Abstract
Our purpose in this study was to examine whole body composition, using dual energy x-ray absorptiometry (DEXA) during dietary intervention in preterm infants (≤1750 g birthweight, ≤34 wk gestation). At discharge, infants were randomized to be fed either a preterm infant formula (discharge–6 mo; group A) or a term formula (discharge–6 mo; group B), or the preterm formula (discharge–term) and the term formula (term–6 mo; group C). Nutrient intake was measured between each clinic visit. To measure body composition, DEXA was used at discharge, term, 12 wk, 6 mo, and 12 mo corrected age. The data were analyzed by ANOVA. At discharge, no differences were noted in patient characteristics between groups A, B, and C. Although energy intakes were similar, protein and mineral intakes differed between groups (A > C > B;p< 0.0001). During the study, weight gain and LM gain were greater in group A than B. At 12 mo, weight, LM, FM, and BMM but not % FM or BMD were greater in group A than B. However, the effects of diet were confined to boys, with no lasting effects seen in girls. In summary, therefore, DEXA was precise enough to detect differences in whole body composition during dietary intervention. Increased weight gain primarily reflected an increase in LM and is consistent with the idea that the preterm formula more closely met protein and/or protein-energy needs in rapidly growing preterm male infants.
Similar content being viewed by others
Main
DEXA provides a relatively easy and noninvasive way to measure body composition. Although the methodology has been questioned (1–3), recent data suggest improved accuracy and precision with new updated software (4, 5). However, the value of DEXA remains unproved, particularly in preterm infants in which accuracy and precision may be affected by body size.
We previously reported that preterm infant boys fed a preterm formula after initial hospital discharge grew better than did those fed a term formula (6). Because better growth was related to increased blood-urea-nitrogen concentrations, it was speculated that improved growth reflected increased protein intake and, therefore, increased LM.
This paper examines the changes in whole body composition when DEXA is used during dietary intervention in the same group of preterm infants and tests the hypothesis that better growth in infants fed the preterm formula primarily reflected an increase in LM.
METHODS
This study was prospective, blind, randomized, and controlled. The study began immediately before initial hospital discharge and lasted until 12 mo corrected age. The study was approved by the Ethics Committee of the Newcastle and North Tyneside Health Authority, and informed consent was obtained from the parent(s) or legal guardian.
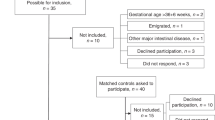
Preterm infants with a gestational age of ≤34 wk and a birthweight of ≤1750 g were considered eligible for the study. Those with no evidence of systemic disease, requiring no medication, and growing normally at the time of hospital discharge;i.e.,≥25 g/d, were enrolled.
At hospital discharge, infants were stratified according to birthweight (<1250 g or ≥1250 g) and were randomized to be fed either 1) a standard preterm infant formula (Cow and Gate Nutriprem LBW, Nutricia Ltd) between discharge and 6 mo corrected age (group A), 2) a standard term infant formula (Cow and Gate Premium, Nutricia Ltd) between discharge and 6 mo corrected age (group B), or 3) a preterm formula between discharge and term, and then the term formula between term and 6 mo corrected age (group C).
The preterm formula differed in several respects from the term formula, e.g., energy (80 vs 66 kcal/100 ml for the preterm and term formulas), protein (2.2 vs 1.4 g/100 ml), calcium (108 vs 54 kcal/100 ml), and phosphorus (54 vs 27 mg/100 ml).
Ready-to-feed formula was provided in cartons containing 24 × 100 mL bottles. Infants were discharged with a 2- to 3-wk supply of preweighed cartons and instructions to thoroughly shake each bottle before feeding and to feed the formula on demand. A 24-h service was provided to deal with any problems.
Infants were seen at the outpatient clinic every 2 wk between discharge and term and monthly between term and 6 mo. At each visit, intake was determined. Body composition was measured at discharge, term, 12 wk, 6 mo, and 12 mo corrected age.
