Abstract
Irreversible congenital heart block (CHB) and the transient rash of neonatal lupus are strongly associated with maternal antibodies to SSA/Ro and SSB/La proteins; however, the precise mechanism by which these antibodies mediate organ-specific injury is not yet defined. Culturing of keratinocytes has provided critical insights. Accordingly, successful culturing of human fetal cardiac myocytes at high yield would constitute a powerful tool to directly examine conditions that promote expression of the target autoantigens. To accomplish this aim, fetal cardiac myocytes from 18- to 22-wk abortuses were established in culture using a novel technique in which cells were isolated after perfusion of the aorta with collagenase in a Langendorff apparatus. After preplating to decrease fibroblast contamination, cardiocytes were grown in flasks and slide chambers. Staining with monoclonal anti-sarcomeric α-actinin revealed the expected striations typical of cardiac myocytes in 70-90% of the cells after 4 d in culture. Furthermore, the cells were observed to beat at rates varying between 25-75 beats per minute (bpm) after the addition of 1.8 mM CaCl2. An average yield of 45-60 × 106 cells was obtained from a 3- to 5-g heart. Cellular localization of SSA/Ro and SSB/La by indirect immunofluorescence and demonstration of mRNA expression by reverse transcriptase polymerase chain reaction supports the feasibility of cultured cardiac myocytes for the study of congenital heart block. In contrast to the increased expression of SSA/Ro reported for keratinocytes, incubation of cultured human cardiac myocytes with either 17β-estradiol or progesterone did not alter mRNA expression or cellular localization of 48 kD SSB/La, 52 kD SSA/Ro, or 60 kD SSA/Ro. In summary, we describe a novel method to successfully culture human fetal cardiac myocytes that should provide a valuable resource for investigation of the molecular mechanism(s) contributing to the development of congenital heart block. Differential constitutive and estradiol-induced expression of 52 and 60 kD SSA/Ro in human cardiac myocytes compared with keratinocytes may be a factor contributing to the marked discordance of clinically detectable injury in these two target tissues.
Similar content being viewed by others
Main
Heart block detected before or at birth, in the absence of structural abnormalities, is strongly associated with maternal autoantibodies to SSA/Ro and/or SSB/La ribonucleoproteins, independent of whether the mother has systemic lupus erythematosus or Sjögren's syndrome, or is totally asymptomatic (1–3). Autoimmune-associated CHB is most often identified between 16 and 24 wk of gestation in an otherwise normally developing heart (4). Limited autopsy data reveal fibrosis of the AV node (5,6) and an associated inflammatory infiltrate in cases of early intrauterine demise (7). Although varying degrees of block can occur, third degree block is irreversible and carries a substantial morbidity and mortality (8). Despite exposure to the identical circulating antibodies, the maternal heart is never affected. Anti-SSA/Ro-SSB/La antibodies are also associated with transient rashes in the neonate but, unlike CHB, these dermatologic manifestations of neonatal lupus disappear with the clearance of the maternal antibodies from the infant's circulation (1–3).
The candidate autoantigens are ubiquitous and present in all cells. The 60 kD SSA/Ro contains an RNA-binding protein consensus motif (9,10), which could account for its direct interaction with small cytoplasmic hY-RNAs (11). More recent studies demonstrate that the "zinc finger" in human 60 kD SSA/Ro is not conserved across species (12). It has been suggested that 60 kD SSA/Ro may function as part of a novel quality control or discard pathway for 5S rRNA production in Xenopus oocytes (13). Anti-SSB/La antibodies recognize a 48 kD polypeptide that does not share antigenic determinants with either 52 or 60 kD SSA/Ro (14,15). SSB/La facilitates maturation of RNA polymerase III transcripts (16), directly binds a spectrum of RNAs, and associates at least transiently with 60 kD SSA/Ro (17). It has recently been shown to be required for 3′ endonucleolytic cleavage that matures tRNA yeast precursors (18). In addition to the well-characterized 60 kD SSA/Ro and 48 kD SSB/La autoantigens, another target of the autoimmune response in mothers whose children have CHB is the 52 kD SSA/Ro protein (19). The full-length protein, 52α, has three distinct domains: an N-terminal region rich in cysteine/histidine motifs, containing two distinct zinc fingers known as RING finger and B-box; a central region containing two coiled coils with heptad periodicity, one a leucine zipper with potential for intramolecular dimerization; and a C-terminal "rfp-like" domain (20,21). An alternatively spliced transcript has recently been identified, 52β (22), in which exon 4 encoding amino acids 168-245, inclusive of the leucine zipper and an immunodominant epitope (23,24), has been deleted. In vitro-translated 52β is immunoprecipitated by antisera from mothers whose children have CHB (22), which is consistent with reports of an additional N-terminal epitope on 52α remaining present in 52β (23,24). mRNA expression of 52β is maximal in the human fetal heart between 14 and 16 wk of gestation (22,25).
Although accumulating clinical data leave little doubt regarding the association of anti-SSA/Ro and/or SSB/La antibodies with the development of CHB, additional lines of evidence are now available to suggest that the putative autoantibodies are not simply "clinical markers." In one case of fatal CHB, maternal IgG molecules bearing anti-SSB/La idiotypes were demonstrated on the surface of the fetal myocardial fibers (26), and in another, anti-SSA/Ro antibodies were eluted from the affected fetal heart (27). Several studies have demonstrated the arrhythmogenic effects of anti-SSA/Ro-SSB/La antibodies and inhibition of calcium currents (28–30). However, the mechanism by which anti-SSA/Ro-SSB/La antibodies interact with their sequestered intracellular antigens remains unclear. The availability of keratinocytes in a culture system has defined conditions that induce expression of SSA/Ro and SSB/La antigens and their translocation to the cell surface. Similar molecular insights should be gained in human fetal cardiac myocytes if current methods of culturing could be improved to produce a reliably high cell yield. Accordingly, we detail a novel method for culturing human cardiac myocytes that have been isolated from fetal hearts (gestational age 18-22 wk) using the Langendorff apparatus. To confirm the feasibility of cultured cardiac myocytes for the study of CHB, mRNA expression of 48 kD SSB/La, 52 kD SSA/Ro, 52β, and 60 kD SSA/Ro was evaluated by RT-PCR. Because fetal tissue is normally exposed to high circulating levels of 17β-estradiol and progesterone, and augmented expression of SSA/Ro-SSB/La has been demonstrated in keratinoyctes (31,32), initial studies aimed at defining conditions that might alter expression of the candidate autoantigens included incubation with female sex hormones. This method should provide a valuable resource to investigators interested in cardiac culturing and CHB.
