Abstract
Almitrine is a piperazine derivative known to stimulate breathing in the adult but cause apnea in fetal sheep. In fetal sheep (127-133 d gestation; term = 147 d) we confirmed this finding, but found that almitrine (4 mg/kg, i.v. or intra-arterial) had a biphasic effect, briefly stimulating and then suppressing breathing movements for at least 3 h. In 2- to 3-d-old (n = 4) and 7- to 14-d-old (n = 4) lambs almitrine increased both tidal volume and breath frequency, increased arterial partial pressure of oxygen and pH, and decreased partial pressure of carbon dioxide. The changes of tidal volume, partial pressure of oxygen and partial pressure of carbon dioxide were less in the 2- to 3-d-old compared with the 7- to 14-d-old lambs. The distribution of the nuclear phosphoprotein FOS, a marker of neuronal activation was examined in fetal and newborn brains. FOS protein was increased in cardiorespiratory areas of the medulla and pons, in the periaqueductal region of the midbrain, and in the supraoptic and paraventricular regions of the hypothalamus. In the pons, FOS protein was increased in the medial parabrachial and subcoeruleus nuclei in the fetuses but not in the 2- to 3- or 7- to 14-d-old lambs. These observations are similar to those reported for hypoxia, and consistent with the hypothesis that both almitrine and hypoxia inhibit fetal breathing movements by an action on a select group of pontine neurons. Whether these neurons respond directly to these stimuli or receive input from the other centers is yet to be elucidated. The mechanisms that change the almitrine (and hypoxia) response from inhibition to excitation at birth have not been identified, but may be important in preventing apnea in the newborn.
Similar content being viewed by others
Main
Almitrine is a piperazine derivative that stimulates respiration in the adult via an excitatory action on the peripheral chemoreceptors(1). However, in fetal sheep this substance has the paradoxical effect of inhibiting respiratory movements(2,3), and its actions are thus similar to those of hypoxia that also inhibits breathing movements in the fetus(4) despite having an excitatory effect on the peripheral chemoreceptors(5). Further similarities extend to the fact that lesions within the lateral pons of the fetal sheep eliminate the inhibitory effects of both almitrine and hypoxia, and indeed, each of them then cause stimulation of respiratory movements(6,7). These results have led to the suggestion that there is a neuronal pool within the lateral pons that inhibits respiratory movements in the fetus(2,6,7) and follows the earlier observation that total collicular transection of the fetal brainstem eliminated the depressive effects of hypoxia on breathing movements(8).
FOS is the phosphoprotein product of the early immediate gene c-fos, and its presence in neuronal nuclei has been widely used as an index of neuronal activation(9). Increased expression of the c-fos gene has been shown in selected neuronal populations after trans-synaptic activation in both the peripheral(10) and central nervous systems(9,11–14). In fetal sheep arterial hypoxia caused an increase of expression of FOS protein in a group of cells in the medial parabrachial/subceruleus region of the rostral pons(15). This cell group extends from below the locus ceruleus to the ventral tip of the lateral parabrachial complex, and corresponds to the areas of the Kolliker-Fuse, medial parabrachial, and subcoerulus nuclei in other species(16). We have hypothesized that it is these pontine neurons that cause respiratory inhibition in the fetus(17). Importantly, these cells did not express FOS in newborn lambs, where arterial hypoxemia results in hyperventilation, as expected(15).
The purpose of this study was 3-fold. First, we hypothesized that almitrine would increase the expression of FOS protein in the same areas of the pons of the fetal brain as shown for maternally induced hypoxia(15), and that increased FOS expression would not occur in this region in newborn lambs. Second, we wanted to know more fully the extent of the effects of almitrine on the brain, and we therefore also examined the distribution of FOS staining in the medulla, midbrain, and hypothalamus. Third, although it is evident that the effect of almitrine on breathing must change from inhibition to augmentation at some time between prenatal and postnatal life, the exact time that this change occurs is not known. Therefore, we examined the effect of almitrine on breathing and FOS expression in 2- to 3-d-old and 7- to 14-d-old lambs to determine whether the "fetal" or "neonatal" response was present in the early neonatal period.
MATERIALS AND METHODS
Animals and surgical procedures
Fetuses and newborn lambs of time-mated Border-Leicester/Merino cross ewes were used in accordance to the Standing Committee on Ethics in Animal Experimentation of Monash University, approval number 94/087. The pregnant ewes were brought to the departmental animal house and held under artificially lit conditions on a 12-h light/dark cycle for at least 5 d before surgery. They were housed in the company of other sheep and given free access to lucerne chaff, hay, and water.
Eight ewes, 119-126 d gestation, were initially anaesthetized using 1 g of thiopentone in 20 mL of sterile water by an i.v. injection. Once anesthesia was established the ewes were intubated and anesthesia was then maintained for the duration of the surgery using 1.5 to 2.0% halothane in 50:50 (vol/vol) nitrous oxide in oxygen delivered through a closed system (Midget, CIG, Medisheild, N.S.W., Australia) and ventilated with a positive pressure ventilator (Campbell, ULCO Engineering, NSW, Australia). Using sterile techniques, the fetal head was exteriorized through midline abdominal and uterine incisions, and polyvinyl catheters (outside diameter 1.52 mm; internal diameter 0.86 mm; Dural Plastics, NSW, Australia) filled with heparinized saline (5000 IU/mL) were inserted into a jugular vein and carotid artery, and advanced toward the heart. A saline filled catheter (outside diameter 2.7 mm; internal diameter 1.5 mm) was inserted into the trachea through a small hole made between the cartilaginous rings and advanced 4-5 cm toward the lungs; this was used to record fetal breathing movements from the changes of intrathoracic pressure, after electronic subtraction of amniotic pressure measured from a catheter sutured to the skin of the fetal neck(8,18). A pair of electrodes for measuring electrocortical (ECoG) activity, made from insulated multistranded stainless steel wire (AS632, Cooner Wire Co., Chatsworth, CA) was inserted into 1-mm holes drilled through the skull above the parietal cortex and fixed in place with a drop of cyanoacrylate glue. EMG activity was recorded from a pair of electrodes sutured into the dorsal nuchal muscles. After the fetus was replaced in the amniotic sac and the uterine membranes were repaired, the catheters and leads were exteriorized through a small incision in the right flank of the ewe. The abdominal incision was then repaired, and the ewe was allowed to recover for at least 5 d; the experiments were performed between 127 and 133 d gestation.