Intake between visits was calculated from the differences in weight between the delivered and returned cartons and were averaged for the 2- to 4-wk period. Body composition was measured by using DEXA (Hologic QDR 2000, Hologic Inc, Waltham, MA), with a special radiation filter on top of the normal scanning couch. This filter plate is optimized for a body weight range of 1–10 kg. Scan analysis was performed with Infant Whole Body Software, version 5.56P.
The scan procedure was as follows. Before the arrival of the infant, the scanning couch of the densitometer was moved to the starting position with the radiation filter plate in place. The scanning area was then covered with a commercial absorbent paper material, and the calibration phantom (the “T” bar) was placed at the edge of the scanning area. The phantom was constructed of PMMA (Perspex) and aluminium, and was designed to simulate three thicknesses of soft tissue and three densities of bone overlying soft tissue.
After arrival, when the baby was settled, she/he was undressed, swaddled in a light cotton sheet, and placed supine onto the scanning surface, parallel to the long axis of the couch with the head about 2 cm from the starting point of the scan as marked on the positioning aid. The scan was begun when the infant was quiet; a research nurse was constantly in attendance.
Scan precision was assessed by scanning the torso and arm sections of the pediatric anthropometric phantom seven times. The phantom had been positioned according to standard protocol for a 1-y-old child. During analysis of each completed baby scan, the head, chest, pelvis, legs, and arms were identified as distinct regions of interest, with boundaries drawn with the software provided. All scans were performed by one of two operators with an intra-operator variability of 1.36 ± 1.0 (mean ± SD) and 0.92 ± 0.7% for operators 1 and 2, respectively.
The data were analyzed by ANOVA, with group, age, and sex as blocking variables. Posthoc comparisons were examined by Bonferroni-Dunn test.
RESULTS
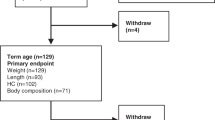
One hundred and twenty-nine infants (group A = 48, B = 54, C = 26; boys = 73, girls = 56;n= 450 scans) were studied. The characteristics of the infants at the time of entry into the study are presented in Table 1; no significant differences between the treatment groups were detected.
Mean energy intakes (108 ± 36 vs 106 ± 32 vs 105 ± 32 kcal/kg/d for A, B, and C) were similar, but protein (2.9 ± 0.9 > 2.3 ± 0.8, 2.4 ± 1.0 g/kg/d; A > and C), calcium (142 ± 44 > 87 ± 30, 102 ± 53 mg/kg/d; A > and C) and phosphorus (71 ± 22 > 43 ± 14, 51 ± 26 mg/kg/d; A > B and C) intakes differed between the groups (p< 0.001).
Between discharge and term, weight gain, LM gain, FM gain, and BMM gain were similar in groups A and C. In term-12 mo infants, weight gain, LM gain, FM gain, BMM gain, body weight, etc., were similar in groups B and C. For ease and clarity of presentation, therefore, the paper will concentrate on a direct comparison between infants in group A, i.e., those fed the preterm formula throughout the study, and infants in group B, i.e., those fed the term formula throughout the study.
Growth velocity data are presented in Figure 1. Growth rates were greatest between discharge and term and declined significantly thereafter (p< 0.0001). Among girls, no differences were detected in growth velocities between those in groups A and B. Among boys, weight gain, LM gain, and FM gain were greater in group A than B, primarily reflecting differences between discharge and term.
Body composition data are also presented in Figure 1. Weight, LM, and FM increased in both groups during the study (p< 0.0001). Percent FM increased between discharge and 6 mo (p< 0.0001) but declined significantly between 6 and 12 mo (p< 0.05). In girls, weight (<0.01) and LM (p< 0.01), but not FM or % FM differed, between the groups (A > B). These differences primarily reflected measurements at term and 12 wk, because at 6 and 12 mo, values were similar.
In boys, increased weight (p< 0.001), LM (p< 0.005) and FM (p< 0.01), but not % FM, were noted in group A, compared with B. At 12 mo corrected age, weight (9645 ± 1255 > 8683 ± 827 g), LM (7224 ± 717 > 6764 ± 605) and FM (2420 ± 757 > 1919 ± 518 g) were greater in group A than B. No differences were detected in % FM (25 ± 6%vs 22 ± 5%) between groups A and B.
