Abstract
The objective of the present study was to explore whether a free radical spin trap agent, α-phenyl-N-tert-butyl nitrone (PBN), influences bioenergetic failure induced in the 20-day-old fetal brain by 30 min of intrauterine ischemia in Wistar rats. Fetal brains were frozen in situ at the end of ischemia and after 1, 2, and 4 h of recirculation for analysis of ATP, ADP, AMP, and lactate. PBN or vehicle was given 1 h after recirculation. Tissue oxygen tension was evaluated in placental and fetal cerebral tissues throughout the whole periods of 30 min of ischemia and 4 h of recirculation. Ischemia was associated with a decrease in ATP concentration and an increase in lactate concentration (p < 0.001). Recirculation (1 and 2 h) led to a recovery of ATP concentration, but continued reflow (4 h) was associated with a secondary deterioration of high-energy phosphates (p < 0.01). Lactate concentration increased during this recovery period. This deterioration was prevented by PBN (p < 0.05). After 30 min of ischemia, tissue oxygen tension in placenta and fetal brain decreased to about 30% and 50% of control, respectively. However, recirculation brought about a recovery of oxygen delivery. The results indicate that although during the early time period after ischemia fetal cerebral energy metabolism is maintained by an acceleration of the anaerobic glycolytic rate, secondary deterioration of cellular bioenergetic state develops in the immature fetal brain. This deterioration may be due to mitochondrial dysfunction, which may be induced by oxygen-derived free radicals, and not by compromised microcirculation.
Similar content being viewed by others
Main
Maternal hypertension, chronic renovascular diseases, and several other conditions, which are associated with reduced uterine blood flow during pregnancy, appear to be important pathophysiologic events in the mechanism of injury to the immature fetal brain (1–3). Indeed, an increasing number of clinical reports suggest that much of the neurologic deficit apparent in neonates can be attributed to prenatal hypoxic-ischemic events (4, 5).
Intrauterine oxygen shortage leads to anaerobic fetal metabolism. Due to an increase of cerebral blood flow (6) and preferential streaming of well-oxygenated blood in the heart (7), the fetal cerebrum is provided with as much oxygen as conditions allow. However, because in immature fetuses circulatory centralization may be in part ineffective, the brain too resorts to anaerobic metabolism during severe or sustained hypoxia (8). Lack of oxygen impairs mitochondrial function, leading to energy shortage (9, 10);i.e. ATP becomes degraded to AMP and, after dephosphorylation, and deamination, further to adenosine, inosine, and hypoxanthine. Moreover, formation of hypoxanthine and xanthine may aggravate outcome after such an insult, because during reoxygenation hypoxanthine and xanthine act as substrate for the enzyme xanthine oxidase, yielding reactive oxygen species and free radical-mediated damage (11).
Evidence is accumulating in adult (12, 13) and neonatal (14, 15) brain ischemia models that the post-ischemic reperfusion period may be of major pathogenetic importance. It has subsequently been confirmed that ischemia-reperfusion in other species, including the rat, leads to production of free radicals (16). These reactive oxygen species and their product, lipid peroxides, are thought to be among the important causes of cell membrane destruction and cell damage (10, 17, 18). In addition, free radical scavengers such as dimetylthiourea and allopurinol (19), as well as the enzymes superoxide dismutase and catalase (20, 21) have been shown to ameliorate the ischemic brain damage. Our recent results obtained from adult rats also demonstrated that α-phenyl-N-tert-butyl-nitrone (PBN), a free radical spin trap, reduces the infarct size (22, 23) and prevents the secondary energy failure (24) and mitochondrial dysfunction (25), which otherwise occur after focal ischemia. PBN also ameliorates the pan-necrotic lesion of the substantia nigra, pars reticulata (SNPR), which is incurred in rats subjected to status epilepticus of more than 30 min duration (26).
In the fetus, however, relatively little is known about the effect of antioxidants and the metabolic conditions in brain during and after a prolonged period of hypoxia-ischemia. In the present experiments, we sought to assess whether there is primary or secondary energy failure in the immature fetal brain during intrauterine ischemia-reperfusion and whether PBN ameliorates the subsequent damage. Because this proved to be the case, the results led to the question whether the energy failure in this situation is due to gradual compromise of microcirculation. To explore this possibility, we measured tissue oxygen tension before, during, and after ischemia.