Lambs were either 1 d old (n = 6), or 4-8 d old (n = 8) when they were anaesthetized with 2-3% halothane in 50:50 (vol/vol) nitrous oxide in oxygen delivered to the lamb via a face mask. They were then intubated and anesthesia was maintained using 1.5-2.0% halothane in 50:50 (vol:vol) nitrous oxide in oxygen. Catheters were placed in a femoral artery and vein. Lambs (1 d old) were allowed to recover for 24 h and the experiments were performed at 2-3 d of age, although the older lambs (4-8 d) were allowed to recover for at least 3 d before experiments were performed at 7-14 d of age.
Experimental protocols
Fetuses. ECoG and nuchal muscle EMG activities were recorded on a polygraph (Neotrace, Neomedix Systems, NSW, Australia) using purpose-built differential amplifiers. The ECoG signal was filtered (bandpass 1-15 Hz) and displayed directly on the polygraph, and the EMG signal was displayed as a running mean using a "leaky" integrator. Arterial and tracheal pressures were recorded after electronic subtraction of the amniotic pressure to minimize pressure artefacts caused by movement of the ewe and the occasional weak contractions of the uterus. Heart rate was derived from the arterial pulse using a tachometer circuit. Paper speed of the polygraph recorder was usually 5 mm/min for the fetal recordings and 10 mm/min for the lamb recordings; higher chart speeds were sometimes used to allow particular aspects of the responses to be examined more closely. On the day of the experiment, control recordings were obtained for at least 3 h before infusion of almitrine (Servier Laboratories, Gichy, France) into either the jugular vein (n = 2 fetuses) or the carotid artery (n = 2 fetuses). The dose of almitrine was 4 mg/kg calculated from the fetal weight estimated for the gestational age on the day of experiment and recalculated from the weight obtained at autopsy. The actual dose given was 3.8 ± 0.3 mg/kg. The drug was made up in 0.01 M HCl, in a volume of approximately 1 mL/kg and injected manually into the fetus over 1 min. Control experiments were performed in four fetuses where a similar volume of the HCl vehicle was given (i.a, n = 3; i.v, n = 3). Fetal arterial blood samples (0.8 mL) were taken at one hour and immediately before the administration of the almitrine or vehicle, and at every subsequent hour for measurement of (PO2), carbon dioxide (PCO2) and pH, which were corrected for a fetal temperature of 39°C. The total volume of blood taken was less than 5 mL. The polygraph recordings continued for a further 3 h, and the ewe and fetus were then killed by a 20 mL i.v. injection of sodium pentobarbitone (325 mg/mL, Lethobarb, Virbac, NSW, Australia) given to the ewe. Three hours was selected as an appropriate time for FOS protein to accumulate in the neuronal nucleus based upon 1) previous studies in adult animals(9); 2) our previous studies in fetal sheep(15,19); and 3), the duration of the physiologic changes induced by almitrine (see "Results" and "Discussion"). The fetal head was perfused transcardially with 1 L of 0.9% saline, followed by 2 L of 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). The fetal brain was then removed from the skull, postfixed for 2-4 h at 4°C in fresh 4% paraformaldehyde. The brain was then cut into blocks containing the medulla, pons, midbrain, and hypothalamus. Each block was placed in 20% sucrose in 0.1 M phosphate buffer (pH 7.4) and left overnight at 4°C.
Lambs. Each lamb was placed in a canvas hammock that allowed their legs to hang freely, and they rested comfortably with their head on a pillow of towels. Arterial pressure and heart rate were recorded on a polygraph, together with respiratory movements recorded from inductance bands placed around the chest and abdomen (Respitrace Ambulatory Monitoring, New York, NY). This record was calibrated by measuring the actual tidal volume at the beginning and end of the recording session using a pneumotach (size 0, Fleisch, Switzerland) attached to a face mask held over the lamb's nose for 2-3 min. Control recordings were obtained over a period of 30-60 min. Almitrine, 4 mg/kg, or an equivalent volume of the HCl vehicle was then infused into the femoral vein over approximately 1 min, and was flushed in by 1 mL of saline. Recordings continued for a further 3 h, and 0.8 mL arterial blood samples were taken at subsequent hourly intervals throughout the experiment for measurement of blood gases and pH, corrected for a body temperature of 38.5°C. The maximum volume of blood taken did not exceed 5 mL during the duration of the experiment. The lamb was then killed by a 10 mL i.v. injection of sodium pentobarbitone (365 mg/mL) and the brain perfused and fixed as described above for the fetuses.
Analysis of polygraph records
Fetuses. Fetal breathing movements were recorded as the negative changes of tracheal pressure, and were analyzed quantitatively from the trace using a digitizing tablet and stylus connected to a minicomputer. The recording was marked into consecutive 1 h epochs, and the amplitude of the tracheal pressure deflections was measured at 1-min intervals for the entire recording. Tracheal pressure changes <1 mm Hg were treated as zero values by the computer. A data file was then constructed showing the number of minutes within each hour that breathing movements were present, together with their mean amplitude. The ECoG recording was divided into the episodes of high and low voltage activity by visual analysis(8,18) and the number of minutes each type of activity was present for each hour was calculated. Similarly, the number of minutes that nuchal EMG activity was present was tabulated for 1-h epochs for the entire record of each fetus.