METHODS
Isolation and culture of human fetal heart cells. Human fetal hearts were aseptically obtained after elective termination of normal pregnancy by dilation and evacuation. This was done in accordance with the guidelines of the Institutional Review Board and after consent from the mothers. No cardiac toxic drugs were administered to the mothers during these procedures. Gestational age was defined by sonographic measurement of biparietal diameter and femur length. The hearts used in this study were as follows: 18 wk (normal), 20 wk (Down syndrome), 22 wk (Down syndrome) for cardiocyte culturing and mRNA isolation; 19 wk (Down syndrome), 19 wk (normal), 21 wk (normal) for cardiocyte culturing and immunofluorescence staining. The hearts, weighing 3-5 g each, were immediately dissected from the thoracic cavity with the great vessels intact and transported to our laboratory within 15 min on iced Hanks' balanced salt solution minus calcium and magnesium (GIBCO BRL, Gaithersburg, MD) containing 100 U/mL of heparin.
The hearts were then cannulated through the aorta for continuous perfusion of the coronary arteries with calcium-free Tyrode's solution (117 mM NaCl, 11 mM glucose, 4.4 mM NaHCO3, 5.7 mM KCl, 1.5 mM KH2PO4, 1.7 mM MgCl2, HEPES 20 mM adjusted to pH 7.4 with NaOH) at 37°C bubbled with 100% O2 as described for the Langendorff preparation (30,33). After 15 min of washing to clear the blood from the heart, fresh calcium-free Tyrode's solution containing 1.5 mg/mL collagenase A (type III) (GIBCO BRL) was recirculated for 20 min until the heart was completely pale and flaccid. The heart was then removed from the Langendorff apparatus and cells were gently dissociated with forceps in calcium-free Tyrode's solution. Additional dissociation was achieved by stirring of cells for 10-15 min at 37°C in 15 mL Hanks' balanced salt solution containing 0.25% trypsin, 1 mM EDTA (GIBCO BRL). The resulting cell suspension was then poured over a cell strainer, 70-µm nylon (Fischer, Pittsburgh, PA), after which the trypsin-dissociated cells were centrifuged and the cell pellet resuspended in 20 mL of culture medium [Dulbecco's modified Eagle's medium supplemented with 15% FCS, 50 U/mL penicillin, 50 U/mL streptomycin, 100 µg/mL gentamicin, 1 mM nonessential amino acid (GIBCO BRL), 0.1 mM essential medium vitamins (GIBCO BRL), 2 mM glutamine (GIBCO BRL), and 0.1 mM Na pyruvate (GIBCO BRL)]. To deplete fibroblasts and minimize subsequent overgrowth in the cardiac cultures, preplating of the cells was done in 15% FCS for 20-30 min at 376°C. The nonadherent cells were then plated at approximately 3 × 106 cells per 25-cm2 culture flask (34), and grown in a humidified incubator containing 10% CO2 at 37°C.
Cardiocyte beating. To evaluate cell contractility and therefore the purity of cardiocyte culture, medium containing 1.8 mM CaCl2 was added to the cultured cells on d 4 and 14. Flasks were maintained on a warmed microscope stage. Morphologic analysis was conducted using a Scientific Imaging Solutions workstation (BDS Inc., Bethesda, MD) consisting of a Macintosh IIfx personal computer equipped with a Pixel Pipeline video acquisition board (Perceptics, Inc, Knoxville, TN), a video camera (CCD72, MTI, Fremont, CA), and TCL-image software (Oncre Imaging Systems, Rockville, MD). Backscattered electron images were acquired through the video camera directly from the screen of a high-resolution image acquisition system and projected onto a VCR-TV unit. Using the imaging work station, the gray-scale images were adjusted for contrast and brightness, and cells beating in culture could be observed and recorded.
Incubation of cells with hormones. Cardiac myocytes were established in culture for 4 d without the addition of exogenous estradiol or progesterone to allow for washout of maternal hormones. For immunofluorescence studies of cardiocyte cultures, cells were first trypsinized and then transferred from flasks to chamber slides for incubation with hormones and ease of staining. A total of 4 mL of prewarmed trypsin was added to a 5-cm2 flask at d 4 of culture and maintained at 37°C in 10% CO2 for 20 min, followed by the addition of 10 mL of culture medium to inactivate the trypsin. Cell suspensions were then centrifuged. Medium alone or medium containing of 10-7 M 17β-estradiol (Fisher) or 10-6 M progesterone (Fisher) was added to the pellets, and approximately 6 × 105 cells were placed in each well of a four-chamber slide and allowed to adhere to the surface overnight. Medium was changed daily, and the concentration of 17β-estradiol or progesterone was measured using the DPC Immulite Chemiluminescent Enzyme Immunoassay (Corning-Metpath, Corning, NY) in select chambers after 24 h in culture just before addition of fresh medium. For evaluation of mRNA expression, 3 × 106 cardiac myocytes were grown in culture flasks, and at d 4 incubated in medium alone or in the presence of 17β-estradiol (10-5-10-9 M), or with progesterone (10-6 M-10-7 M) for 1-7 d as described in "Results." Culture medium was changed daily. Cardiac myocytes were then harvested with the use of trypsin for RNA isolation.
