
Rights and permissions
About this article
Cite this article
Temesvári, P., Karg, E., Bódi, I. et al. Impaired Early Neurologic Outcome in Newborn Piglets Reoxygenated with 100% Oxygen Compared with Room Air after Pneumothorax-Induced Asphyxia. Pediatr Res 49, 812–819 (2001). https://doi.org/10.1203/00006450-200106000-00017
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-200106000-00017
This article is cited by
-
Versorgung und Reanimation des Neugeborenen nach der Geburt
Notfall + Rettungsmedizin (2021)
-
Normoxic post-ROSC ventilation delays hippocampal CA1 neurodegeneration in a rat cardiac arrest model, but does not prevent it
Experimental Brain Research (2020)
-
aEEG and neurologic exam findings correlate with hypoxic–ischemic brain damage severity in a piglet survival model
Pediatric Research (2019)
-
Die Versorgung und Reanimation des Neugeborenen
Notfall + Rettungsmedizin (2015)
-
Decreased GABAB receptor function in the cerebellum and brain stem of hypoxic neonatal rats: Role of glucose, oxygen and epinephrine resuscitation
Journal of Biomedical Science (2011)