Lambs. Mean tidal volume and respiratory rate were calculated at 1-min intervals throughout the entire recording. These values were then used to calculate mean values over 1-h epochs, or were used to show changes at 1-, 5-, or 10-min intervals, where appropriate. Mean arterial pressure was calculated from the diastolic and systolic pressures determined from the trace at 1-h intervals.
Immunohistochemistry. Frozen coronal sections (40 µm) of the medulla, pons, midbrain, and hypothalamus were cut using a freezing microtome (Leitz Wetzlar, Germany). Every sixth serial section was prepared for analysis by FOS immunohistochemistry. Free floating sections were washed (3 × 5 min) in 0.05 M phosphate buffer and placed in 10% normal goat serum (NGS) in 0.1 M phosphate buffer (pH 7.4) for 30 min. Sections were then placed in a 1:500 dilution of rabbit polyclonal FOS antibody prepared using 0.1 M phosphate buffer/2% NGS/0.3% Triton X-100 (Sigma Chemical Co., St. Louis, MO) for 48 h at 4°C. The antibody was raised against the N-terminal 4-17 amino acid sequence of human FOS protein (Oncogene Science Inc., MA). Sections were then washed in buffer (3 × 5 min) and incubated for 45 min in a solution of a secondary biotinylated anti-rabbit antibody (Vectastain, Vector Laboratories Inc., CA) diluted 1:200 in 0.1 M phosphate buffer containing 2% NGS. After another wash in buffer (3 × 5 min), the tissues were transferred to a solution containing 1:1:100 dilution of avidin-biotin peroxidase complex in 0.1 M phosphate buffer for 45 min. The sections were then washed (3 × 5 min) in buffer, and incubated for 10 min in 0.05% diaminobenzidine and 0.04% nickel ammonium sulfate (Sigma) in 0.1 M phosphate buffer. Finally, FOS protein was visualised by adding 30 µL hydrogen peroxide (BDH Chemicals, Vic., Australia) to the incubation solution for 6 min. The sections were then washed in buffer (3 × 5 min) and mounted onto gelatinised (1%) glass slides, air dried, and were then counterstained with 0.01% thionin, dehydrated, cleared, and coverslipped. Control experiments for the immunohistochemical procedure were done by replacing the FOS antibody with nonimmune serum; this always resulted in the absence of staining.
Presentation of the anatomical data. All of the sections stained for FOS protein from all of the animals were examined, and the individual FOS-positive cells identified and counted. Particular nuclei at each level of the brain were identified with the aid of published anatomical drawings for the fetal(20,21) and adult sheep brain(22,23), and from previous work done in this laboratory(15,19) (Nitsos I, Joseph S, Walker DW, unpublished observations) (stereotaxic coordinates cannot not be used because cranial size is quite variable at any gestational age and with breed/sire differences in sheep flocks). The outline of each brain section from one animal was traced using a microscope stage attached to a digitiser (M.D. Optical Encoder, Minnesota Datametrics Corp., Minnesota, MN) and computer, using a software package (M.D. Plot, v3.1, Minnesota Datametrics Corp.) which allowed the position of each FOS-positive cell to be accurately marked within the section. The subsequent file was then manipulated using a graphics program (Corel Draw!, Corel Corp., Dublin, Ireland). Sections were also photographed under bright field illumination.
Statistics. All data are presented as mean ± SEM. A two way analysis of variance with repeated measures was used to compare the effects of almitrine and vehicle treatment on blood gases, arterial pressure, heart rate, fetal breathing movements, tidal volume, and breath frequency. Where a significant effect of treatment was detected the Student-Newman-Keuls test was used to determine differences between the treatments at each time point.
The number of FOS-positive cells for each region of the brain was counted in every section that had been prepared for FOS straining from each animal. For each specific brain region the results were averaged across the number of sections in which that region was represented to obtain a value typical for that animal; the results from all animal in each group were then averaged. The results for the treated and control fetuses and lambs were then compared using the unpaired t test. p < 0.05 was considered to be statistically significant.
RESULTS
Respiratory and cardiovascular effects of almitrine
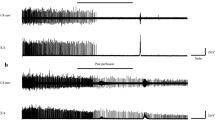
Fetuses. There was no consistent difference between the experiments where almitrine was given either i.v or i.a, and the results have been combined and statistical analysis performed on this pooled data. As reported previously by others(2,3) almitrine caused a prolonged suppression of breathing movements and nuchal muscle activity. However, in most experiments we also observed that almitrine had a biphasic effect, first stimulating breathing movements and nuchal muscle activity for a short period of time before the onset of prolonged apnea and muscle atonia (Fig. 1). The breathing movements began 2 ± 1 min after injection of almitrine and lasted 10.5 ± 2.9 min before the onset of the apneic period that then lasted 108 ± 27.2 min (eight trials, four fetuses). The injections were always done during low voltage ECoG activity but when breathing movements were not present. The incidence of breathing movements decreased progressively after injection of almitrine and were significantly lower in the third hour compared with the control period (Table 1).
The effect of almitrine (12 mg) on breathing movements (shown as negative deflections of tracheal pressure), nuchal muscle EMG activity, arterial pressure, and heart rate in a fetus at 132 d of gestation. Almitrine (ALM) was given i.v. over 1 min at the time indicated by the arrow. Asterisk marks the time an arterial blood sample was taken.