Immunocytochemistry. After 4 d in culture, cardiac myocytes were trypsinized, transferred to chamber slides, and allowed to adhere overnight. The adherent cells were washed in PBS-C, pH 7.4, fixed with 4% paraformaldehyde for 20 min, and permeabilized by exposure to 50% acetone/50% methanol for 20 min. Cells were again washed for 5 min in PBS-C. To determine purity of the cardiocyte culture, actinin staining was done at d 4 and 14. The cultured cells were incubated with monoclonal anti-α-actinin (sarcomeric) mouse IgG1 (Sigma Chemical Co., St. Louis, MO) at a dilution of 1:500 in PBS-C for 1 h at room temperature. Monoclonal anti-α-actinin antibody is specific for α-skeletal and cardiac muscle actinin. It stains Z lines and dots in stress fibers of myotubes in skeletal and cardiac muscle, but not in nonsarcomeric muscle elements present in connective tissue, epithelium, nerves, or smooth muscle. Cells were again washed for 5 min with PBS-C and incubated with either goat anti-mouse IgG (whole molecule) fluorescein isothiocyanate-conjugate (Sigma) or goat anti-mouse IgG Texas Red-conjugate (Molecular Probes, Eugene, OR) at 1:500 dilution in PBS-C for 30 min. After additional washing for 5 min, the cells were mounted in gelvatol and cover slips applied. To evaluate the cellular localization of SSA/Ro and SSB/La, cells were incubated with one of the following antisera at a dilution of 1:100 for 1 h in PBS-C: human autoimmune sera containing anti-48 kD SSB/La antibodies alone [Ze (20) or Lew] or 52 and 60 kD SSA/Ro antibodies alone [Ge (20), Ohl, or Di], or human sera with no known autoantibodies. In select experiments cells were stained with affinity purified anti-60 kD SSA/Ro or anti-SSB/La antibodies. Briefly, these antibodies were isolated from sera by affinity column chromatography using the respective histidine-tagged recombinant proteins coupled to cyanogen bromide-activated Sepharose 4B as antigens. Cells were washed and incubated with goat anti-human IgG fluorescein isothiocyanate-conjugate (Antibodies Inc., Davis, CA) or Texas Red Conjugated AffiniPure goat anti-human IgG (Accurate Chemical Corp, Westbury, NY) at 1:500 dilution. As an additional control, the first stage antibody was omitted. For optimal staining with anti-SSA/Ro antibodies, cells were permeabilized with 100% acetone alone for 5 min.
To evaluate the effect of sex hormones on the topology of the autoantigens, cells cultured on chamber slides were incubated with medium alone or in the presence of 10-7 M 17β-estradiol or 10-6 M progesterone. After 3 d of hormonal exposure (7 d from initial isolation), the cells were fixed and immediately stained or permeabilized with acetone/methanol or acetone alone, and then washed with PBS-C in preparation for staining as described above.
RNA isolation and RT-PCR. Total RNA was purified from each flask containing approximately 3 × 106 cells using the RNEasy kit (Qiagen, Chatsworth, CA), which is particularly useful for extracting RNA from limited quantities of cells. First strand synthesis was accomplished using ClonTech 1st Strand Synthesis Kit (ClonTech, Palo Alto, CA) and approximately 2 µg of total RNA. PCR was performed using a TC9600 Cycler (Perkin-Elmer, Foster City, CA). Briefly, the 50 µL reaction in 1X Taq buffer contains 2 U Taq polymerase (GIBCO BRL), 2.5 mM MgCl2, 200 mM of each dNTP, 20 pmol of each primer, and 1 µL of first strand synthesis reaction cDNA. Amplification was started by heating for 2 min to 94°C. Thirty cycles followed, each consisting of 1 min at 94°C, 2 min at 55°C, and 1.5 min at 72°C. The temperature was then held for 10 min at 72°C and cooled down to 4°C. A total of 13 µL of the respective products was mixed with 1.5 µL of sample buffer (50% glycerol, 0.25% bromophenol blue in H2O) and applied to a 1.5-1.8% agarose gel in Tris borate EDTA buffer for analysis. For determination of the molecular weights of the PCR products, 250 ng of φX174 RF DNA HaeIII digest and 250 ng λ DNA HindIII fragments (GIBCO BRL) were run in parallel. The gels were subsequently stained with ethidium bromide and photographs taken with Polaroid film 667.
For the detection of 52α and 52β, RT-PCR was performed as described using a sense primer spanning bases 423-443 (5′-TATGTGCCCAGTCTCGGAAAC-3′) located upstream of exon 4 and an anti-sense primer covering bases 1377-1396 (5′-GGCACATTCAGAGAAGGAGT-3′) downstream of exon 4 (22,25). Positive control plasmids encoding 52α and 52β cDNAs and the identical primers were run for direct comparison to ensure that the RT-PCR products were accurate representations. RT-PCR to identify the mRNA for 48 kD SSB/La was performed using a sense primer spanning bases 72-89 (5′-AGAATTCATGGCTGAAAATGGTGA-3′) located at the methionine start site for translation, and an anti-sense primer spanning bases 1348-1362 (5′-AATATGTCGACTTAAAAGCCCCGCAAAC-3′) of the full-length sequence for 48 kD SSB/La (14). RT-PCR was performed to identify the mRNA for 60 kD SSA/Ro using a sense primer spanning bases 386-406 (5′-GGCAGAGGATGTGAAGTGAT-3′) and an anti-sense primer spanning bases 1609-1629 (5′-TGCAAGGCTCTATCATCTGG-3′) of the full-length sequence for 60 kD SSA/Ro (9).
The mRNA of the housekeeping gene encoding for GAPDH was selected as an internal control. Oligonucleotide primers were synthesized with a model 394 DNA synthesizer (Applied Biosystems Inc., Foster City, CA). Two sets of primers were used: the sense primer 5′-TGGTATCGTGGAAGGACTCATGAC-3′ and anti-sense primer 5′-ATGCCAGTGAGCTTCCCGTTCAGC; and the sense primer 5′-TGAAGGTCGGAGTCAACGGATTTGGT-3′ and anti-sense primer 5′-CATGTGGGCCAT GAGGTCCACCAC-3′.
Densitometry. Densitometry was performed using Image QuaNT (Molecular Dynamics, Sunnyvale, CA).
RESULTS
Generation of human fetal cardiac cultures. Fetal hearts (18-22 wk) were digested by perfusion of the aorta with collagenase in a Langendorff apparatus. The cells released during the subsequent trypsin digestion were recovered and cultured on standard tissue culture-grade plasticware. Initial inspection of the dissociated fetal heart cells by phase-contrast light microscopy revealed a homogeneous population of rounded cells. After 24 h in culture with Dulbecco's modified Eagle's medium containing 15% FCS, the cells could be observed to adhere, and by d 3 a clear monolayer of 75-85% confluent cells was apparent. This method allows for a yield of approximately 45-60 × 106 cells per 3-5 g fetal heart.
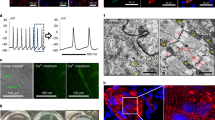
Staining with monoclonal anti-sarcomeric α-actinin was done at d 4 of culture to assess purity of the cells. At this time approximately 70-90% of the plated cells displayed the expected striations characteristic of differentiated cardiac myocytes (Fig. 1, A-C). However, after 12-14 d in culture, the percentage of cells expressing sarcomeric α-actinin was decreased by approximately 90% (Fig. 1D). Although dedifferentiated cardiac myoblasts do not express contractile proteins and have the capacity to proliferate, the cells were considered to be cardiac fibroblasts because of their characteristic flat appearance and large nuclei (34).