MATERIALS AND METHODS
The study was approved by the Ethical Committee for Animal Experiment in our university. Animal care complied with the “Guideline of Experimental Animal Care” issued from the Office of the Prime Minister of Japan.
Animal preparation.
The experiments were performed on 10-wk-old pregnant Wistar rats (Sanyo Lab Service Tokyo, Japan) weighing 250–300 g. The rats were housed separately at ambient temperature with a 12-h light cycle, and were allowed free access to water and food. On 20 d of gestation (term 21.5 d), the animals were anesthetized with 3% halothane in a mixture of N2O: O2 (70: 30) after overnight fasting. They were then intubated and artificially ventilated on 1.0% to 1.5% halothane during the operation. The tail artery was cannulated to measure arterial blood gases, pH, blood glucose, and blood pressure. A midline abdominal incision was performed and the two uterine horns were exposed and kept moist with saline. Transient uterine artery occlusion was induced according to the technique of Tanaka et al. (27). Briefly, two microvascular clips were used to occlude the uterine vessels near the lower and upper ends of the right uterine horn. The clips were removed after 30 min of ischemia. During the operation core temperature was regularly maintained at 37.0°C with a heating pad. For each experiment the fetuses in the right uterine horn served as the ischemia group and those in the left horn as the control group. After predetermined times of recovery (see below) the animals were re-anesthetized, tracheotomized, and artificially ventilated. After the physiologic parameters had been stabilized for at least 5 min, fetuses were delivered by cesarean section and were immersed immediately into liquid nitrogen to measure high-energy phosphates and glycolytic intermediates. To keep the fetal condition stable, only one fetus was sampled from the middle portion of each uterine horn. Because this surgical procedure was performed by two investigators, the time interval from the delivery to the immersion of the fetuses into liquid nitrogen lasted only 5 s.
Drug administration and experimental groups.
PBN, purchased from Sigma Chemical Co. (St. Louis, MO), was dissolved in saline (10 mg · ml−1). Six groups of pregnant animals (n = 36) were studied. One group was used as control (n = 6), in which procedures were identical to those in the experimental groups except that uterine artery occlusion was not induced. In the other group of animals, uterine artery occlusion was induced for 30 min. At the end of 30 min of ischemia, the fetuses of one group of pregnant rats (n = 6) were frozen in situ; the other animals were allowed recovery periods of either 1 h (n = 6), 2 h (n = 6 with saline), or 4 h (n = 6 with saline;n = 6 with PBN) of recirculation. PBN (100 mg · kg −1) or vehicle (saline) was administered intraperitoneally to pregnant rats after 1 h of recirculation.
Enzymatic methods.
At the time intervals chosen, corresponding to the end of 30 min of uterine artery occlusion or 30 min of uterine artery occlusion followed by 1, 2, or 4 h of recirculation, respectively, the brain was frozen in situ. Cerebral cortical tissue was removed from the frozen brain at −2°C, weighed (100–200 mg), extracted with 1.0 mL HCl-methanol (0.1 mol/L HCl) and then at 0 with 4.0 mL (0.3 mol/<l) perchloric acid, as described by Folbergrova et al. (28). The homogenate was centrifuged at 3000 rpm for 10 min. The supernatant was neutralized with KOH-imidazole (1.5 M KOH, 0.4 M imidazole, 0.3 M KCl). To obtain a complete precipitation of potassium perchlorate, the neutralized supernatant remained in ice water for 1 h and was then centrifuged again at 3000 rpm for 10 min. The fluorometric enzymatic techniques of Lowry et al. (29) were used for measurement of ATP, ADP, AMP, and lactate, with analytical conditions as previously described (28).
Measurement of tissue oxygen tension.
In a separate series of experiments, the tissue oxygen tension was continuously monitored by a microelectrode to evaluate the blood flow changes on placental and fetal cerebral tissues throughout the whole period of 30 min of ischemia and 4 h of recirculation. Both oxygen tension monitorings were performed simultaneously in a total of six animals.