Almitrine also caused a sustained increase in the mean arterial pressure and heart rate (Table 1), but had no significant effect on the arterial PO2 (20.2 ± 6.1 mm Hg), PCO2 (38.1 ± 1.4 mm Hg), or pH (7.34 ± 0.01). Infusion of the vehicle solution had no significant effect on blood gases, heart rate, breathing movements, or nuchal muscle activity. There was no effect of almitrine on the mean incidences of high and low voltage ECoG activities.
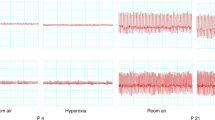
Lambs. Tidal volume and breath frequency were significantly increased by almitrine in both 2- to 3- and 7- to 14-d-old lambs, resulting in alteration of the arterial blood gases and pH. Arterial PO2 and pH increased, and PCO2 decreased significantly in both lamb groups but the increase of PO2 and decrease in PCO2 occurred earlier and was greater in the older lambs compared with the younger lambs (Fig. 2). Infusion of the vehicle solution had no effect on tidal volume, breath frequency, or blood gases and pH at either postnatal age. In both groups of lambs almitrine was associated with a significant increase of the arterial pressure (Table 1). Heart rate tended to increase but this was not significant.
FOS immunohistochemistry
In both fetal and neonatal brain sections, FOS-positive neurons were clearly identifiable as cells with a dark nucleus surrounded by clear cytoplasm. They were easily distinguished from the occasional red blood cell which exhibit a dark stain, hollow center and a concave appearance. FOS staining was always confined to neurons except in the dorsal motor nucleus of the vagus (DMV) where a number of very small, darkly staining cells were observed in both the fetuses and neonates. These cells may be glia. In all fetuses and lambs numerous FOS-positive cells were observed in the SCN confirming previous work(19) and providing a positive control for sections from untreated animals where few or no FOS cells were observed in some sections.
Medulla. The distribution of FOS-positive cells for the fetuses, and the 2- to 3- and 7- to 14-d-old lambs is shown in Figure 3. In the control fetuses lightly stained FOS-positive cells were seen within the AP, in the commissuralis subnucleus of the caudal NTS, and also more rostrally in the NTS in the region adjacent to the AP and VLM (Table 2). The intensity of staining and the number of FOS immunoreactive cells in the control fetuses was variable and in two of the four fetuses no FOS staining was identified in this region of the brain.
Line drawings of coronal sections of the medulla at the level of the facial nerve tract from control (right) and almitrine-treated (left) fetuses and newborn lambs. Results have been combined from two consecutive sections taken 240 µm apart from one animal; nuclei that express FOS protein are marked (·). Scale bar = 5 mm. ECu, external cuneate nucleus; Fl, lateral fasiculus; IO, inferior olive; LRt, lateral reticular nucleus; NnXII, nucleus of hypoglossal nerve; NTSv, nucleus of the spinal tract of the trigeminal nerve; nXII, hypoglossal nerve; Py, pyramids; TS, tractus solitarius; TSv, spinal tract of the trigeminal nerve.
In the almitrine-treated fetuses a significant increase in the number of FOS-positive cells was present in nuclei involved in cardiorespiratory control located in the rostral medulla, compared with the control tissue (Table 2; Fig. 3). An increased number of FOS-positive cells were found within the intermediate region of the NTS, located rostral to the obex, and in the rostral portion of the VLM. FOS labeled cells were present in the lateral DMV, in contrast to the control fetuses where little or no staining was present. A similar level of FOS staining was detected in the AP of both the almitrine-treated and control fetuses.
In both the treated and control newborn lambs, sparse and lightly stained FOS-positive cells were present in the NTS, VLM, and AP (Fig. 3). Almitrine treatment did not consistently change the level of FOS staining in these medullary nuclei in the lambs at both postnatal ages. The mean number of FOS-positive cells detected in the medullary nuclei of the 7- to 14-d-old lambs is shown in Table 2.
Pons. Sparsely distributed FOS-positive cells were observed in the rostral pons of the control fetuses (Fig. 4). These cells were located ventromedially in the TB and in the nucleus pontis. In two of these fetuses a small number of FOS-positive cells were observed within the LC. Sparsely distributed FOS-positive cells were observed in the ventral part of the LPBN.
Line drawings of coronal sections of the rostral pons from control (right) and almitrine treated (left) fetuses and newborn lambs. Results have been combined from four consecutive sections each 240 µm apart, taken from one animal; nuclei that express FOS protein are marked (·). Scale bar = 5 mm. MCP, middle cerebellar peduncle; NP, nucleus pontis.
In the almitrine treated fetuses, the number of FOS-positive cells was clearly increased in two regions adjacent to the SCP (Figs. 4 and 5). Labeled cells extended in a continuous band along the lateral border of the SCP, i.e. in the LPBN. Medial and ventral to the SCP a band of FOS-positive cells extended from below the LC to the ventral pole of the SCP. This band of cells thus corresponds to the positions of the MPBN and SC nuclei, and in its ventrolateral extent corresponds to the position of the KF nucleus. The number of FOS-positive cells was somewhat greater ventrolaterally near the region of the KF than it was dorso-medially near the LC. FOS staining in the LPBN and MPBN/SC extended caudally from the level of the middle cerebellar peduncle rostrally to the pontine-midbrain junction (Table 2).
Line drawings of coronal sections of the lateral pons at the level of the superior cerebellar peduncle (SCP) and locus coeruleus (LC) showing the distribution of FOS labeled cells in (a) vehicle-treated fetus 132 d gestation; (b) almitrine treated fetus 132 d gestation; and (c) almitrine treated lamb 14 d old. Scale bar for all drawings is 0.5 mm. FOS staining in the nuclei of neuronal cell bodies in the region of the medial parabrachial nucleus of an almitrine treated fetus (132 d gestation) is shown in (d); scale = 100 µm.