Immunofluorescence staining of human fetal cardiac myocytes with monoclonal anti-α-actinin. Immunofluorescence staining of the cells was done on d 4 culture with monoclonal anti-α-actinin which labels Z lines and dots in stress fibers of myotubes in cardiac muscle. A, B, and C demonstrate the expected striations typical of cardiac myocytes, as seen in ∼70-90% of the cultured cells. In C a cardiac fibroblast can be seen growing alongside the cardiac myocytes, as evidenced by the absence of stained striations. D demonstrates the overgrowth of cardiac fibroblasts that occurs by d 14, as supported by the absence of α-actinin. Intensity of staining may not be appreciated due to differences in depth of field.
The purity of cultures was also evaluated by the induction of contractility. On d 4 of culture, 1.8 mM of CaCl2 were added to the medium and the cardiac myocytes were placed on a warmed stage to maintain an approximate temperature of 37°C. The cells were viewed in an enhanced computer-generated image (250×). In the majority of fields, more than 90% of cells were observed to contract in synchrony at rates of 25-75 bpm. However, the number of beating cells per field become progressively fewer over time with only isolated groups of beating cells at d 14.
Cellular localization of 48 kD SSB/La, 52 kD SSA/Ro, and 60 kD SSA/Ro in cultured cardiac myocytes. The cellular locations of the SSA/Ro and SSB/La proteins were determined by conventional immunofluorescence and scanning confocal microscopy. Two human antisera, each containing 48 kD SSB/La antibodies and no detectable anti-52 or 60 kD SSA/Ro by ELISA, immunoblot, or immunoprecipitation, stained the nucleus of cardiac myocytes cultured from fetal hearts aged 19-21 wk (Fig. 2A). The nuclear immunofluorescence was homogeneous with no detectable staining of the cytoplasm. Two human antisera, each containing anti-SSA/Ro antibodies (52 and 60 kD), also demonstrated nuclear fluorescence with minor staining of the cytoplasm (Fig. 2B). Double-labeling with anti-α-actinin confirmed that the cells being stained were cardiac myocytes (Fig. 2, A and B, right). In fields where fibroblasts were observed, the cellular topology of SSA/Ro and SSB/La was identical to that seen in the cardiac myocytes.
Cellular localization of SSB/La and SSA/Ro in cultured human cardiac myocytes. Cells in A, B, and C were double-labeled. In A the cells were stained with both Lew (anti-La) and mouse anti-actinin and labeled with goat anti-human IgG FITC and goat anti-mouse IgG Texas Red conjugate. Cells were viewed under FITC (left) and Texas Red (right) filters. In B the cells were stained with Ohl (anti-Ro) and anti-actinin. In C the cells were stained with control serum (left) and anti-actinin (right). Single arrows point to representative cardiac myocytes as evidenced by staining with anti-actinin. Double arrows point to representative cells which stain only for SSA/Ro or SSB/La and not actinin, and are presumed to be fibroblasts. Fields were chosen to illustrate that staining of SSA/Ro and SSB/La is equivalent in cardiocytes and fibroblasts.
Cardiocyte mRNA expression of 48 kD SSB/La, 52 kD SSA/Ro, and 60 kD SSA/Ro after incubation with 17β-estradiol or progesterone. The successful culturing of human fetal cardiac myocytes allowed us to determine whether sex hormones modulate expression of the autoantigens. This was accomplished by evaluating the mRNA expression of 48 kD SSB/La and 60 kD SSA/Ro in addition to the alternative transcripts of 52 kD SSA/Ro after exposure to varying concentrations of 17β-estradiol. Because the fetal heart is normally exposed to high circulating levels of maternal hormones, the cardiac myocytes were established in culture for 4 d without the addition of exogenous estradiol or progesterone, to allow for washout. Approximately 3 × 106 cardiac myocytes were incubated in medium alone or in the presence of 17β-estradiol (10-6-10-9 M) for 1 to 7 d, after which they were harvested from culture flasks using trypsin. Total RNA was purified and mRNA expression analyzed using RT-PCR with sequence-specific primers for 48 kD SSB/La, 52 kD SSA/Ro, and 60 kD SSA/Ro cDNA. GAPDH mRNA expression was initially evaluated to normalize the RNA used in each reaction.
Figures 3 and 4 represent the RT-PCR results obtained after incubation of cell cultures from a 20-wk heart in medium alone or in the presence of 10-7 M 17β-estradiol [a physiologic concentration approached at the end of the third trimester of pregnancy (35)], respectively. In addition to a dominant 1.0 kb mRNA transcript representing the full-length 52α SSA/Ro, a transcript of 0.78 kb, representing the 52β form, was also expressed in the cardiac myocytes (Fig. 3A and Fig. 4B). The high ratio of 52α to 52β mRNA expression in cells isolated from hearts of 18-22 wk gestation is consistent with our previous findings in the intact heart, in which 52β was maximal between 14-16 wk with less expression thereafter (22,24). As assessed by densitometry of each amplification product, there was a trend toward increased expression of both isoforms of 52 kD SSA/Ro mRNA over time in cells cultured in medium alone (Fig. 3A). Similar results were seen when exogenous estradiol was added (Fig. 4B). In no case was there an increase over baseline expression (d 0) of more than 3-fold. mRNA expression of 60 kD SSA/Ro was also readily detected at baseline. Equivalent to the results observed for 52 kD SSA/Ro, there were no appreciable changes in expression after incubation with estradiol (Fig. 4C) compared with incubation over time in medium alone (Fig. 3B). mRNA expression of 48 kD SSB/La was detected at baseline and, as observed with 52 kD SSA/Ro or 60 kD SSA/Ro mRNA, never increased more than 3-fold. Similar results were seen in the 18- and 22-wk hearts (data not shown), with no differences between Down syndrome and normal. Decreased concentrations of estradiol to approximate levels present during the second trimester (10-8 M) or mid menstrual cycle (10-9 M) did not effect expression of any SSA/Ro-SSB/La component after 1 to 7 d of incubation (data not shown).
