Abstract
This study compares two models for examining ependymal ciliary function: rat brain slices cut from the fourth ventricle and primary ependymal cells in culture. The cilia from both preparations were very reproducible; each preparation had cilia beating at a constant frequency of between 38 and 44 Hz. With the brain slices, ciliary stasis occurred after 5 d in culture. However, ependymal cells had fully functional cilia for up to 48 d in culture. The pneumococcal toxin, pneumolysin, caused a dose-dependent inhibition of cilia beat frequency within 15 min in both models. There were no significant differences in the mean log 50% inhibitory concentration (pIC50) slice = 0.65 ± 0.05, equivalent to 4.4 hemolytic units (HU)/mL; cells = 0.57 ± 0.14, equivalent to 3.7 HU/mL. There were also no significant differences in the mean Hill slope factors for the curves (slice = 1.4 ± 0.05; cells = 1.6 ± 0.4). These data demonstrate that both models can be used to examine the acute (15-min) effects of pneumolysin on cilia beat frequency. The main advantage of the primary ependymal culture model is that considerably more cultured ependymal cells (∼70%) are available, compared with the number of ependymal cells on the brain slices (∼2%), thus reducing the number of animals used. A pure ependymal culture was not achieved (∼30% of the cells were not ciliated). The increased survival time of the ependymal cells compared with the brain slices make cultured ependymal cells more useful for examining long-term ciliary function, whereas brain slices may be more useful for examining the interactions between ependymal and other nearby cells.
Similar content being viewed by others
Main
Despite the introduction of antibiotics and modern intensive care, a high morbidity and mortality rate is still associated with meningitis, particularly pneumococcal meningitis (1). Although animal models have been used in the investigation of meningitis, there is still a need for other relevant models of the disease to enable dissection of the mechanisms of pathogenesis.
The ependymal epithelium is ciliated to varying degrees, depending on the ventricular location: for example, the cells lining the ventricular floor are more ciliated than those lining the circumventricular organs such as the choroid plexus (2). A recent report has shown that ciliated ependymal cells may be adult neuronal stem cells from which other neuronal cell phenotypes originate (3). In addition, the ependyma is believed to act as a filter relaying macromolecules to and from the CSF. However, the exact function of the ependyma is unknown, as is its response to toxic insult.
We have developed a system whereby brain slices are prepared with an intact ependymal lining. The ciliary beat frequency of the ependymal cilia can be measured directly and continually. The advantage of such a technique is that ependymal cells are relevant, in that they are adjacent to the CSF, which is infected in meningitis. Continual observation of ependymal ciliary movement enables us to assess the function and integrity of ependymal cells, thus allowing rapid screening for toxins. However, the use of this tissue has disadvantages.
The aim of this work, therefore, was to prepare a primary ependymal culture model that would allow the measurement of CBF. The advantage of such a method would be that it would enable one to study only ependymal cells, without the presence of neuronal tissue associated with the brain slice model. In addition, the cells might survive over a longer period of time, permitting extended time course experiments to be performed. A cell culture model may also yield more ependymal cells, hence reducing the number of animals needed for the experiments.
This study describes the development of a tissue culture model of the ependyma and its assessment, alongside the existing brain slice method, as a means of measuring toxicity on CBF. The acute effects of pneumococcal toxin, pneumolysin, was used to compare the two experimental models.
MATERIALS AND METHODS
Brain slice.
Wistar rats (14–17 d old) were killed by cervical dislocation. Their cerebellum was removed and mounted on a vibratome under ice-cold M199 medium containing penicillin (100 IU/mL) and streptomycin (100 μg/mL). The brain was sliced (250 μm) through the medulla oblongata and pons into the floor of the fourth ventricle so that the ciliated V-shaped floor was clear. The slices were mounted in 2 mL of prewarmed M199 medium before use.
Cell culture.
To grow the ependymal cells, we used an adaptation of the method by Wiebel et al. (4). Eight-well (25 × 35 mm) tissue culture trays were coated with bovine fibronectin (75 μg/2 mL; ≅1 μg/cm2) and were incubated at 37°C in 5% CO2 for 2 h before use.