In the control lambs at both postnatal ages FOS-positive cells occurred in the LPBN, TB, medial longitudinal fasciculus (mlf), and along the dorsal margin of the pons (Fig. 4) Staining in the midline raphe nuclei was also prominent, an area that was devoid of staining in the fetuses. Almitrine treatment did not clearly increase FOS expression in the lambs, except in the 2- to 3-d-old lambs where the FOS staining extended laterally and ventrally from the LPBN to join with the FOS-positive cells present in the TB. It was notable that there was little or no FOS staining in the ventral MPBN and SC region of the pons in the lambs treated with almitrine, compared with the significant increase that occurred in these regions in the fetuses (Table 2).
Midbrain. Very few FOS-positive cells were present in the control fetuses in sections of the caudal midbrain (Fig. 6). In contrast, FOS staining was consistently found at this level of the brain in the almitrine-treated fetuses at these ages. These cells were located in regions ventral and ventrolateral to the central canal, which include the ventrolateral-PAG and the nucleus of occulomotor nerve (NnIII) (Fig. 6). Cells in the red nucleus (RN) did not show FOS staining. FOS staining was present in the ventrolateral-PAG in the control lambs, but there was no significant change after almitrine treatment (Table 2).
Line drawing of coronal sections of the caudal midbrain from control (right) and almitrine-treated (left) fetuses and newborn lambs. Results have been combined from two consecutive sections each 240 µm apart, taken from one animal; nuclei that express FOS protein are marked (·). Scale bar = 10 mm. CS, superior colliculus; FTC, central tegmentum field; NnIII, nucleus of oculomotor nerve.
Hypothalamus. FOS-positive cells were present throughout the SCN of the control fetuses (Fig. 7). A few cells were observed in the SON, PVN, and Pv. After almitrine treatment intense FOS staining was observed throughout the SON and PVN, and also more dorsally in the Pv adjacent to the lateral ventricle (Fig. 7). The increased level of FOS staining in the dorsal PVN occurred in both magnocellular and parvocellular neurons. This intense FOS staining was observed at all levels of the hypothalamus, from the caudal pole of the mamillary bodies rostrally to the optic chiasm. The significant increase in the mean cell counts for the SON, PVN, and Pv in the almitrine treated fetuses is shown in Table 2.
Line drawing of coronal sections of the rostral hypothalamus at the level of the optic chiasm from control (right) and almitrine-treated (left) fetuses and newborn lambs. Results are from one 40-µm section from one animal; nuclei which express FOS protein are marked (·). Scale bar = 10 mm. F, fornix; OC, optic chiasm; III, third ventricle.
In hypothalamic sections from the almitrine treated lambs at both2–3 and7–14 d of age there was an increased number of FOS-positive cells in the SON, PVN and Pv compared with the control fetuses (Fig. 7). There was no clear difference in the number of FOS positive cells in these areas in the almitrine-treated and control lambs, nor was there an apparent difference between lambs at the two postnatal ages.
DISCUSSION
The effect of almitrine in decreasing the incidence of breathing movements and causing apnea in fetal sheep of late gestation is well documented(2,3). Our results confirm that almitrine causes a prolonged decrease in breathing movements and other muscle activities. There was no change in the duration of high and low voltage ECoG activities, suggesting that the drug acts on pathways other than those that modulate activity via sleep-related mechanisms. However, we consistently observed that breathing movements and nuchal muscle activity increased before the period of suppression. Two previous studies(2,3) did not report such observations although we contend that it can be discerned in the traces presented in these reports; a third study on two fetal sheep reported only that almitrine stimulated fetal breathing movements(5). It is possible that almitrine initially stimulates the peripheral chemoreceptors, but this response is only transient or is subsequently inhibited by another mechanism of slower onset. The peripheral chemoreceptors respond to both almitrine and hypoxia in the fetal sheep(5,24). In adult cats bolus injections of almitrine into a carotid artery caused a marked excitation of the carotid sinus nerve that was maximal for approximately 2 min, but sustained at a lower level for 40 min(25). However, the absence of breathing movements and muscle activity for much of the next 3 h is consistent with the proposal that almitrine has a second action in the fetus that inhibits respiratory activity, perhaps overriding the effect of the increased peripheral chemoreceptor activity. There were no changes in fetal blood gases or pH after almitrine treatment, ruling out the possibility that the effects were secondary to systemic hypoxia or acidemia. The changes in heart rate and blood pressure that also followed almitrine treatment is consistent with stimulation of the aortic chemoreceptors, because similar responses occur after injection of cyanide into the aortic arch in anaesthetised fetal sheep(26), and during experimentally induced hypoxemia in the unanesthetized fetus(4).
Almitrine stimulated ventilation in both newborn lamb groups examined in this study. An increase in both tidal volume and breath frequency was exhibited at all ages in response to treatment, causing an increase in arterial PO2 and pH, and a decrease in PCO2, which lasted for at least 2 h after the administration. During hyperventilation, younger lambs tended to elevate their respiratory rate rather than increase tidal volume compared with the older groups, and it was evident that the increase of PO2 and decrease of PCO2 was less in younger lambs. This could be due to the relative inefficiency of gas exchange in the lungs at this time. Despite this difference, these results show that lambs no more than 72 h old are capable of producing a hyperventilatory response to a respiratory agent such as almitrine. Further work is required to show precisely when the inhibitory response to almitrine shown in the fetus is lost; whether this occurs during late gestation, or sometime during parturition.