mRNA expression of 52 and 60 kD SSA/Ro and GAPDH in cardiac myocytes cultured from a 20-wk fetus after incubation in medium alone. First strand synthesis was performed using mRNA isolated from cardiac myocytes cultured from a 20-wk heart as described in the methods. Day 0 is representative of cells that have been in culture for 4 d (in medium alone) after initial isolation from the fetal heart. The cells were maintained in culture for an additional 4 d as depicted above each lane. Each PCR represents RNA isolated from 3 × 106 cells. In A, cDNA was amplified with a sense primer spanning bases 423-443 located upstream of exon 4 and an anti-sense primer covering bases 1377-1396 of 52 kD SSA/Ro. Two products were amplified: a larger transcript of 1.0 kb, corresponding to the full-length 52α; and a second of 0.78 kb, corresponding to 52β. cDNA controls for 52α and β forms are shown at the extreme right. In B, cDNA was amplified with a sense primer spanning bases 386-406 and an anti-sense primer spanning bases 1609-1629 of the full-length sequence for 60 kD SSA/Ro. cDNA control for 60 kD SSA/Ro is shown on the extreme right. In C, GAPDH expression is shown as reference for each condition. Densitometric units (× 103) are shown under each lane.
mRNA expression of 48 kD SSB/La, 52 kD SSA/Ro, and 60 kD SSA/Ro in cardiac myocytes cultured from a 20-wk fetus after incubation with 17β-estradiol. First strand synthesis was performed using mRNA isolated from cardiac myocytes cultured from a 20-wk heart (same heart as that used in Figs. 3 and 5) after exposure to 10-7 M 17β-estradiol for 1 to 4 d (as shown above each lane). Day 0 is representative of cells that have been in culture for 4 d (in medium alone) after initial isolation from the fetal heart. Each PCR represents RNA isolated from 3 × 106 cells. In A, the resulting cDNA was amplified with a sense primer spanning bases 72-89 and an anti-sense primer spanning bases 1348-1362 of the full-length sequence for 48 kD SSB/La. cDNA control for 48 kD SSB/La is shown on the extreme right. Although there appears to be a doublet, this is due to gel distortion and should not be interpreted as two separate bands. In B, cDNA was amplified with a sense primer spanning bases 423-443 located upstream of exon 4 and an anti-sense primer covering bases 1377-1396 of 52 kD SSA/Ro. cDNA controls for 52α and β forms are shown at the extreme left. In C, cDNA was amplified with a sense primer spanning bases 386-406 and an anti-sense primer spanning bases 1609-1629 of the full-length sequence for 60 kD SSA/Ro. cDNA control for 60 kD SSA/Ro is shown on the extreme right. In D, GAPDH expression is shown as reference for each condition. Densitometric units (× 103) are shown under each lane.
Figure 5 represents the RT-PCR results obtained after incubation of cell cultures from a 20-wk heart with 10-6 M progesterone, a physiologic concentration reached in the late third trimester of pregnancy (35). mRNA expression of 48 kD SSB/La, 52 kD SSA/Ro (α or β isoforms), or 60 kD SSA/Ro was not altered after 1 to 4 d of incubation. Equivalent results were observed in cultures obtained from an 18- and 22-wk heart (data not shown).
mRNA expression of 48 kD SSB/La, 52 kD SSA/Ro, and 60 kD SSA/Ro in cardiac myocytes cultured from a 20-wk fetus after incubation with progesterone. RNA was isolated from cardiac myocytes cultured from a 20-wk fetal heart after exposure to 10-6 M progesterone for 1 to 4 d. Day 0 is representative of cells cultured in medium without additional estradiol or progesterone for 4 d. Each PCR represents RNA isolated from 3 × 106 cells. The format is identical to that described for Figure 4. Densitometric units (× 103) are shown under each lane.
There were no changes in the cellular localization of 48 kD SSB/La, 52 kD or 60 kD SSA/Ro after incubation for 3 d with 10-7 M estradiol (Fig. 6, D-H) or 10-6 M progesterone (data not shown). Moreover, there was no detectable surface staining of these autoantigens, in either the presence (Fig. 6, J and L) or absence of exogenously added hormones (Fig. 6, I and K), in cardiac myocytes that were not permeabilized before incubation with the antibodies.
Cellular localization of SSB/La and SSA/Ro in human cardiac myocytes cultured with 17β-estradiol. Cells were isolated from a 19-wk fetal heart and cultured for 4 d in medium alone. Over the subsequent 3 d the cells were cultured in the absence (A, C, E, G, I, and K) or presence of 10-7 M 17β-estradiol (B, D, F, H, J, and L). Cells were either fixed with 4% paraformaldehyde and permeabilized with 100% acetone before staining (A-H) or stained in the absence of permeabilization (I-L). Cells were stained with specific antibodies as described for each panel, labeled with goat anti-human IgG FITC and viewed by scanning confocal microscopy. In A and B, cardiac myocytes were stained with normal human serum containing no known autoantibodies. In C and D, and I and J, cells were stained with affinity purified anti-SSB/La antibodies. In E and F, and K and L, cells were stained with serum Di which recognizes 52 kD SSA/Ro on immunoblot but immunoprecipitates in vitro translated 52 and 60 kD SSA/Ro. In G and H, cells were stained with affinity purified 60 kD SSA/Ro antibodies. In nonpermeabilized cardiac myocytes stained with normal human serum or affinity purified anti-60 kD SSA/Ro antibodies results were identical to those shown in I-L (data not shown).
To confirm that the cardiac myocytes had been exposed to the expected concentrations of hormone, estradiol and progesterone were measured in the culture medium after 24 h of incubation with the cells. Medium without added hormone contained 0.165-0.353 ng/mL of 17β-estradiol and 0.480-0.520 ng/mL of progesterone. Medium in which cells were incubated with 10-7 M 17β-estradiol or 10-6 M progesterone contained 16.2 ng/mL estradiol or 186 ng/mL progesterone, respectively.
DISCUSSION
While the mechanism by which antibodies to sequestered nuclear antigens cause tissue damage is of considerable interest for autoimmune diseases in general, it is particularly relevant to the pathogenesis of cardiac injury in the developing fetus. Research efforts to define the molecular basis for the near universal linkage of maternal antibodies reactive with components of the SSA/Ro-SSB/La ribonucleoprotein complex to CHB have been limited by the availability of target tissue. Culturing of human fetal cardiac myocytes represents a major advance. Accordingly, the main objective of this report is to provide sufficient detail for reproducible high yield cell culturing. We have applied the Langendorff technique to isolate cardiac myocytes and to subsequently establish cells in culture. The presence of cardiac myocytes was confirmed by two independent assays. Staining with a monoclonal antibody that recognizes sarcomeric α-actinin was used to track the percentage of cardiac myocytes at different days in culture. Contractility, after the addition of physiologic calcium and warming, assured that the cells were functionally viable cardiac myocytes.
