Abstract
Leptin is a metabolic signal that may be involved in signaling adequacy of energy metabolism for the onset of reproductive function. The aim of this study was to investigate the relationship between leptin serum levels and pubertal development in girls with progressive central precocious puberty (CPP). We investigated longitudinally 14 girls with CPP before and during treatment with depot leuprorelin acetate. Mean (± SEM) chronological age and bone age at start of therapy were 6.0 ± 0.6 y and 9.5 ± 0.7 y, respectively. Leptin was determined by RIA. Girls with CPP showed no significant difference in leptin levels at pretreatment and after 1 and 2 y of treatment compared with healthy girls of the same body mass index (BMI). Mean leptin SD score adjusted for BMI was 0.31 ± 0.4, 0.24 ± 0.2, and 0.49 ± 0.3, respectively (not significant). In a stepwise regression analysis model with BMI, bone age, chronological age, basal and stimulated LH, estradiol, dehydroepiandrosterone, androstenedione, and clinical pubertal signs, BMI was the only parameter that showed a significant correlation with leptin (p = 0.006). In conclusion, these data suggest that serum leptin levels are not significantly elevated at the onset of CPP compared with normal girls. Treatment with depot gonadotropin releasing hormone agonist seems to have no influence on leptin concentrations. As in normal girls, serum leptin levels in girls with CPP are mainly determined by BMI. Thus, we have no evidence that alterations of leptin are related to premature onset of puberty.
Similar content being viewed by others
Main
Leptin is the protein product of the obese (ob) gene. It is secreted as a hormone mainly from adipocytes and interacts with its receptor in the hypothalamus to signal the state of energy stores to the brain. Serum levels of leptin are correlated with the degree of obesity and are regulated by food intake and fasting status(1–4).
Homozygous defects in ob/ob mice or db/db mice, which are unable to produce leptin or to express the leptin receptor, result in adiposity, infertility, short stature, and abnormally high circulating levels of glucose, insulin, and cortisol. Administration of leptin to ob/ob mice corrects these features(5,6). Injection of low doses of leptin leads to an accelerated sexual maturation and earlier onset of reproductive function in female mice(7), although an adequate nutritional status is a prerequisite for pubertal growth and sexual development. Calorie restriction in animals leads to a suppression of reproductive function(7–9). Leptin administration to malnourished prepubertal rats only partially normalizes the delay in pubertal maturation(8,9). Thus, leptin plays an important role in the control of reproductive function in the rodent model and seems to be a signal to allow for sexual maturation to proceed under adequate metabolic conditions(10).
Studies on leptin-deficient humans show that leptin is essential for the initiation of puberty and establishment of secondary sexual characteristics. Patients who are homozygous for a mutation in the leptin receptor gene show no signs of pubertal development and only limited secretion of growth and thyrotropic hormones(11,12). A homozygous missense mutation in the leptin gene results in morbid obesity(13) and impaired sexual function(14).
In humans, longitudinal and cross-sectional studies show increasing leptin levels before the onset of puberty(15–23). In boys, longitudinal and cross-sectional studies show that leptin levels rise by approximately 50% just before the onset of puberty compared with baseline prepubertal levels, and decrease to baseline levels after the initiation of puberty(16,22,23). In girls, leptin levels increase after the onset of puberty, remain constant in midpuberty, and rise to a peak in late puberty(15). In all pubertal stages, leptin concentrations correlate positively with body mass index (BMI) and fat mass(21). It has been demonstrated that leptin levels and body fat mass are inversely related to age at menarche in girls(24). Reference ranges for leptin were constructed in relation to BMI(21). All these investigations emphasize that leptin has a key signaling function in regulating pubertal development, not only in laboratory animals but also in normal children.
The aim of our study was to investigate the relationship between leptin levels and pathologic early onset of pubertal development in girls with central precocious puberty (CPP). In a longitudinal trial, we examined the leptin concentrations in girls with CPP before start of therapy, when the hypothalamic-pituitary-gonadal axis was fully activated, and after 1 and 2 y of treatment with the depot gonadotropin releasing hormone (GnRH) agonist, leuprorelin (leuprolide) acetate, when puberty was completely suppressed.