Miniature monopolar electrodes for oxygen tension were constructed according to the method described by Rossem et al. (30). Pure platinum wire (length 15 mm, diameter 0.1 mm) was soldered to a gold connector. After staining with black ink, the wire was insulated with cyanoacrylate glue by dipping. The measuring tip of the electrode was created manually under the microscope. First, the insulation was removed at the free end of the wire over a length of 0.2 mm by using a scalpel. The naked tip was then covered with a cellulose acetate membrane by dipping it repeatedly into a cellulose acetate solution (5% cellulose diacetate in 33.3% ethanol in acetone solution).
On 20 d of gestation, pregnant rats were kept under artificial ventilation with 0.5% to 1.0% halothane and continuous infusion of muscle relaxant (vecuronium bromide, 2 mg/h) throughout the experiment. The tail artery was cannulated to measure arterial blood gases, pH, blood glucose, and blood pressure. The right uterine horn was revealed through a midline incision. Because the uterine wall and the fetal cranial vault of the skull were thin, the fetal bregma and the other superficial structures could be seen through the uterine wall. Thereafter, the microelectrode was stereotaxically implanted through the uterine wall and fetal skull for fetal cerebral tissue oxygen monitoring. The tip of the microelectrode was placed on the right side 2 mm lateral to bregma and inserted 1.5 mm below the fetal brain surface into the parietal cortex. The position of the microelectrode tip was previously determined by anatomical observation of the fetal brain. For placental tissue oxygen monitoring, the adjoining placenta in the right horn was used. The microelectrode was carefully inserted into the placental tissue through the uterine wall (2 mm in depth). A reference electrode was placed in the upper abdominal space. After implantation, the electrode was connected to the recording circuit and polarized at −700 mV. A 15- to 30-min stabilization period was permitted before initiating 30 min of uterine artery occlusion. Then tissue oxygen tensions were measured continuously throughout the whole period of 30 min occlusion and 4 h recirculation. The changes in tissue oxygen tension were expressed as a percentage of the control level.
Statistical analysis.
All data were expressed as mean standard deviation (SD). One-factor ANOVA, followed by Scheffé's F test, was used to compare the values within each vehicle-treated rats experimental groups. The effect of PBN at 4 h of recirculation was evaluated with unpaired t test. Differences with a p value of <0.05 were considered to be statistically significant.
RESULTS
Physiologic Parameters
Physiologic parameters of experimental groups are shown in Table 1. Core temperature, blood pressure, arterial Po2, Pco2, pH, and glucose concentrations were maintained close to the control level during operation. There were no significant differences in these parameters among the experimental groups. The rats given PBN showed no significant difference from vehicle-treated rats in core temperature during and after uterine artery occlusion.
Changes in Metabolites
Table 2 shows tissue concentrations of labile metabolites in control and ischemic animals.
Non-ischemic horn.
As shown in Table 2, there were no significant changes in concentrations of high-energy phosphates and glycolytic intermediates during the whole period of 30 min of ischemia and 4 h of recirculation.
Ischemic horn.
After 30 min of uterine artery occlusion, the ATP concentration decreased to 26% and ADP concentration decreased to 55% of sham-operated controls, respectively, but AMP concentrations rose. The sum of adenine nucleotides (ΣAdn) decreased to 58% of sham-operated controls. There was a 2-fold increase in lactate concentration (Table 2).
ATP concentration markedly improved, increasing to normal values after 1 h of recirculation, with partial recovery of the ΣAdn. After 2 h of recirculation, ATP, ADP, and AMP concentrations were maintained close to control values, but deteriorated again with respect to ATP and ΣAdn after 4 h of recirculation. The data on vehicle-treated animals suggest that a secondary deterioration of cellular energy state occurs after 4 h of recovery or longer. Furthermore, lactate concentration still increased during this recovery period.
Animals Injected with PBN
As shown in Table 2, treatment with PBN had a beneficial effect on the recovery process. After 4 h of recirculation (i.e. 3 h after PBN injection), there was a significant recovery of ATP and ΣAdn (86% and 84% of controls), compared with 4 h of recirculation in vehicle-treated animals (75% and 76% of controls). PBN treatment remarkably reduced lactate levels (from 204% to 133% of controls) (Table 2).