Although the Langendorff technique has been used to obtain mammalian cardiac myocytes for patch clamping and recording of ion channels (30,36), to our knowledge all published methods for the culturing of human fetal heart cells describe initial mechanical dispersion (minced pieces) of tissue followed by incubation with various proteases (34,37–41). Moreover, little information is provided on cell yields per heart for use in primary cultures. Cannulation of the aorta with subsequent perfusion of the heart via the coronary arteries facilitates greater accessibility of proteolytic enzyme to the tissue. Several culture systems have been developed to generate progenitor cells of cardiac myocytes (myoblasts) to address questions of proliferation and subsequent differentiation by evaluation of serum withdrawal and different growth factors (34,39,40). In contrast, our goal was to set up high yield primary cultures of a differentiated phenotype as evidenced by spontaneous regular contractions and expression of muscle-specific markers. The establishment of human fetal cardiac myocytes in culture facilitates two categorical approaches to the study of CHB: developmental (i.e. comparison to cardiac myocytes isolated from adult hearts) and environmental.
mRNA and protein expression of the candidate antigens were readily demonstrated in the cultured cardiac myocytes. Based on indirect immunofluorescence using human antisera, both SSB/La and SSA/Ro autoantigens were located in the cell nucleus. The results for SSB/La are consistent with those reported by others in a myriad of different cell types (42,43). However, the cellular localization of SSA/Ro has been discrepant, in part based on the differences in fixation technique, human substrate, and the specificity of the antibodies used for immunostaining. Several studies have demonstrated predominantly nuclear and minor cytoplasmic localization for both the 52 and 60 kD SSA/Ro antigens (44–48). One study found predominantly cytoplasmic localization of 60 and nuclear of 52 in HeLa and HEp-2 cells (49). In a recent study by Yell and colleagues (50) using highly purified antisera, distinct differences were found between 52 and 60 kD SSA/Ro, the former predominantly cytoplasmic and the latter nuclear. The topology reported for SSA/Ro herein does not separate 52 kD reactivity from 60 kD SSA/Ro, which will be addressed in future studies.
Based on the hypothesis that inductive events in utero can alter the gene expression of SSA/Ro and/or SSB/La and promote their surface expression uniquely during cardiac development, we initiated a systematic evaluation of conditions that increase mRNA expression and/or facilitate the surface expression of SSA/Ro and/or SSB/La polypeptides. The rationale for incubations with estradiol and progesterone was based on several established observations. Both sex steroid hormones interact with high-affinity intracellular receptors and translocate to the nucleus to affect gene transcription and protein translation. The presence of estrogen and progesterone receptors has been established in rat and baboon aorta and myocardium (51,52). Furthermore, one mismatch consensus estrogen response element was identified upstream of the transcriptional start sites in the gene encoding 52 kD SSA/Ro, and an estrogen response element similar to that of c-myc was detected in the human gene encoding 60 kD SSA/Ro (32). Of further relevance to CHB, the highest levels of estrogen and progesterone occur during pregnancy: 25 ng/mL is the peak maternal concentration of 17β-estradiol achieved at 36 wk of gestation, and 400 ng/mL is the peak maternal concentration of progesterone achieved at the end of gestation (35). Despite these predictive factors, incubation of the cultured human cardiac myocytes with either 17β-estradiol or progesterone did not alter mRNA expression of 52 kD SSA/Ro (α or β), or 60 kD SSA/Ro compared with cells cultured in medium alone or result in cellular translocation. However, in preliminary experiments using this novel culture system, induction of apoptosis in the cardiac myocytes resulted in clustering of SSA/Ro and SSB/La in surface blebs.
In contrast to the results obtained with fetal cardiac myocytes, hormonal modulation of SSA/Ro and SSB/La has been demonstrated in keratinocytes and other cell lines (31). Most recently, Wang and Chan (32) demonstrated that 17β-estradiol at concentrations of 10-8 to 10-7 M induced up to a 5-fold increase in the expression of 52α and 60 mRNA in human keratinocytes isolated from neonatal foreskins, compared with untreated cells in which basal expression was low. Moreover, hormonal depletion of the human breast cancer cell line MCF-7 showed decreased levels of SSA/Ro mRNA and protein, and the addition of estradiol led to increased SSA/Ro expression. Notably, 52β mRNA was not expressed in the keratinocytes or MCF-7 cell line under any conditions.
The apparent discordance between cardiac myocytes and keratinocytes regarding increased mRNA expression and translocation of SSA/Ro-SSB/La proteins after hormonal exposure may account, in part, for clinical differences observed for the varied manifestations of the neonatal lupus syndromes (1). Available data, based on previous literature (2,8) and a recently established national research registry of over 120 cases of neonatal lupus (Buyon JP, unpublished data), support an equal sex distribution for CHB although rashes are more common in girls. Furthermore, cardiac disease is permanent, yet the skin lesions are transient and disappear coincidently with clearing of the maternal antibodies from the neonatal circulation. Bradyarrhythmias are detected in utero and rarely if even after birth, in contrast to cutaneous disease which is most often apparent several weeks after birth. Variable dependence on hormone modulation as a means of promoting accessibility of the candidate autoantigens in neonatal lupus may contribute to the clinically observed differences between cardiac and cutaneous manifestations particularly regarding sex predominance and timing. The issue of reversibility may also relate to the inability of conduction tissue cells from mid and late fetal stages to restore function once injured, although skin cells retain reparative capabilities.
Although it is acknowledged that damage specific to the conducting system of the human fetal heart is characteristically associated with maternal anti-SSA/Ro-SSB/La antibodies, it is anticipated that the culture system described can be adapted for isolated SA and AV nodal cells. However, the working myocardium is also a target of injury. Mononuclear cell infiltration has been demonstrated in the myocardium of a fetus dying in utero at 18 wk of gestation (7), and patchy lymphoid aggregates observed throughout the myocardium of an infant delivered at 30 wk and dying in the immediate postnatal period (53). Moreover, immunofluorescent studies have shown deposition of IgG and complement (including Clq, C4, C3d, C6, and C9) in the working myocardium as well as the conducting system (5,53,54). Litsey et al. (5) identified IgG deposits in the epicardial, myocardial, and endocardial tissue of the right atrium on postmortem analysis of a neonate with CHB. Extensive review of cases in the Research Registry for Neonatal Lupus, established in October 1994, revealed that pancarditis can also be part of the spectrum of disease as well as intractable cardiomyopathy requiring transplantation (55).