Newborn (1- to 2-d-old) Wistar rats were killed by cervical dislocation, and their brains were removed by careful dissection. The cerebellum was removed, as were small (3-mm) edge regions of the frontal cortex and the left and right cortical hemispheres. The remaining brain regions (containing ependymal cells and ventricles) were mechanically dissociated by passing the tissue through an 18-gauge needle in 2 mL of tissue culture medium.
The growth medium was serum-free minimum essential medium (GIBCO Life Technologies, Paisley, Scotland) containing penicillin (100 IU/mL), streptomycin (100 μg/mL), Fungizone (2.5 μg/mL), bovine serum albumin (5 μg/mL), insulin (5 μg/mL), transferrin (10 μg/mL), selenium (5 μg/mL), and from day 3 onward, thrombin (0.5I U/mL).
Dissociated tissue from a single brain was seeded (500 μL/well) into eight-well (25 × 35 mm) tissue culture trays, each well containing 2.5 mL of medium. The medium was replaced 3 d after seeding. The adherent ependymal cells were fed by the replacement of 2 mL of fresh medium two times a week.
The ciliated ependymal cell colonies were identified at day 5, and experiments with pneumolysin were performed when cells were between 14 and 17 d old and cell proliferation was optimal (4).
Cilia beat frequency.
To determine ciliary beat frequency, both the cells in culture and the brain slices were placed in a humidified (80–90%) incubation (37°C) chamber and were observed via an inverted microscope system (Diphot, Nikon, UK). Tissue was allowed to equilibrate for 30 min before readings. Beating cilia were recorded with a digital high-speed video camera (Kodak Ektapro Motion Analyzer, model 1012) at a rate of 400 frames/s, with a shutter speed of 1 in 2000. The camera allows video sequences to be recorded and played back at reduced frame rates or frame by frame. Ciliary beat frequency can be determined by timing a given number of individual cilia beat cycles. Basal ciliary beat frequency was measured at 30 min. Each time point represents the measurement of four individual cilia from each slice or each well. Calculation of CBF (Hz): 400 (number of frames/sec)/5 (frames elapsed for five ciliary beat cycles) × 5 (conversion per beat cycle).
Pneumolysin purification.
Pneumolysin was purified as previously described (5), and was made up in M199. One milliliter of pneumolysin solution was added to the tissue, plus a further 1 mL of medium. Repeated gentle pipetting mixed the medium, and the tissues were incubated for 15 min before final measurement of CBF.
Electron microscopy.
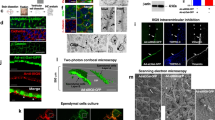
For scanning electron microscopy, the tissues were fixed in Sorensen's phosphate-buffered (pH 7.4) glutaraldehyde (4% wt/vol). After postfixation in osmium tetroxide (1% wt/vol), samples were dehydrated through graded ethanols and immersed in hexamethyldisilazane. The hexamethyldisilazane evaporated, leaving dry tissue with no phase boundary damage (damage caused to the tissue by pressure differences of the air/liquid interface). To visualize the most severe effects of pneumolysin on the ependyma, the upper concentration (1 μg) was selected for electron microscopy.
Statistics.
All data presented are mean ± SEM of six independent experiments. Statistical analysis was performed as appropriate. Individual curves were analyzed by GraphPad PRIZM (nonlinear regression, variable slope), and were tested by ANOVA. We compared the log 50% inhibitory concentration (pIC50) and Hill slope factors, using unpaired Student's t tests.
RESULTS
There was no significant (unpaired t test, p = 0.12) difference in the ciliary beat frequency between the two ependymal models (slice = 38.5 ± 0.7 Hz; cells = 40.7 ± 0.9 Hz). Indeed, reproducibility of the cilia beat frequency (measured once daily for each tissue) with the two models was very good for the first 3 days in culture (Fig. 1A). With regard to the brain slices, ciliary slowing had occurred after 2 days in culture, and complete ciliary stasis occurred on day 5. In contrast, the cilia on the ependymal cells in culture maintained a beat frequency of 38–44 Hz for up to 48 d (Fig. 1A). The proportion of the cells in culture that were ciliated, i.e. of ependymal origin, was approximately 70%.