Changes in Tissue Oxygen Tension
Core temperature, blood pressure, arterial Po2, Pco2, pH, and glucose concentrations were maintained close to the control level during the experiment (data not shown). There were no significant differences in these parameters during the experiment.
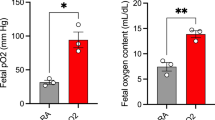
Figure 1 shows relative changes of tissue oxygen tension during and after 30 min of uterine artery occlusion. Immediately after the occlusion, tissue oxygen tension in placenta fell to about 30% of the control level. Oxygen tensions in fetal cerebrum showed a delayed decrease to about 50% of control level. After recirculation, tissue oxygen tensions in placenta and fetal cerebrum increased gradually to near-control values and remained almost constant throughout the whole period of 4 h of recirculation. Thus, tissue oxygen tensions remained close to control during the recirculation period.
Changes in tissue oxygen tension of placenta (top) and fetal cerebrum (bottom) during ischemia and recirculation. Values are presented as the means ± SD. During the early ischemic period, tissue oxygen tension in fetal cerebrum showed delayed decrease compared with those in placenta. At the end of occlusion, placental and fetal cerebral tissue oxygen tension decreased to about 30% and 50% of control levels, respectively. After recirculation, both tissue oxygen tensions increased gradually close to control values and remained almost constant throughout the whole period of 4 h of recirculation.
DISCUSSION
Maternal uterine artery ligation has frequently been used to study the consequences of reduced uterine blood flow on the fetus. This procedure has been shown to produce fetal hypoxia, hypercapnia, and acidosis (31–34), which likely affect fetal cellular metabolism. The present results indicate that although during the early time period after ischemia fetal cerebral energy metabolism is maintained by an acceleration of the anaerobic glycolytic rate, secondary deterioration of cellular bioenergetic state develops in the immature fetal brain. This deterioration is palliated by PBN, given 1 h after start of recirculation.
Cellular Bioenergetic Response to Ischemia and Reperfusion
Ischemic period.
It has been known that the developing brain is particularly resistant to hypoxic-ischemic brain injury, but the mechanisms that are responsible for this resistance are not completely clear. Our results demonstrate that the ATP concentration decreased to 26% of sham-operated controls at the end of 30 min of transient ischemia. Recently, Kunievsky et al. (35) demonstrated that a transient and complete occlusion of the circulation of a single uterine horn was accompanied by slow and moderate dissipation in the energy levels in fetal brain. The more moderate decline in ATP levels during in utero ischemia including our study was concordant with the changes in high-energy phosphates in the fetal rat brain as previously measured in vivo by 31P-NMR spectroscopy (36). A similar moderate fall in energy metabolites has been noticed after short episodes of uterine blood flow arrest in the near-term guinea pig (37). These data from fetal brain are in contrast to the adult brain in which a precipitous fall in ATP levels is noticed after complete cerebral ischemia (38, 39). The slow and moderate dissipation of energy metabolites is a reflection of the resistance of the developing brain to ischemic insults and is presumably the underlying mechanism for the apparent protection. The delay in the decrease in tissue oxygen tension in fetal cerebrum, which was observed in our results during the ischemic period, may be implicated in the slow and moderate dissipation of energy metabolites, suggestive of circulatory centralization.
The measured rise in cerebral lactate concentration at the end of ischemia in this study is agreement with measurements in adult animals, in which the increase in tissue lactate concentration was the earliest sign of tissue hypoxia (40, 41). However, the rise in cerebral concentration of lactate during hypoxemia is less in fetuses than in adults (42, 43). This may be due to lower fetal cerebral metabolic rate.
Early reperfusion period.
The complete restoration of ATP during the early reperfusion period (1 and 2 h of recirculation) in the present study is similar to that previously reported in fetal hypoxia-ischemia model (35). This is in contrast to the partial restoration that has been reported in models of adult ischemia, particularly with respect to ATP concentrations and the sum of nucleotides (24, 44). Our results on lactate, which remained high during the whole period of recirculation, are also in good agreement with previous experimental studies in fetus of rats and primates (35, 37, 45). A more complete recovery of lactate level is obtained in adult models of ischemia during the early reperfusion period (24). It seems likely that the expense of anaerobic glycolysis is dominant for maintenance of fetal cerebral energy metabolism at these time points.