In summary, we have developed a novel method to successfully culture human fetal cardiac myocytes. Differential constitutive and estradiol-induced expression of 52α, 52β, and 60Ro in human cardiac myocytes and keratinocytes may be a factor contributing to the marked discordance of clinically detectable injury in these two target tissues. This culture system should provide an invaluable tool for defining the molecular mechanism whereby anti-SSA/Ro and/or anti-SSB/La antibodies, or perhaps autoantibodies yet to be identified, bind target antigens and damage fetal heart tissue. Because only 1 to 2% of mothers with anti-SSA/Ro and/or SSB-La antibodies give birth to children with CHB (2,56), the search for unique fetal factors may be facilitated by the use of cardiac myocyte cultures.
Abbreviations
- CHB:
-
congenital heart block
- AV:
-
atrioventricular
- bpm:
-
beats per minute
- GAPDH:
-
glyceraldehyde 3-phosphate dehydrogenase
- FCS:
-
fetal calf serum
- PBS-C:
-
PBS containing 0.1 mM CaCl2
- RT-PCR:
-
reverse transcriptase polymerase chain reaction
References
Buyon JP 1992 Neonatal lupus syndromes. In: Lahita R (ed) Systemic Lupus Erythematosus. Churchill Livingstone, New York, 419–435.
Lee LA 1993 Neonatal lupus erythematosus. J Invest Dermatol 100: 9S–13S
Buyon JP, Winchester R 1990 Congenital complete heart block: a human model of passively acquired autoimmune injury. Arthritis Rheum 33: 609–614
Buyon JP, Waltuck J, Kleinman C, Copel J 1995 In utero identification and therapy of congenital heart block (CHB). Lupus 4: 116–121
Litsey SE, Noonan JA, O'Connor WN, Cottrill CM, Mitchell B 1985 Maternal connective tissue disease and congenital heart block. N Engl J Med 312: 98–100
Ho SY, Esscher A, Anderson RH, Michaelsson M 1986 Anatomy of congenital complete heart block and relation to maternal anti-Ro antibodies. Am J Cardiol 58: 291–294
Herreman G, Galezewski N 1985 Maternal connective tissue disease and congenital heart block. [letter] N Engl J Med 312:1329
Waltuck J, Buyon JP 1994 Autoantibody-associated congenital heart block: Outcome in mothers and children. Ann Intern Med 120: 544–551
Ben-Chetrit E, Gandy BJ, Tan EM, Sullivan KF 1989 Isolation and characterization of a cDNA clone encoding the 60-kD component of the human SS-A/Ro ribonucleoprotein autoantigen. J Clin Invest 83: 1284–1292
Deutscher SL, Harley JB, Keene JD 1988 Molecular analysis of the 60 kDa human Ro ribonucleoprotein. Proc Natl Acad Sci USA 85: 9479–9483
Lerner MR, Boyle JA, Hardin JA, Steitz JA 1981 Two novel classes of small ribonucleoproteins detected by antibodies associated with lupus erythematosus. Science 211: 400–402
Wang D, Buyon JP, Chan EKL 1996 Cloning and expression of mouse 60 kDa ribonucleoprotein SS-A/Ro. Mol Biol Rept 23: 205–210
O'Brien CA, Wolin SL 1994 A possible role for the 60-kD Ro autoantigen in a discard pathway for defective 5s rRNA precursors. Genes Dev 8: 2891–2903
Chan EKL, Sullivan KF, Tan EM 1989 Ribonucleoprotein SS-B/La belongs to a protein family with consensus sequences for RNA-binding. Nucleic Acids Res 17: 2233–2244
Chambers JC, Kenan D, Martin BJ, Keene JD 1988 Genomic structure and amino acid sequence domains of the human La autoantigen. J Biol Chem 263: 18043–18051
Boire B, Craft J 1990 Human Ro ribonucleoprotein particles: characterization of native structure and stable association with the La polypeptide. J Clin Invest 85: 1182–1190
Gottlieb E, Steitz JA 1989 Function of the mammalian La protein: evidence for its action in transcription termination by RNA polymerase III. EMBO J 8: 851–861
Yoo CJ, Wolin SL 1997 The yeast La protein is required for the 3′ endonucleolytic cleavage that matures tRNA precursors. Cell 89: 393–402
Ben-Chetrit E, Chan EKL, Sullivan KF, Tan EM 1988 A 52 kD protein is a novel component of the SS-A/Ro antigenic particle. J Exp Med 162: 1560–1571
Chan EKL, Hamel JC, Buyon JP, Tan EM 1991 Molecular definition and sequence motifs of the 52-kD component of human SS-A/Ro autoantigen. J Clin Invest 87: 68–76
Itoh K, Itoh Y, Frank MB 1991 Protein heterogeneity in the human Ro/SSA ribonucleoproteins. J Clin Invest 87: 177–186
Chan EKL, DiDonato F, Hamel JC, Tseng CE, Buyon JP 1995 52-kD SS-A/Ro: Genomic structure and identification of an alternatively spliced transcript encoding a novel leucine zipper-minus autoantigen expressed in fetal and adult heart. J Exp Med 182: 983–992
Buyon JP, Slade SG, Reveille JD, Hamel JC, Chan EKL 1994 Autoantibody responses to the "native" 52kD SS-A/Ro protein in neonatal lupus syndromes, systemic lupus erythematosus and Sjögren's syndrome. J Immunol 152: 3675–3684
Blange I, Ringertz NR, Pettersson I 1994 Identification of antigenic regions of the human 52kD Ro/SS-A protein recognized by patient sera. J Autoimmun 7: 263–274
Buyon JP, Tseng CE, DiDonato F, Rashbaum W, Morris A, Chan EKL 1997 Cardiac expression of 52β, an alternative transcript of the congenital heart block-associated 52-kD SS-A/Ro autoantigen, is maximal during fetal development. Arthritis Rheum 40: 655–660
Horsfall AC, Venables PJW, Taylor PV, Maini RN 1991 Ro and La antigens and maternal autoantibody idiotype on the surface of myocardial fibres in congenital heart block. J Autoimmun 4: 165–176
Reichlin M, Brucato A, Frank MB, Maddison PJ, McCubbin VR, Wolfson-Reichlin M, Lee LA 1994 Concentration of autoantibodies to native 60-kD Ro/SS-A and denatured 52-kD Ro/SS-A in eluates from the heart of a child who died with congenital complete heart block. Arthritis Rheum 37: 1698–1703