(A) CBF measurements of ependymal cells (□) and brain slices (▪) in culture. Data are mean ± SEM of three to five preparations up to day 33. All subsequent data are from a single culture. (B) Pneumolysin inhibition of CBF in ependymal cells (▪) and brain slices (□). Data are mean ± SEM of six individual experiments.
To examine the response of the cilia to a known bacterial toxin, we studied the acute effects of pneumolysin at a clinically relevant concentration. The upper concentration of pneumolysin that we selected was 1 μg/mL (149 HU), which can be released from D39 wild-type bacteria at 2 × 107 colony-forming units/mL, a bacterial concentration that is observed commonly in the CSF of patients with pneumococcal meningitis (6). Pneumolysin caused a dose-dependent inhibition of ependymal ciliary beat frequency in both models (Fig. 1B). There was no significant difference (unpaired t test, p = 0.58) in the log of the 50% inhibitory concentration (slice = 0.65 ± 0.05, which is equivalent to 4.4 HU/mL; cells = 0.57 ± 0.14 equivalent to 3.7 HU/mL). The Hill slope factors were not significantly different (unpaired t test, p = 0.6) (slice = 1.4 ± 0.08; cells = 1.6 ± 0.4) for the two models. Scanning electron microscopy revealed similar ependymal cell and ciliary structure in both brain slices (Fig. 2A) and cell culture (Fig. 2B). With the addition of pneumolysin [at 1 μg/mL (149 HU) for 15 min] to brain slices, we observed widespread destruction of the epithelium. After treatment with pneumolysin, there were sparse and disrupted cilia, which seemed to be fused (Fig. 2A, before and after pneumolysin). After the addition of pneumolysin (1 μg/mL: 149 HU for 15 min) to ependymal cells in culture, we observed that the cilia were stripped from the cell surface. Unciliated ependymal cell debris remained in the place of heavily ciliated colonies (Fig. 2B, before and after pneumolysin).
DISCUSSION
Ex vivo rat brain slices had ciliated ependymal cells that beat at 38–44 Hz for the first 3 d in culture. However, the brain slice loses approximately 65% of its ciliary function on day 4, and at day 5, no functional cilia were evident. This is consistent with a study examining postmortem respiratory cilia, which showed that there was a dramatic reduction in CBF 7 days after death (7). The degree of ciliation in the ependymal cells in culture was 70%, in agreement with results obtained by other workers (4).
Ciliary function is a good indicator of ependymal viability, and CBF measurements can be made quickly and used as an indicator of toxic potential. This technique will be useful for identifying and screening potential virulence factors active in diseases of the CSF. In addition, destruction of the ciliary function may play a direct role in the disease process; thus, a determination of means to reverse this destruction could lead to clinical interventions.
The effect of pneumolysin on respiratory epithelial ciliary function has been studied previously (8). It was shown to cause rapid ciliary stasis when applied to nasal brush biopsy samples. We have also shown that this is an effect common to the cilia lining the fourth ventricle of rat brain (9). In this study, the two dose-response curves for the inhibition of CBF in the two models essentially overlapped, which demonstrates that the pneumolysin was equipotent. The steep slope factors for the dose-response curves may indicate positive cooperativity in the action of pneumolysin; however, further experiments are required to confirm this. To visualize the most severe effects of pneumolysin on the ependyma, the upper concentration (1 μg) was selected for electron microscopy. The toxic effects of pneumolysin (10, 11) on the ependymal models can be seen on the electron micrographs, which show that almost complete tissue destruction has been caused by 1 μg of pneumolysin after 15 min in both models.
It is clear from the data presented here that for long-term studies, the ciliated ependymal cells in culture represent a major improvement over brain slices. In addition, ependymal cells in culture are advantageous in that the proportion of ciliated cells is increased over nonciliated cells when compared with brain slices, thus reducing the number of animals used for experiments.
However, the brain slice model is ideal for acute studies of ependymal ciliary function in which rapid measurements of CBF are required, because the transverse section of ependyma can be visualized readily, without need for 14 d of culture. Brain slices would also be advantageous in experiments studying neuronal-ependymal cell communication because of the presence of the underlying neuronal tissue.