Other components of the resistance to ischemia in fetuses may include the absence of post-ischemic hyperemia. In adult models of brain ischemia, a pronounced hyperemia occurs in the early post-ischemic reperfusion period (46–48). In contrast, our findings, and a previous report (8), on tissue oxygen tension in fetal cerebrum indicate no hyperemia or hypoperfusion. The absence of post-ischemic hyperemia may be beneficial in light of observations by Kuroiwa et al. (49), who observed a decrease in tissue edema and blood-brain barrier disruption when post-ischemic hyperemia was prevented by constriction of the middle cerebral artery after the ischemic episode. The spontaneous limitation of post-ischemic blood flow in the fetus may have a similar protective effect.
Although these unique characteristics of the intrauterine environment during ischemia and reperfusion exist, secondary deterioration of cellular bioenergetic state developed in the immature fetal brain after 4 h of recirculation. These findings are in good agreement with the results reported by Folbergrováet al. (24), who used 2 h of middle cerebral artery occlusion in adult rats. To explain the secondary deterioration, they suggested two possible mechanisms: secondary microcirculatory obstruction and delayed mitochondrial dysfunction. In the adult model, the first possibility seems less likely in view of reports showing an adequate return of blood flow to tissue (50) and the marked increase in tissue oxygen tension during recirculation (46–48). Our present results in the fetal model confirm and extend these observations inasmuch as tissue oxygen tension remained unchanged during the whole period of 4 h recirculation although secondary bioenergetic failure developed in the immature fetal brain. This leaves us with the likely possibility that secondary mitochondrial dysfunction is what causes bioenergetic failure.
Effect of Delayed Treatment with PBN
Recently, it was shown that transient forebrain ischemia in adult gerbils is accompanied by enhanced production of free radicals, as assessed by hydroxylation of salicylate, and by production of a free radical adduct of the spin trapping agent PBN, as well as by oxidation of proteins, the latter being estimated from the carbonyl content in the soluble protein fraction, and from glutamine synthetase activity (51–53). In that species, PBN also ameliorated functional and morphologic brain damage, suggesting that free radicals play an important pathophysiological role in the ischemic damage (52, 54).
In the present study, the decrease in ATP content at 4 h of recirculation in vehicle-treated animals is the major cause of the decrease in the sum of adenine nucleotides. This is the expected result of an increase in the concentration of (free) AMP, which is dephosphorylated and deaminated to yield nucleosides and free bases (e.g. hypoxanthine, xanthine, inosine) (55, 56). PBN increased the adenine nucleotide pool, but the mechanisms remain to be elucidated.
Because PBN is one of the classical spin trapping agents, it seems likely that it acts by scavenging free radicals (51). However, because no other free radical scavenger has comparable effects, or gives a therapeutic window as wide as PBN in the adult model, one must presume that the effect of the nitrone reflects its partition in cellular organelles or membrane phases. Theoretically, these beneficial effects could reflect accumulation of PBN in mitochondria, where it conceivably could prevent free radical damage to components of the respiratory chain. Indeed, it has been shown that the delayed treatment with PBN attenuated mitochondrial dysfunction, which occurs after transient focal cerebral ischemia in adult rats (25). Thus, PBN probably prevents the secondary deterioration of the bioenergetic state of the tissue in adult and developing brain by protecting the mitochondria against oxidative attack.
The results obtained with PBN in the adult model (52, 54), together with the present data, which demonstrate a pronounced effect of PBN on metabolic recovery, indicate a wide therapeutic window for nitrones of the PBN type of potentially large clinical importance.
In conclusion, our results indicate that the secondary deterioration of cellular bioenergetic state develops in the immature fetal brain during intrauterine ischemia-reperfusion. This deterioration may be due to damage of mitochondrial membranes and/or of the mitochondrial respiratory chain enzymes, which may be induced by oxygen-derived free radicals, and not by compromised microcirculation.