METHODS
Patients. Fourteen girls (mean age, 6.0 ± 0.6 y; range, 2.1-8.7 y) with progressive idiopathic CPP were studied. This study was approved by the Ethical Committee of the Medical Faculty of the University of Kiel. Main criteria for the diagnosis of CPP were 1) secondary pubertal signs before the age of 8 y, 2) growth rate above the 75th percentile, 3) an acceleration of bone age (BA) more than 1 y above chronological age (CA) and ΔBA/ΔCA > 1.2, and 4) a pubertal response to exogenous GnRH with LH peak > 11 IU/L(25) and a ratio of stimulated LH/stimulated FSH > 1.0(26,27).
GnRH agonist treatment was initiated after an observation period of 6 mo. The absence of an organic course of CPP was demonstrated by magnetic resonance. Patients were treated for progressive CPP within a German multicenter trial with depot leuprorelin acetate (Enantone, Takeda Pharma, Aachen, Germany). For ethical reasons, an untreated patient control group was not available. Injections were given every 30 ± 2 d s.c. in a dose of 0.5 or 1 ampule (1.88 mg for body weight <20 kg and 3.75 mg for body weight >20 kg). Adequate suppression of pituitary-gonadal function was defined as an estradiol level <50 pM and a stimulated plasma LH after GnRH stimulation <5 IU/L.
Measurements. Standing height was determined by stadiometer. BA was estimated centrally by one experienced investigator by the method of Greulich and Pyle(28). Prospective adult height was calculated according to Bayley and Pinneau(29). German longitudinal normative data were used as standards for height and growth velocity(30,31). The normative data of Rolland-Cachera et al.(32) were used for BMI.
Expected leptin levels and leptin SD score, both adjusted for BMI, were calculated according to the following equations: (Equation 1) as reported by Blum et al.(21).

Plasma gonadotropins and estradiol and serum IGF-1 were determined by RIA or enzyme immunoassay at the Endocrine Laboratory of the University Department of Pediatrics in Kiel. Sensitivity of LH and FSH assay was 0.3 IU/L. A value of 0.2 IU/L was assigned to LH and FSH levels below the detection limit. The detection limit of estradiol was 5 pg/mL. A value of 4 pg/mL was assigned to samples below the detection limit.
Serum leptin was measured by RIA. Assay components and assay procedure were described previously(21). Assay sensitivity was 0.03 ng/mL. Intraassay and interassay variability was 0.8 and 8.5%, respectively (n = 10)(21).
Statistics. Statistical comparison of data at start of treatment and 1 and 2 y after start of therapy was performed by ANOVA. In those cases in which the data showed a significant deviation from a normal distribution, Friedman's ranked analysis of variance was applied. For evaluation of statistical differences between two different times, the Student-Newman-Keuls test was used as a post hoc test (SigmaStat 1.0, Jandel Scientific, Erkrath, Germany). Multiple stepwise regression analysis was performed to test which parameters contributed to the variation of leptin levels. Data are presented as mean and SEM, and a p < 0.05 was considered significant.
RESULTS
The clinical characteristics of the 14 girls with CPP are summarized in Table 1. Mean CA and BA at start of treatment were 6.0 ± 0.6 y and 9.5 ± 0.7 y, respectively. During the pretreatment observation period, ΔBA/ΔCA was 2.52 ± 0.4. Patients showed a pubertal growth rate of 13.0 ± 1.5 cm/y, equivalent to a velocity of 7.8 ± 1.7 height SD score. The stimulated LH/FSH ratio at the time of diagnosis was 1.8 ± 0.2, indicating central activation of the hypothalamic-pituitary-gonadal axis.
During GnRH agonist therapy, the BMI SD score for CA increased significantly from 1.9 ± 0.5 to 2.7 ± 0.5 after 1 y and to 2.8 ± 0.4 after 2 y of treatment (p < 0.05 versus pretreatment). Sufficient suppression of the hypothalamic-pituitary-gonadal axis was achieved in all patients. GnRH-stimulated LH levels >5 IU/L (n = 83 GnRH tests with n = 15 not completely suppressed) and estradiol levels >50 pM (n = 109 estradiol levels with n = 7 >50 pM) were only seen during the first few months of treatment.
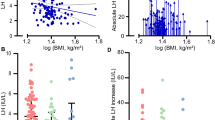
Serum leptin levels and expected leptin values of healthy girls of the same BMI referenced to our patients are demonstrated in Table 2. There was a significant increase in serum leptin levels after 1 y (8.1 ± 1.7 ng/mL; p < 0.05) and 2 y of treatment (11.2 ± 3.0 ng/mL; p < 0.05) compared with pretreatment levels (7.5 ± 2.3 ng/mL).