In conclusion, the brain slices were very useful for acute experiments with pneumolysin. However, for longer-term low-dose recovery experiments, it is clear that ependymal cells in culture offer an improved alternative. The potency of pneumolysin-induced ciliary stasis remained constant for both models tested.
Abbreviations
- CBF:
-
ciliary beat frequency
- CSF:
-
cerebrospinal fluid
- HMDS:
-
hexamethyldisilazane
- HU:
-
hemolytic units
References
Quagliarello V, Scheld MW 1992 Bacterial meningitis: pathogenesis, pathophysiology and progress. N Engl J Med 327: 864–872
Del Bigio MR 1995 The ependyma: a protective barrier between brain and cerebrospinal fluid. Glia 14: 1–13
Johansson CB, Momma S, Clarke DL, Reisling M, Lendahl U, Frisen J 1999 Identification of a neural stem cell in the adult mammalian central nervous system. Cell 96: 25–34
Weibel M, Pettmann B, Artault JC, Sensenbrenner M, Labourdette G 1986 Primary culture of rat ependymal cells in serum-free defined medium. Dev Brain Res 25: 199–209
Mitchell TJ, Walker JE, Saunders FK, Andrew PW, Boulnois GJ 1989 Expression of the pneumolysin gene in Escherichia coli : rapid purification and biological properties. Biochim Biophys Acta 1007: 67–72
Bingen E, Lambert-Zechovsky N, Mariani-Kurkdjian P, Doit C, Aujard Y, Fournerie F, Mathieu H 1990 Bacterial counts in cerebrospinal fluid of children with meningitis. Eur J Clin Microbiol Infect Dis 9: 278–281
Lee RMKW, Rossman CM, O'Brodovich H 1987 Assessment of postmortem respiratory ciliary motility and ultrastructure. Am Rev Respir Dis 136: 445–447
Feldman C, Mitchell TJ, Andrew PW, Boulnois GJ, Read RC, Todd HC, Cole PJ, Wilson R 1990 The effect of Streptococcus pneumoniae pneumolysin on human respiratory epithelium in vitro. Microb Pathog 9: 275–284
Mohamed BJ, Mitchell TJ, Andrew PW, Hirst RA, O'Callaghan C 1999 The effect of the pneumococcal toxin, pneumolysin, on brain ependymal cilia. Microb Pathog 275: 303–309
Paton JC, Andrew PW, Boulnois GC, Mitchell TJ 1993 Molecular analysis of the pathogenicity of Streptococcus pneumoniae : the role of pneumococcal proteins. Annu Rev Microbiol 47: 89–115
Paton JC 1996 The contribution of pneumolysin to the pathogenicity of Streptococcus pneumonia. Trends Microbiol Sci 43: 103–106
Author information
Authors and Affiliations
Additional information
This study was funded by a British United Providence Association (BUPA, UK) research grant to C.O'C. and P.W.A. and approved by the University of Leicester.
Rights and permissions
About this article
Cite this article
Hirst, R., Rutman, A., Sikand, K. et al. Effect of Pneumolysin on Rat Brain Ciliary Function: Comparison of Brain Slices with Cultured Ependymal Cells. Pediatr Res 47, 381–384 (2000). https://doi.org/10.1203/00006450-200003000-00016
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-200003000-00016
This article is cited by
-
The effect of ethanol and acetaldehyde on brain ependymal and respiratory ciliary beat frequency
Cilia (2013)
-
The effect of halothane and pentobarbital sodium on brain ependymal cilia
Cilia (2012)
-
ciliaFA: a research tool for automated, high-throughput measurement of ciliary beat frequency using freely available software
Cilia (2012)
-
Analysis of ependymal ciliary beat pattern and beat frequency using high speed imaging: comparison with the photomultiplier and photodiode methods
Cilia (2012)
-
Intracerebroventricular antisense knockdown of Gαi2 results in ciliary stasis and ventricular dilatation in the rat
BMC Neuroscience (2007)