Abbreviations
- PBN:
-
α-phenyl-N-tert-butyl nitrone
References
Ting P, Yamaguchi S, Bacher JD, Killens RH, Myers RE 1983 Hypoxic-ischemic cerebral necrosis in midgestational sheep fetuses: pathophysiologic correlation. Exp Neurol 80: 227–245.
Wagner RE, Ting P, Westfall MV, Yamaguchi S, Bacher JD, Myers RE 1986 Brain metabolic correlates of hypoxic ischemic cerebral necrosis in mid gestational sheep fetuses: significance of hypotension. J Cereb Blood Flow Metab 6: 425–434.
Vannucci RC, Lyons DT, Vasta F 1988 Regional cerebral blood flow during hypoxia-ischemia in immature rats. Stroke 19: 245–250.
Bejor R, Wozniak P, Allard M 1988 Antenatal origin of neurologic damage in newborn infants. Am J Obstet Gynecol 159: 357–363.
Scher MS, Belfar H, Martin J, Painter MJ 1991 Destructive brain lesion of presumed fetal onset: antepartum courses of cerebral palsy. Pediatrics 88: 898–906.
Peeters LLH, Sheldon RE, Jones MD, Makowski EL, Meschia G 1979 Blood flow to fetal organs as function of arterial oxygen content. Am J Obstet Gynecol 135: 637–646.
Rudolph AM 1985 Distribution and regulation of blood flow in the fetus and neonate lamb. Circ Res 57: 811–821.
Chao CR, Hohimer AR, Bissonnette JM 1991 Fetal cerebral blood flow and metabolism during oligemia and early postoligemic reperfusion. J Cereb Blood Flow Metab 11: 416–423.
Younkin DP, Wagerle LC, Chance B, Maria J 1987 31P-NMR studies of cerebral metabolic changes during graded hypoxia in newborn lambs. J Appl Physiol 62: 1569–1574.
Siesjö BK, Siesjö P 1996 Mechanisms of secondary brain injury. Eur J Anaesthesiol 13: 247–268.
MacCord JM 1985 Oxygen derived free radicals in post-ischemic tissue injury. N Engl J Med 312: 159–163.
Yoshida S, Inoh S, Asano T, Sano K, Kubota M, Shimazaki H, Ueta N 1980 Effect of transient ischemia on free fatty acids and phospholipids in gerbil brain: lipid peroxidation as a possible cause of postischemic injury. J Neurosurg 53: 323–331.
Siesjö BK 1981 Cell damage in the brain: a speculative synthesis. J Cereb Blood Flow Metab 1: 155–185.
Rosenberg AA, Murdaugh E, White CE 1989 The role of oxygen free radicals in postasphyxia cerebral hypoperfusion in newborn lambs. Pediatr Res 26: 215–219.
Thordstein M, Bagenholm R, Thiringer K, Kjellmer I 1993 Scavengers of free oxygen radicals in combination with magnesium ameliorate perinatal hypoxic-ischemic brain damage in the rat. Pediatr Res 34: 23–26.
Zini I, Tomasi A, Grimaldi R, Vannini V, Agnati LE 1992 Detection of free radicals during brain ischemia and reperfusion by spin trapping and microdialysis. Neurosci Lett 138: 279–282.
Kontos HA 1985 Oxygen radicals in cerebral vascular injury. Cir Res 57: 508–516.
Siesjö BK, Agardh C-D, Bengtsson F 1989 Free radicals and brain damage. Cerebrovasc Brain Metab Rev 1: 165–211.
Martz D, Rayos G, Schielke GP, Betz AL 1989 Allopurinol and dimethylthiourea reduce brain infarction following middle cerebral artery occlusion in rats. Stroke 20: 488–494.
Liu TH, Beckman JS, Freeman BA, Hogan EL, Hsu CY 1989 Polyethylene glycol-conjugated superoxide dismutase and catalase reduce ischemic brain injury. Am J Physiol 256:H589–H593.
Chan PH 1996 Role of oxidants in ischemic brain damage. Stroke 27: 1124–1129.
Cao X, Phillis JW 1994 α-Phenyl-tert-butyl-nitrone reduced cortical infarct and edema in rats subjected to focal ischemia. Brain Res 644: 267–272.