Almitrine caused an increase in FOS expression at all levels of the brain between the medulla and anterior hypothalamus in both the fetuses and lambs. The high level of FOS expression in the brains of the control newborn lambs compared with the fetuses was presumably due to the higher levels of activity in autonomic and respiratory pathways after birth compared with in utero conditions. This limited our ability to assess the effect of almitrine in nuclei which showed a high level of basal expression. However, some clear conclusions can be drawn for most regions of the brain. In the fetal medulla, there was an substantial increase in FOS expression after almitrine treatment in nuclei involved in respiratory and cardiovascular control; i.e. the NTS, DMV, and VLM. The increase in FOS immunoreactivity in the subnucleus commisuralis of the NTS, the site of termination of carotid and aortic receptor afferents(25) is consistent with the stimulation of the peripheral chemoreceptors. In the pons there was an increase in FOS expression in the LPBN of the fetuses. A clear topographic organization of cardiovascular responses exist within the LPBN(27), and the activation of this area of the pons may be related to the tachycardia and increase in mean arterial blood pressure which followed the administration of almitrine. In the midbrain, almitrine elicited an increase of FOS staining in the caudal areas corresponding to the ventrolateral-PAG, a region that has a role in mediating responses to threatening and painful stimuli(12,28–30). In the hypothalamus there was an increase of FOS expression in the PVN and SON in the treated fetuses compared with controls. Although almitrine has been previously described only as an agent that increases ventilation via stimulation of the peripheral chemoreceptors(1), we have thus shown that it has more extensive effects involving the midbrain and hypothalamus. At this point we cannot conclude whether the increased activity in these more rostral areas was dependent on stimulation arising from the peripheral chemoreceptors. This could be studied in animals in which the peripheral chemoreceptors have been denervated. Because the NTS has ascending connections with pontine and hypothalamic neurons(31,32), it is possible that the drug can stimulate more rostral centers via these pathways. Alternatively, it has been suggested that almitrine crosses the blood brain barrier, and it is possible that it can act on higher centers directly(2).
It is important to note that absence of FOS staining does not necessarily imply that neurons were unaffected by the drug. Almitrine may have had effects that did not induce FOS expression. Although many studies have shown restricted and specific distribution of FOS protein after the application of discrete stimuli (e.g.Refs. 9–15), other significant effects on the nervous system cannot be ruled out.
The main difference between the fetal and postnatal brains was the presence of FOS immunoreactivity in the MPBN and SC regions in the pons of the fetuses, and its absence in the newborn lambs after injection of the drug. In the fetuses FOS staining occurred in a band of cells extending from below the LC to the area designated as the KF in other species(33). The FOS staining was more intense in the region of the KF. In contrast, FOS expression was present in the lambs dorsomedially near the LC, but not ventrolaterally near the KF. These results are essentially the same as those obtained by Breen et al.(15) who demonstrated that hypoxia increased FOS expression in cells in the MPBN/SC region of the pons in fetal sheep, but not in newborn lambs. The MPBN and the KF have been considered as components of the "pneumotaxic center" in the adult brain, an area thought to be involved with the timing of respiratory rhythm(34,35). Stimulation of this area of the pons electrically or using microinjections of glutamate caused an increase in the duration of both the expiratory and inspiratory phases of the respiratory cycle(36). Thus, it is possible that this neuronal pool, when activated by either hypoxia or almitrine, has a role in decreasing breathing movements in the fetus.
Because almitrine also caused an increase of FOS expression in midbrain and hypothalamic nuclei, it is necessary to consider if these regions could have also contributed to the reduction of breathing movements and motor activity in the fetus, even though we could detect no difference in the distribution of FOS staining in the fetuses and newborn lambs at these levels of the brain. The prominent FOS staining present in the ventrolateral-PAG area of the midbrain is of interest because, in conscious rats, microinjection of the excitotoxin kainic acid caused cessation of spontaneous activity and reduced motor and vocal reactivity(29), a fall in resting arterial blood pressure and heart rate(28), and analgesia(37). Furthermore, deep noxious pain has been shown to elicit an increase in FOS immunoreactivity in the ventrolateral-PAG of adult rats(12). Thus, the ventrolateral-PAG appears to be a significant mediator of passive behavioral responses, inducing quiescence, hypotonia, and hypotension in response to stressful stimuli. We had also expected to observe staining either within or adjacent to the RN, as electrical stimulation of this area has been reported to inhibit breathing in adult cats(30), and bilateral lesions of the RN abolish the decrease in respiratory output during the biphasic response to hypoxia in newborn rabbits(38). However, FOS expression was never observed in or near the RN in any of the fetal or postnatal groups. In the hypothalamus there was an increase of FOS expression in the PVN and SON. It has been shown in adult cats that neurons within and adjacent to the PVN are intrinsically sensitive to hypoxia(39), and these chemosensitive neurons could therefore activate pontine neurons during both hypoxia- and almitrine-induced stimulation. Psychological and physical stress also produce an increase in c-fos mRNA in the parvocellular and magnocellular cells of the PVN(13), and in the SON(11). The activation of these areas in the fetus is likely to be the cellular counterpart of the vasopressin and ACTH release which occurs during hypoxemia and acidemia(20,40). The high level of basal FOS expression in the control newborn lambs, perhaps due to the greater degree of awareness of the lambs during the conduct of the experiments, made it difficult to detect the specific effects of the almitrine treatment. Also, some of this extensively distributed labeling could be secondary to the cardiovascular and behavioural effects of the treatment. In the conscious rat subjected to hypoxia or severe hypercapnia(41), FOS staining was observed throughout the brainstem, and it was considered that it could have arisen in part from the stress and cardiovascular effects of these particular treatments.
The intense FOS expression observed in the SCN was expected, as the experiments were performed during the day and we have previously shown that FOS staining is higher during the day compared with the night in both fetuses and newly born lambs(19).