Alexander E, Buyon JP, Provost TT, Guarnieri T 1992 Anti-Ro/SS-A antibodies in the pathophysiology of congenital heart block in neonatal lupus syndrome, an experimental model: in vitro electrophysiologic and immunocytochemical studies. Arthritis Rheum 35: 176–189
Garcia S, Nascimento JH, Bonfa E, Levy R, Oliveira SF, Tavares AV, Campos deCarvalho AC 1994 Cellular mechanism of the conduction abnormalities induced by serum from anti-Ro/SSA-positive patients in rabbit hearts. J Clin Invest 93: 718–724
Boutjdir M, Chen L, Zhang Z, Tseng C, DiDonato F, Rashbaum W, Morris A, El-Sherif N, Buyon JP 1997 Arrhythmogenicity of IgG and anti-52kD SSA/Ro affinity purified antibodies from mothers of children with congenital heart block. Circ Res 80: 354–362
Furukawa F, Lyons MB, Lee La, Coulter SN, Norris DA 1988 Estradiol enhances binding to cultured human keratinocytes of antibodies specific for SSA/Ro and SSB/La. J Immunol 141: 1480–1488
Wang D, Chan EKL 1996 17β-estradiol increases expression of 52 kDa and 60 kDa SSA/Ro autoantigens in human keratinocytes and breast cancer cell line MCF-7. J Invest Dermatol 107: 610–614
Langendorff O 1895 Untersuchungen am überlebenden Saugetierherzen. Arch Gesamte Physiol Mens Tiere Pflügers 61: 291–332
Kohtz DS, Dische NR, Inagami T, Goldman B 1989 Growth and partial differentiation of presumptive human cardiac myoblasts in culture. J Cell Biol 108: 1067–1078
Levitz M, Young BK 1977 Estrogens in pregnancy. Vitam Horm 35: 109–147
Tytgat J 1994 How to isolate cardiac myocytes. Cardiovasc Res 28: 280–283
Hassall CJ, Penketh R, Rodeck C, Burnstock, G 1990 The intracardiac neurones of the fetal human heart in culture. Anat Embryol 182: 329–337
Bkaily G, Sculptoreanu A, Jacques D, Economos D, Menard D 1992 Apamin, a highly potent fetal L-type Ca2+ current blocker in single heart cells. Am J Physiol 262: H463–H471
Goldman B, Mach A, Wurzel J 1996 Epidermal growth factor promotes a cardiomyoblastic phenotype in human fetal cardiac myocytes Exp Cell Res 228: 237–245
Goldman BI, Wurzel J 1992 Effects of subcultivation and culture medium on differentiation of human fetal cardiac myocytes. In Vitro Cell Dev Biol 28A: 109–119
Huang MH, Friend DS, Sunday ME, Singh K, Haley K, Austen KF, Kelly RA, Smith TW 1996 An intrinsic adrenergic system in mammalian heart. J Clin Invest 98: 1298–1303
Chan EKL, Andrade LEC 1992 Antinuclear antibodies in Sjögren's syndrome. Rheum Dis Clin North Am 118: 551–565
Alspaugh MA, Talal N, Tan EM 1976 Differentiation and characterization of autoantibodies and their antigens in Sjögren's syndrome. Arthritis Rheum 19: 216–222
Ben-Chetrit E, Chan EKL, Sullivan KF, Tan EM 1988 A 52-kD protein is a novel component of the SS-A/Ro antigenic particle. J Exp Med 167: 1560–1571
Slobbe RL, Pruijn GJM, Damen WGM, Van der Keemp JWCM, Van Venrooij WJ 1991 Detection and occurrence of the 60- and 52-kD Ro (SS-A) antigens and of autoantibodies against these proteins. Clin Exp Immunol 86: 99–105
Peek R, van Venrooij WJ, Simons F, Pruijn G 1994 The SS-A/SS-B autoantigenic complex: localization and assembly. Clin Exp Rheumatol 12( suppl 11): S15–S18
Casciola-Rosen LA, Anhalt G, Rosen A 1994 Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med 179: 1317–1330
Wahren M, Mellqvist E, Vene S, Ringertz NR, Pettersson I 1996 Nuclear colocalization of the Ro 60 kDa autoantigen and a subset of U snRNP domains. Eur J Cell Biol 70: 189–197
Kelekar A, Saitta MR, Keene JD 1994 Molecular composition of Ro small ribonucleoprotein complexes in human cells. J Clin Invest 93: 1637–1644
Yell JA, Wang L, Hongliang Y, McCauliffe DP 1996 Disparate locations of the 52- and 60-kDa Ro/SS-A antigens in cultured human keratinocytes. J Invest Dermatol 107: 622–626
Lin AL, Gonzalez R, Carey KD, Shain SA 1986 Estradiol-17β affects estrogen receptor distribution and elevated progesterone receptor content in baboon aorta. Arteriosclerosis 6: 495–504
Ciocca DR, Vargas-Roig LM 1995 Estrogen receptors in human nontarget tissues: biological and clinical implications. Endocrine Rev 16: 35–62
Lee LA, Coulter S, Erner S, Chu H 1987 Cardiac immunoglobulin deposition in congenital heart block associated with maternal anti-Ro antibody. Am J Med 83: 793–796
Taylor PV, Scott JS, Gerlis LM, Path FRC, Esscher E, Scott O 1986 Maternal antibodies against fetal cardiac antigens in congenital complete heart block. N Engl J Med 315: 667–672
Buyon JP, Hiebert R, Copel J, Craft J, Friedman D, Katholi M, Lee L, Provost T, Reichlin M, Rider L, Rupel A, Weston W, Skovron ML 1998 Autoimmune-associated congenital heart block: mortality, morbidity, and recurrence rates obtained from a national neonatal lupus registry. J Am Coll Cardiol 31: 1658–1666
Silverman ED 1993 Congenital heart block and neonatal lupus: prevention is the goal. J Rheum 20: 1101–1104
Acknowledgements
The authors thank Dr. Jack Ricci for technical expertise.
Author information
Authors and Affiliations
Additional information
Supported in part by Grants AR41803 and AR42455 from the National Institutes of Health and an Arthritis Foundation Fellowship to C.T.
Rights and permissions
About this article
Cite this article
Tseng, CE., Miranda, E., Di Donato, F. et al. mRNA and Protein Expression of SSA/Ro and SSB/La in Human Fetal Cardiac Myocytes Cultured Using a Novel Application of the Langendorff Procedure. Pediatr Res 45, 260–269 (1999). https://doi.org/10.1203/00006450-199902000-00018
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199902000-00018