Zhao Q, Pahlmark K, Smith M, Siesjö BK 1994 Delayed treatment with the spin trap a-phenyl-N-tert-butyl nitron (PBN) reduces infarct size following transient middle cerebral artery occlusion in rats. Acta Physiol Scand 152: 349–350.
Folbergrova J, Zhao Q, Katsura K, Siesjö BK 1995 N-tert-butyl-α-phenylnitrone improves recovery of brain energy state in rats following transient focal ischemia. Proc Natl Acad Sci USA 92: 5057–5061.
Kuroda S, Katsura K, Hillered L, Bates TE, Siesjö BK 1996 Delayed treatment with α-phenyl-N-tert-butyl-nitrone (PBN) attenuates secondary mitochondrial dysfunction after transient focal ischemia in the rat. Neurobiol Dis 3: 149–157.
He Q-P, Smith M, Li P-A, Siesjö BK 1997 Necrosis of the substantia nigra, pars reticulate, in flurothyl-induced status epilepticus is ameliorated by the spin trap alfa phenyl-N-tert-butyl nitrone. Free Radic Biol Med 22: 917–922.
Tanaka M, Natori M, Ishimoto H, Miyazaki T, Kobayashi T, Nozawa S 1994 Experimental growth retardation produced by transient period of uteroplacental ischemia in pregnant Sprague-Dawley rats. Am J Obstet Gynecol 171: 1231–1234.
Folbergrova J, MacMillan V, Siesjö BK 1972 The effect of moderate and marked hypercapnia upon the energy state and upon the cytoplasmic NADH/NAD+ ratio of the rat brain. J Neurochem 19: 2497–2505.
Lowry OH, Rosebrough N, Farr A, Randall R 1951 Protein measurement with phenol reagent. J Biol Chem 193: 265–275.
Rossem K, Vermarien H, Bourgain R 1992 Construction, calibration and evaluation of PO2 electrodes for chronical implantation in the rabbit brain cortex. Adv Exp Med Biol 316: 85–101.
Wigglesworth JS 1964 Experimental growth retardation in the foetal rat. J Pathol Bacteriol 88: 1–13.
Ogata ES, Bussey M, Finley S 1989 Altered gas exchange, limited glucose and branched chain amino acids, and hypoinsulinemia retard fetal growth in the rat. Metabolism 35: 970–977.
Yavin E, Inserte-Igual J, Gil S 1995 Ischemia-triggered translocation and inactivation of protein kinase C isoforms in the fetal brain. J Neurochem 65: 2594–2602.
Ishimoto H, Natori M, Tanaka M, Miyazaki T, Kobayashi T, Yoshimura Y 1997 Role of oxygen-derived free radicals in fetal growth retardation induced by ischemia-reperfusion in rats. Am J Physiol 272: 701–705.
Kunievsky B, Pretsky A, Yavin E 1994 Transient rise of glucose uptake in the fetal rat brain after brief episodes of intrauterine ischemia. Dev Neurosci 16: 313–320.
O'Shaughnessy CT, Lythgoe DJ, Butcher SP, Kendall L, Wood B, Steward MC 1992 Effect of hypoxia on fetal rat brain metabolism studied in utero by 31P-NMR spectroscopy. Brain Res 551: 334–337.
Berger R, Jensen A, Krieglstein J, Steigelmann J-P 1993 Cerebral energy metabolism in fetal guinea pigs during moderate maternal hypoxemia at 0:75 of gestation. J Dev Physiol 19: 193–196.
Nordstrom C-H, Siesjö BK 1978 Influence of phenobarbital on changes in the metabolites of the energy reserve of the cerebral cortex following complete ischemia. Acta Physiol Scand 104: 271–280.
Onodera H, Iijima K, Kogure M 1986 Mononucleotide metabolism in the rat brain after transient ischemia. J Neurochem 46: 1704–1710.
Siesjo BK, Nilsson L 1971 The influence of arterial hypoxemia upon labile phosphates and upon extracellular and intracellular lactate and pyruvate concentrations in the rat brain. Scand J Clin Lab Invest 27: 83–96.