Thus, the major differences between the fetus and newborn was the appearance of FOS-positive neurons in the MPBN and SC region of the pons in fetal brain, but not in the newborn lambs. The distribution of FOS-positive neurons in the MPBN and SC regions after almitrine treatment was similar to that observed after hypoxia in the fetal sheep(15) and strengthens, but does not prove, the proposal that these neurons mediate the inhibition of breathing and motor activity in the fetus in response to both almitrine and hypoxia. A direct synaptic connection between the MPBN and the phrenic motor nucleus of the adult rat has been demonstrated using retrograde tracing techniques(42). In late gestation fetal sheep the parabrachial cells that express FOS in response to hypoxia and that have a direct spinal projection have also been shown to be tyrosine hydroxylase positive(43). At some point during the transition from fetal to newborn life the response to almitrine changes from inhibition to excitation. This could occur either just before, during, or soon after parturition, but the mechanisms that regulate this change remain unexplained. We cannot rule out that there is rapid degeneration (apoptosis) of a select subpopulation of neurons in the region of the MPBN and SC, but this is unlikely because of the rapidity of the change from the fetal to neonatal response. Alternatively, endocrine or chemical changes that occur at birth because of the removal of the placenta (e.g. decrease of plasma concentrations of progesterone, prostaglandins, adenosine, etc.), or the increase in arterial PO2 at the onset of gaseous ventilation, could change the response of these pontine neurons to substances such as almitrine, or to acute hypoxia. Failure of this pontine network to "switch off" at birth would have serious consequences for the infant, since respiratory inhibition as a response to hypoxia could persist postnatally.
Abbreviations
- AP:
-
area postrema
- EMG:
-
electromyogram
- i.a.:
-
intra-arterial
- KF:
-
Kolliker-Fuse nucleus
- LC:
-
locus coeruleus
- LPBN:
-
lateral parabrachial nucleus
- MPBN:
-
medial parabrachial nucleus
- NGS:
-
normal goat serum
- NTS:
-
nucleus tractus solitarius
- PAG:
-
periaqueductal gray
- PCO2:
-
partial pressure of carbon dioxide
- PO2:
-
partial pressure of oxygen
- Pv:
-
periventricular nucleus
- PVN:
-
paraventricular nucleus
- SC:
-
subcoeruleus nucleus
- SCN:
-
suprachiasmatic nucleus
- SCP:
-
superior cerebellar peduncle
- SON:
-
supraoptic nucleus
- TB:
-
trapezoid body
- VLM:
-
ventrolateral medulla
References
Laubie M, Schmitt H 1980 Long-lasting hyperventilation induced by almitrine: Evidence for a specific effect on carotid and thoracic chemoreceptors. Eur J Pharmacol 61: 125–136.
Johnston BM, Moore PJ, Bennet L, Hanson MA, Gluckman PD 1990 Almitrine mimics the effect of hypoxia in fetal sheep with pontine lesions. J Appl Physiol 69: 1330–1335.
Moore PJ, Hanson MA, Parkes MJ 1989 Almitrine inhibits breathing movements in fetal sheep in utero. J Dev Physiol 11: 277–281.
Boddy K, Dawes GS, Fisher R, Pinter S, Robinson JS 1974 Fetal respiratory movements, electrocortical and cardiovascular responses to hypoxemia and hypercapnia in sheep. J Physiol (Lond) 243: 599–618.
Blanco CE, Hanson MA, McCooke HB 1983 Effects of almitrine bismethylate on chemoreceptor activity in fetal sheep and newborn lambs. Eur J Respir Dis 64: suppl 126 313–317.
Gluckman PD, Johnston BM 1987 Lesions in the upper lateral pons abolish the hypoxic depression of breathing in unanaesthetised fetal lambs in utero. J Physiol (Lond) 382: 373–383.
Johnston BM, Gluckman PD 1993 Peripheral chemoreceptors respond to hypoxia in pontine-lesioned fetal lambs in utero. J Appl Physiol 74: 1–8.
Dawes GS, Gardner WN, Johnston BM, Walker DW 1983 Breathing in fetal lambs: the effect of brain stem section. J Physiol (Lond) 335: 535–553.
Sagar SM, Sharp FR, Curran T 1988 Expression of c-fos protein in the brain: metabolic mapping at the cellular level. Science 240: 1328–1331.
Hunt SP, Pini A, Evan G 1987 Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature 328: 632–634.
Imaki T, Shibasaki T, Hotta M, Demur H 1992 Early induction of c-fos precedes expression of corticotropin-releasing factor messenger ribonucleic acid in the paraventricular nucleus after immobilisation stress. Endocrinology 131: 240–246.
Keay KA, Bandler R 1993 Deep and superficial noxious stimulation increase fos-like immunoreactivity in different regions of the midbrain periqueductal grey of the rat. Neurosci Lett 154: 23–26.
Kononen J, Honkaniemi J, Alho H, Koistinaho J, Iadarola M 1992 FOS-like immunoreactivity in the rat hypothalamic-pituitary axis after immobilisation stress. Endocrinology 130: 3041–3047.
Minson JB, Arnoldo LD, Llewellyn-Smith LJ, Pilowsky PM, Suzuki S, Chalmers JP 1996 Immediate early genes in blood pressure regulation. Clin Exp Hyper 18: 279–90.
Breen S, Rees S, Walker DW 1997 Identification of brain stem neurons responding to hypoxia in fetal and newborn sheep. Brain Res 748: 107–121.
Paxinos G, Watson C 1986 The Rat Brain in Stereotaxic Coordinates. Academic Press, San Diego, CA, figs. 21–76.
Walker DW 1995 Hypoxic inhibition of breathing and motor activity in the fetus and newborn. Clin Exp Pharmacol Physiol 22: 533–536.
Clewlow F, Dawes GS, Johnston BM, Walker DW 1984 Changes in breathing, electrocortical and muscle activity in unanaesthetised fetal lambs with age. J Physiol (Lond) 341: 463–476.