Serum leptin levels were in the normal range for BMI and pubertal stage in the majority of the patients on the three occasions they were measured. A leptin level more than +2 SD was found in two of 14 patients at pretreatment, in no patients after 1 y of treatment, and in one of 15 patients after 2 y of treatment (Fig. 1). No patient showed a leptin levels less than -2 SD. Mean leptin SD score adjusted for BMI showed no significant changes during the period of observation.
In a stepwise regression analysis model with BMI, BA, CA, basal and stimulated LH, estradiol, dehydroepiandrosterone, androstenedione, and clinical pubertal signs, BMI was the only parameter that did show a significant correlation with leptin (p = 0.006, Table 3, Fig. 2).
DISCUSSION
We investigated serum leptin concentrations in girls with CPP before the start of treatment and after 1 and 2 y of therapy with depot leuprorelin acetate. This longitudinal study enabled us to elucidate the role of leptin in pathologically early pubertal development and during its suppression.
Several investigations in animals confirm that leptin signals adequate metabolic conditions for sexual maturation to proceed(7–10). Furthermore, investigations in normal children showed that serum leptin levels increase just before the obvious onset of pubertal signs(15,16,21,22).
We found that leptin concentrations had a tendency to be slightly but not significantly higher both at pretreatment and during treatment than the expected leptin concentrations of healthy girls (Table 2; Fig. 1). On the average, leptin serum concentrations were 1.4-fold higher in girls with CPP than in healthy girls of the same BMI(21). Similarly, the mean leptin SD score adjusted for BMI was invariably greater than zero both at diagnosis and during treatment, although the majority of CPP patients had leptin levels in the upper normal range (Fig. 1). To our knowledge, other investigators have not calculated expected leptin levels adjusted for BMI(33).
We found a significant increase in measured and expected leptin concentrations throughout the period of treatment with the depot GnRH agonist while the hypothalamic-pituitary-gonadal axis was permanently suppressed (Table 2). Therefore, suppressive treatment did not affect the tendency for higher than the expected leptin levels. The mean leptin SD score adjusted for BMI remained unchanged during treatment; in particular, no decrease was seen. This absence of decrease in leptin concentrations is in contrast to findings of decreased leptin levels in ovariectomized rats(34) and postmenopausal women(35,36). The assumption that lack of estrogens is responsible for the decrease in leptin is discussed controversially. In fact, other investigators did not find a relationship between estrogen and decreased leptin(37–39). Our findings of a slight increase of leptin levels after initiation of puberty are similar to the observation in healthy girls, who show a continuous rise of leptin concentration after pubertal onset(15,20). In contrast, in healthy boys, leptin concentrations decrease to prepubertal levels after initiation of puberty(16,22,23). Particularly in boys with CPP, a suppression of precocious testosterone production leads to an increase in leptin concentration(33).
In girls with CPP, leptin concentration appeared to be unaffected by the functional status of the hypothalamic-pituitary-gonadal axis and vice versa. Serum leptin concentrations were related to BMI only. Thus, BMI is the major predictive factor for leptin both in CPP patients and in healthy girls(21). In principal, our results confirm those of Palmert et al.(33), who found that estrogen did not play a major role in the regulation of leptin levels. Our study emphasizes that leptin concentrations should be referenced to the major influencing variables BMI and sex in patients with disorders of pubertal development and in cases of disease-specific changes in BMI.
It is well-known that ballet dancers, marathon runners, and patients with chronic diseases suffer from impaired reproductive function(40), which may be related to low leptin levels(10). Thus, leptin is signaling to the brain as to whether or not the conditions for sexual development are adequate. However, in the case of CPP, in which patients may be either lean or obese, it seems unlikely that the onset of CPP is directly induced by leptin. Furthermore, treatment with GnRH agonists seems to have no influence on leptin concentrations in girls with CPP. Thus, we have no evidence that alterations of leptin are related to premature onset of puberty in children.