Bone mineral data are presented in Figure 2. In the girls in groups A and B, no significant differences were noted in BA or BMM gain. In girls, BA and BMM were greater in group A than B (p< 0.001). These differences reflected measurements at term and 12 wk, because at 6 and 12 mo, values were similar. No differences were detected in BMD between groups A and B.
In boys, no significant differences were detected in BA and BMM gain between groups A and B. However, BA (p< 0.001) and BMM (p< 0.001), but not BMD, were greater in A than B. At 12 mo corrected age, BA and BMM in group A were 656 ± 59 cm2 and 202 ± 35 g compared with 621 ± 59 cm2 and 186 ± 23 g in group B.
Energy (105 ± 35 vs 106 ± 32 kcal/kg/d), protein (2.9 ± 0.9 vs 2.9 ± 0.9 g/kg/d), calcium (141 ± 45 vs 142 ± 46 mg/kg/d), and phosphorus (71 ± 23 ±vs 72 ± 22 mg/kg/d) intakes were virtually identical in girls and boys in group A. However, weight gain (p< 0.005), LM gain (p< 0.001), weight (p< 0.001), and LM (p< 0.001) were less in girls than boys (Fig. 3). No differences were detected in absolute FM, but % FM was greater in girls (p< 0.001;Fig. 3). No differences were detected in BA gain, BMM gain, BA, BMM, or BMD between girls and boys in group A.
DISCUSSION
In this study, DEXA was precise enough to detect differences in the rate and nature of growth between treatment groups and between the sexes. Differences in growth largely reflected increased nutrient accretion between discharge and term and not thereafter. This suggests that the timing of dietary intervention may be critical and supports the idea that “critical epochs of growth” occur in these rapidly growing infants.
Compared with boys fed the term formula, increased weight gain in boys fed the preterm formula primarily reflected an increase in LM. Although increased weight gain also reflected an increase in absolute FM, no differences were detected in % FM between the treatment groups. The latter observation is important.
The accuracy of DEXA has been questioned, because a small error in LM (∼5%) results in a large error in FM (∼100%) measurement (2). In this study, a wide variability was noted in % FM, and a type II error may have occurred when % FM was compared between groups. The coefficient of variation in total FM (0.36) was not less than that in % FM (0.67). Because significant differences were detected in the former, a type II error in the latter seems unlikely. Nonetheless, attention should be paid to this possibility when using DEXA to measure whole-body FM.
Differences in weight and LM between boys fed the preterm and term formulas cannot be related to energy intakes, which were similar (108 vs 106 kcal/kg/d for groups A and B). In part, they may be related to differences in protein intake (A, 2.9 > B, 2.1 g/kg/d, p< 0.0001) and are consistent with the idea that protein intake and/or protein: energy ratio more closely mirrored requirements in male infants fed the preterm formula (6).
Boys fed the preterm formula also had a greater weight gain, LM gain, weight, and LM than did girls fed the preterm formula. This cannot be related to differences in energy or protein, calcium, or phosphorus intakes, which were virtually identical, and it raises an important question about the control of growth during infancy.
Term infant boys have increased growth velocity, are larger, and have increased LM but lower % FM than girls (7), trends closely matched by our preterm infants. How this is controlled is not clear. Growth, in terms of LM or ideal body weight for length, is thought to be genetically determined (7, 8). Boys may therefore be programmed to grow faster, and also to have increased LM accretion, than girls.
How this is achieved is less clear. Term boys and girls may regulate dietary intake differently; the way in which a boy or girl sucks may alter the protein and/or energy content of breast milk. However, few data are available that compare dietary intake in breast- or formula-fed term girls and boys. In this study, intakes were similar.
Programming may alter nutrient metabolism. However, energy and nutrient balance studies have not reported systematic differences between the sexes. The chemical composition of LM may change with maturity (9). As the infant matures, protein content and, therefore, energy cost of LM deposition may decrease. If the relative change were greater in boys than girls, that might, in part, explain our findings.