Siesjo BK 1981 Cell damage in the brain: a speculative synthesis. J Cereb Blood Flow Metab 1: 155–185.
Beck T, Krieglstein J 1987 Cerebral circulation, metabolism, and blood-brain barrier of rats in hypocapnic hypoxia. Am J Physiol 252: H504–H512.
Berger R, Gjedde A, Heck J, Muller E, Krieglstein J, Jensen A 1991 Application of the Sokoloff method to calculate cerebral glucose consumption in fetal guinea pigs. J Cereb Blood Flow Metab 11: S869.
Welsh FA, Marcy VR, Sims RE 1991 NADH fluorescence and regional energy metabolites during focal ischemia and reperfusion of rat brain. J Cereb Blood Flow Metab 11: 459–465.
Haan H, Von Reempts J, Vles J, Haan J, Hasaart T 1993 Effect of asphyxia on the fetal lamb brain. Am J Obstet Gynecol 169: 1493–1501.
Nagasawa H, Kogure K 1989 Correlation between cerebral blood flow and histologic changes in a new rat model of middle cerebral artery occlusion. Stroke 20: 1037–1043.
Matsuo Y, Kihara T, Ikeda M, Ninomiya M, Onodera H, Kogure K 1995 Role of Neutrophils in radical production during ischemia and reperfusion of the rat brain: effect of neutrophil depletion extracellular ascorbyl radical formation. J Cereb Blood Flow Metab 15: 941–947.
Nakai A, Kuroda S, Kristian T, Siesjö BK 1997 The immunosuppressant drug FK506 ameliorates secondary mitochondrial dysfunction following transient focal cerebral ischemia in the rat. Neurobiol Dis 4: 288–300.
Kuroiwa T, Shibutani M, Okeda R 1989 Nonhyperemic blood flow restoration and brain edema in experimental focal cerebral ischemia. J Neurosurg 70: 73–80.
Tsuchidate R, He Q-P, Smith M-L, Siesjö BK 1997 Regional cerebral blood flow during and following 2 hours of middle cerebral artery occlusion in the rat. J Cereb Blood Flow Metab 17: 1066–1073.
Floyd RA 1990 Role of oxygen free radicals in carcinogenesis and brain ischemia. FASEB J 4: 2587–2597.
Oliver CN, Starke-Reed PE, Liu GL, Carney JM, Floyd RA 1990 Oxidative damage to brain proteins, loss of glutamine synthetase activity, and production of free radicals during ischemia/reperfusion-induced injury to gerbil brain. Proc Natl Acad Sci USA 87: 5144–5147.
Carney JM, Floyd RA 1991 Protection against oxidative damage to CNS by alpha-phenyl-tert-butyl nitrone (PBN) and other spin-trapping agents: a novel series of nonlipid free radical scavengers. J Mol Neurosci 3: 47–57.
Clough-Helfman C, Phillis JW 1991 The free radical trapping agent N-tert-butyl-alpha-phenylnitrone (PBN) attenuates cerebral ischaemic injury in gerbils. Free Radic Res Commun 15: 177–186.
Kleihues P, Kobayashi K, Hossmann KA 1974 Prine nucleotide metabolism in the cat brain after one hour of complete ischemia. J Neurochem 23: 417–425.
Chapman AG, Westerberg E, Siesjö BK 1981 The metabolism of purine and pyrimidine nucleotides in rat cortex during insulin-induced hypoglycemia and recovery. J Neurochem 36: 179–189.
Author information
Authors and Affiliations
Additional information
This study was supported by the Tokyo Ohka Foundation for the Promotion of Science and Technology (Grant 98106).
Rights and permissions
About this article
Cite this article
Nakai, A., Asakura, H., Taniuchi, Y. et al. Effect of α-Phenyl-N-tert-Butyl Nitrone (PBN) on Fetal Cerebral Energy Metabolism during Intrauterine Ischemia and Reperfusion in Rats. Pediatr Res 47, 451–456 (2000). https://doi.org/10.1203/00006450-200004000-00007
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-200004000-00007
This article is cited by
-
Perinatal asphyxia: Timing and mechanisms of injury in neonatal encephalopathy
Current Neurology and Neuroscience Reports (2001)