Abbreviations
- BA:
-
bone age
- BMI:
-
body mass index
- CA:
-
chronological age
- CPP:
-
central precocious puberty
- GnRH:
-
gonadotropin releasing hormone
References
Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM 1994 Positional cloning of the mouse obese gene and its human homologue. Nature 372: 425–432
Considine RV, Caro JF 1996 Leptin: genes, concepts and clinical perspective. Horm Res 46: 249–256
Beales PL, Kopelman PG 1996 Obesity genes. Clin Endocrinol (Oxf) 45: 373–378
Stephens TW, Caro JF 1996 To be lean or not to be lean: is leptin the answer?. Exp Clin Endocrinol Diabetes 106: 1–15
Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P 1995 Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science 269: 546–549
Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS 1996 Role of leptin in the neuroendocrine response to fasting. Nature 382: 250–252
Vogel G 1996 Leptin: a trigger for puberty?. Science 274: 1466–1467
Chehab FF, Mounzih K, Lu R, Lim ME 1997 Early onset of reproductive function in normal female mice treated with leptin. Science 275: 88–90
Cheung CC, Thornton JE, Kuijper JL, Weigle DS, Clifton DK, Steiner RA 1997 Leptin is a metabolic gate for the onset of puberty in the female rat. Endocrinology 138: 855–858
Kiess W, Blum WF, Aubert ML 1998 Leptin, puberty and reproductive function: lessons from animal studies and observations in humans. Eur J Endocrinol 138: 26–29
Clément K, Vaisse C, Lahlou N, Cabrol S, Pelloux V, Cassuto D, Gourmelen M, Dina C, Chambaz J, Lacorte JM, Basdevant A, Bougnères P, Lebouc Y, Froguel P, Guy-Grand B 1998 A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature 392: 398–401
O'Rahilly S 1998 Life without leptin. Nature 392: 330–331
Montague CT, Farooqi S, Whitehead JP, Soos MA, Rau H, Wareham NJ, Sewter CP, Digby JE, Mohammed SN, Hurst JA, Cheetham CH, Earley AR, Barnett AH, Prins JB, O'Rahilly S 1997 Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature 387: 903–908
Strobel A, Issad T, Camoin L, Ozata M, Strosberg AD 1998 A leptin missense mutation associated with hypogonadism and morbid obesity. Nat Genet 18: 213–215
Clayton PE, Gill MS, Hall CM, Tillmann V, Whatmore AJ, Price DA 1997 Serum leptin through childhood and adolescence. Clin Endocrinol (Oxf) 46: 727–733
Mantzoros CS, Flier JS, Rogol AD 1997 A longitudinal assessment of hormonal and physical alterations during normal puberty in boys. V. Rising leptin levels may signal the onset of puberty. J Clin Endocrinol Metab 82: 1066–1070
Apter D 1997 Leptin in puberty. Clin Endocrinol (Oxf) 47: 175–176
Rogol AD 1998 Leptin and puberty. J Clin Endocrinol Metab 83: 1089–1090
Nagy TR, Gower BA, Trowbridge CA, Denzenberg C, Shewchuk RM, Goran MI 1997 Effects of gender, ethnicity, body composition, and fat distribution on serum leptin concentrations in children. J Clin Endocrinol Metab 82: 2148–2152
Hassink SG, Sheslow DV, de Lancey E, Opentanova I, Considine RV, Caro JF 1996 Serum leptin in children with obesity: relationship to gender and development. Pediatrics 98: 201–203
Blum WF, Enlargo P, Hanitsch S, Juul A, Hertel NT, Müller J, Skakkebæk NE, Heiman ML, Birkett M, Attanasio AM, Kiess W, Rascher W 1997 Plasma leptin levels in healthy children and adolescents: dependence on body mass index, body fat mass, gender, pubertal stage and testosterone. J Clin Endocrinol Metab 82: 2904–2910
Garcia-Mayor RV, Andrade A, Rios M, Lage M, Dieguez C, Casanueva FF 1997 Serum leptin levels in normal children: relationship to age, gender, body mass index, pituitary-gonadal hormones and pubertal stage. J Clin Endocrinol Metab 82: 2849–2855
Carlsson B, Ankarberg C, Rosberg S, Norjavaara E, Albertsson-Wikland K, Carlsson LMS 1997 Serum leptin concentrations in relation to pubertal development. Arch Dis Child 77: 396–400
Matkovic V, Ilich JZ, Skugor M, Badenhop NE, Goel P, Clairmont A, Klisovic D, Nahhas RW, Landoll JD 1997 Leptin is inversely related to age at menarche in human females. J Clin Endocrinol Metab 82: 3239–3245