The results of this study are important. Although the value of DEXA has been questioned, this study suggests that DEXA is precise enough to detect differences in body composition during dietary intervention. Results from our first paper indicated that boys fed a preterm formula grew better than boys fed a term infant formula (6). Data in this paper suggest that improved weight gain primarily reflected differences in LM, an important consideration when feeding different levels of energy and protein.
Differences in the velocity and nature of growth between girls and boys have been recognized in the term infant (7). This is the first study to show similar trends in the preterm infant and to indicate that differences in growth are not explained by differences in nutrient intake. Further studies are needed to more closely examine the regulation of growth in the sexes.
Abbreviations
- DEXA:
-
dual energy x-ray absorptiometry
- LM:
-
lean body mass
- FM:
-
fat mass
- % FM:
-
percent fat mass
- BA:
-
bone area
- BMM:
-
bone mineral mass
- BMD:
-
bone mineral density
- DEXA:
-
dual energy x-ray absorptiometry
References
Brunton JA, Bayley HS, Atkinson SA 1993 Validation and application of dual-energy x-ray absorptiometry to measure bone mass and body composition in small infants. Am J Clin Nutr 58: 839–845.
Roubenoff R, Kehayias JJ, Dawson-Hughes B, Heymsfield SB 1993 Use of dual-energy x-ray absorptiometry in body-composition studies: not yet a “gold standard. Am J Clin Nutr 58: 589–591.
Tothill P, Roubenoff R, Kehayias JJ, Dawson-Hughes B, Heymsfield SB 1993 Limitations of dual-energy x-ray absorptiometry. Am J Clin Nutr 61: 398–400.
Picaud JC, Rigo J, Nyamugabo K, Milet J, Senterre J 1996 Evaluation of dual-energy x-ray absorptiometry for body-composition assessment in piglets and term human neonates. Am J Clin Nutr 63: 157–163.
Brunton JA, Weiler HA, Atkinson SA 1997 Improvement in the accuracy of dual energy x-ray absorptiometry for whole body and regional analysis of body composition: validation using piglets and methodologic considerations in infants. Pediatr Res 41: 590–596.
Cooke RJ, Griffin IJ, McCormick K, Wells JCK, Smith JS, Robinson SJ, Leighton M 1998 Feeding preterm infants after hospital discharge: effect of dietary manipulation on nutrient intake and growth. Pediatr Res 43: 355–360.
Fomon SJ 1993 Size and growth. In: Fomon SJ (ed) Nutrition of Normal Infants. Mosby-Year Book, St. Louis, MO, 36–84.
Ashworth A, Millward DJ 1986 Catch-up growth in children. Nutr Rev 44: 157–163.
Moulton C 1923 Age and chemical development in mammals. J Biol Chem 57: 79–97.
Acknowledgements
The authors thank our research staff (J. S. Smith, M. Henderson, M. Leighton, S. Robinson) and the nursing and medical staff on the Special Care Baby Units of the Royal Victoria Infirmary and Ashington General Hospital for their advice and support in performing this study.
Author information
Authors and Affiliations
Additional information
This study was supported in part by a grant from Nutricia Ltd, Trowbridge, Wilts, United Kingdom.
Rights and permissions
About this article
Cite this article
Cooke, R., McCormick, K., Griffin, I. et al. Feeding Preterm Infants after Hospital Discharge: Effect of Diet on Body Composition. Pediatr Res 46, 461 (1999). https://doi.org/10.1203/00006450-199910000-00017
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199910000-00017
This article is cited by
-
Different doses of phosphorus, calcium, and vitamin D in premature infants and their effect on bone mineralization: systematic review and meta-analysis
Nutrire (2023)
-
Body composition measurement for the preterm neonate: using a clinical utility framework to translate research tools into clinical care
Journal of Perinatology (2022)
-
Improving long-term health outcomes of preterm infants: how to implement the findings of nutritional intervention studies into daily clinical practice
European Journal of Pediatrics (2021)
-
Growth and metabolic outcome in adolescents born preterm (GROWMORE): follow-up protocol for the Newcastle preterm birth growth study (PTBGS)
BMC Pediatrics (2013)
-
Increased gain in bone mineral content of preterm infants fed an isocaloric, protein-, and mineral-enriched postdischarge formula
European Journal of Nutrition (2013)