Breen S, Rees S, Walker DW 1996 The development of diurnal rhythmicity in fetal suprachiasmatic neurons as demonstrated by FOS-immunohistochemistry. Neuroscience 74: 917–926.
Boddy K, Jones CT, Robinson JS 1974 Correlations between plasma ACTH concentrations and breathing movements in fetal sheep. Nature 250: 75–76.
Gluckman PD, Parson Y 1983 Stereotaxic method and atlas for ovine fetal forebrain. J Dev Physiol 5: 101–128.
Tillet Y, Thibault J 1989 Catecholamine-containing neurons in the sheep brainstem and diencephalon: immunohistochemical study with tyrosine hydroxylase and dopamine-β-hydroxylase antibodies. J Comp Neurol 290: 69–104.
Breder CD, Smith WL, Raz A, Masferrer J, Seibert K, Needleman P, Saper C 1992 Distribution and characterisation of cyclooxygenase immunoreactivity in the ovine brain. J Comp Neurol 322: 409–438.
Blanco CE, Dawes GS, Hanson MA, McCooke HB 1984 The response to hypoxia of arterial chemoreceptors in fetal sheep and newborn lambs. J Physiol (Lond) 351: 25–37.
Chitravanshi VC, Kanchroo A, Sapru HN 1994 A midline area in the nucleus commissuralis of the NTS mediates the phrenic nerve responses to carotid chemoreceptor stimulation. Brain Res 662: 127–133.
O'Reagan RG, Majcherczyk S, Przybyszewski A 1983 Effects of almitrine bismesylate on activities recorded from nerves supplying the carotid bifurcation in the cat. Eur J Respir Dis 64: 197–202.
Chamberlain NL, Saper CB 1992 Topographic organisation of cardiovascular responses to electrical and glutamate microstimulation of the parabrachial nucleus in the rat. J Comp Neurol 326: 245–262.
Carrive P, Bandler R 1991 Viscerotopic organisation of neurons subserving hypotensive reaction within the midbrain periqueductal grey: Correlative functional and anatomical study. Brain Res 541: 206–215.
Depaulis A, Keay KA, Bandler R 1994 Quiescence and hyporeactivity evoked by activation of cell bodies in the ventrolateral midbrain periaqueductal grey of the rat. Exp Brain Res 99: 75–83.
Gallman EA, Lawing LL, Millhorn DE 1991 Mesencephalic stimulation elicits inhibition of phrenic nerve activity in cat. J Physiol (Lond) 436: 405–420.
Herbert H, Moga MM, Saper CS 1990 Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J Comp Neurol 293: 540–580.
Moga MM, Herbert H, Hurley KM, Yasui Y, Gray TS, Saper CS 1989 Organisation of cortical, basal forebrain, and hypothalamic afferents to the parabrachial nucleus in the rat. J Comp Neurol 295: 624–661.
Swanson LW 1992 Brain Maps: Structure of the Rat Brain. Elsevier, Amsterdam, pp 20–73
Lumsden T 1923 Observations on the respiratory centres in the cat. J Physiol (Lond) 57: 153–160.
Wang W, Fung M, St John W 1993 Pontine regulation of ventilatory activity in the adult rat. J Appl Physiol 74: 2801–2811.
Lara JP, Parkes MJ, Silva-Carhalo L, Izzo P, Dawid-Milner MS, Spyer KM 1994 Cardiovascular and respiratory effects of stimulation of cell bodies of the parabrachial nuclei in the anaesthetised rat. J Physiol (Lond) 477: 321–329.
Liebeskind JC, Guilbauld G, Besson J, Oliveras J 1973 Analgesia from electrical stimulation of the periaqueductal grey matter in the cat: behavioural observations and inhibitory effects on spinal cord interneurons. Brain Res 50: 441–446.
Ackland GL, Waites BA, Nobel R, Hanson MA 1995 Bilateral lesions in the red nucleus abolish the biphasic respiratory response to hypoxia in decerebrate newborn rabbit. J Physiol (Lond) 483: 89P
Dillon GH, Waldrop TG 1992 In vitro responses of caudal hypothalamic neurons to hypoxia and hypercapnia. Neuroscience 51: 941–950.
Rurak DW 1978 Plasma vasopressin levels during hypoxemia and the cardiovascular effects of exogenous vasopressin in fetal and adult sheep. J Physiol (Lond) 277: 341–357.
Teppema LJ, Veening JG, Kramenburg A, Dahan A, Berkenbosch AAD, Olievier C 1997 Expression of c-fos in the rat brainstem after exposure to hypoxia and to normoxic hyperoxic hypercapnia. J Comp Neurol 388: 169–190.
Dobbins EG, Feldman JL 1994 Brainstem network controlling descending drive to phrenic motoneurons in rat. J Comp Neurol 347: 64–86.
Nitsos I, Rajan R, Walker DW 1997 Characterisation of spinal projecting neurons in the pons which express FOS immunoreactivity during hypoxia in fetal sheep. Soc Neurosci Abstr 23: 436
Acknowledgements
We thank Servier Australia Pty Ltd for the gift of almitrine bismesylate, Alex Satragno and Michelle Mulholland of the Physiology Department, Monash University, and Dr. D. Finklestein, Dr. S. Cheema and Hoa Kha from the Anatomy Department, Monash University for their guidance and support.
Author information
Authors and Affiliations
Additional information
Supported by grants from the National Health and Medical Research Council of Australia, and the National SIDS Council of Australia (D.W.W.).
Rights and permissions
About this article
Cite this article
Lee, B., Nitsos, I. & Walker, D. Effects of the Respiratory Stimulant Almitrine on Breathing and FOS Expression in the Brain of Fetal and Newborn Sheep. Pediatr Res 45, 531–543 (1999). https://doi.org/10.1203/00006450-199904010-00013
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199904010-00013