Partsch CJ, Hümmelink R, Sippell WG 1990 Reference ranges of lutropin and follitropin in the luliberin test in prepubertal and pubertal children using a monoclonal immunoradiometric assay. J Clin Chem Clin Biochem 28: 49–52
Partsch CJ, Hümmelink R, Lorenzen F, Sippell WG 1989 Bedeutung und Charakteristika des LH-RH-Testes in der Diagnostik der vorzeitigen Pubertätsentwicklung bei Mädchen: Der stimulierte LH/FSH-Quotient differenziert zwischen zentraler Pubertas praecox und praematurer Thelarche. Monatsschr Kinderheilkd 137: 284–288
Pescovitz OH, Hench KD, Barnes KM, Loriaux DL, Cutler GB Jr 1988 Premature thelarche and central precocious puberty: the relationship between clinical presentation and the gonadotropin response to luteinizing hormone releasing hormone. J Clin Endocrinol Metab 67: 474–479
Greulich W, Pyle I 1959 Radiographic Atlas of Skeletal Development of the Hand and Wrist. 2nd ed. Stanford University Press, Stanford, pp 125–176
Bayley N, Pinneau SR 1952 Tables for predicting adult height for skeletal age: evidence for use with the Greulich-Pyle hand standards. J Pediatr 40: 423–441( Erratum J Pediatr 41:371)
Reinken L, Stolley H, Droese W, van Oost G 1980 Longitudinale Körperentwicklung gesunder Kinder. II. Größe, Gewicht, Hautfettfalten von Kinder im Alter von 1:5 bis 16 Jahren. Klin Padiatr 192: 25–33
Reinken L, van Oost G 1992 Longitudinale Körperentwicklung gesunder Kinder von 0 bis 18 Jahren. Klin Padiatr 204: 129–133
Rolland-Cachera MF, Cole TJ, Sempe M, Tichet J, Rossignol C, Charraud A 1991 Body mass index variations: centiles from birth to 87 y. Eur J Clin Nutr 45: 13–21
Palmert MR, Radovick S, Boepple PA 1998 The impact of reversible gonadal sex steroid suppression on serum leptin concentrations in children with central precocious puberty. J Clin Endocrinol Metab 83: 1091–1096
Shimizu H, Shimomura Y, Nakanishi Y, Futawatari T, Ohtani K, Sato N, Mori M 1997 Estrogen increases in vivo leptin production in rats and human subjects. J Endocrinol 154: 285–292
Ostlund RE, Yang JW, Klein S, Gingerich R 1996 Relation between plasma leptin concentration and body fat, gender, diet, age, and metabolic covariates. J Clin Endocrinol Metab 81: 3909–3913
Rosenbaum M, Nicolson M, Hirsch J, Heymsfield SB, Gallagher D, Chu F, Leibel RL 1996 Effects of gender, body composition, and menopause on plasma concentrations of leptin. J Clin Endocrinol Metab 81: 3424–3427
Kohrt WM, Landt M, Birge SJ 1996 Serum leptin levels are reduced in response to exercise training, but not hormone replacement therapy, in older women. J Clin Endocrinol Metab 81: 3980–3985
Havel PJ, Kasim-Karakas S, Dubuc GR, Mueller W, Phinney SD 1996 Gender differences in plasma leptin concentration. Nat Med 2: 949–950
Saad MF, Damani S, Gingerich RL, Riad-Gabriel MG, Khan A, Boyadijian R, Jinagouda SD, El-Tawil K, Rude RK, Kamdar V 1997 Sexual dimorphism in plasma leptin concentration. J Clin Endocrinol Metab 82: 579–584
Frisch RE, Wyshak G, Vincent L 1980 Delayed menarche and amenorrhea in ballet dancers. N Engl J Med 303: 17–19
Acknowledgements
The authors thank Stefanie Kasch and Silke Struve for their expert technical assistance in the hormonal analyses and Sigrid Hanitsch and Pierra Enlargo for their expert technical assistance in serum leptin analyses. We also thank Joanna Voerste for linguistic editing of the manuscript, and all the collaborating colleagues who sent us serum and plasma samples and provided us with the main clinical data of their patients.
Author information
Authors and Affiliations
Additional information
Supported by the science budgets of the institutes of the authors.
Rights and permissions
About this article
Cite this article
Heger, S., Partsch, CJ., Peter, M. et al. Serum Leptin Levels in Patients with Progressive Central Precocious Puberty1. Pediatr Res 46, 71–75 (1999). https://doi.org/10.1203/00006450-199907000-00012
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199907000-00012
This article is cited by
-
Serum nesfatin-1 and leptin levels in non-obese girls with premature thelarche
Journal of Endocrinological Investigation (2015)