Abstract
As a model of the meconium aspiration syndrome (MAS) of human infants, adult rabbits and newborn rhesus monkeys received intratracheal instillation of human meconium to induce pulmonary injury. Injured rabbits were ventilated with 100% O2 and divided into four treatment groups, receiving: 1) bronchoalveolar lavages (BAL) with dilute KL4-Surfactant; 2) lavages with equal volumes of sterile saline; 3) a single intratracheal bolus of KL4-Surfactant, 100 mg/kg; and 4) no treatment. The untreated rabbits developed atelectasis, a fall in pressure-volume levels and in partial pressure of O2 in arterial blood (PaO2) from approximately 500 to <100 mm Hg, and severe pulmonary inflammation between 3 and 5 h after instillation of meconium. Rabbits treated by BAL with dilute KL4-Surfactant showed rapid and sustained recovery of PaO2 to approximately 300 mm Hg within minutes, a return toward normal pressure-volume levels, and diminished inflammation. Rabbits receiving BAL with saline failed to show recovery, and rabbits treated with a bolus of surfactant intratracheally exhibited a transient response by 1-2 h after treatment, but then returned to the initial atelectatic state. Newborn rhesus monkeys, after receiving human meconium intratracheally before the first breath, developed severe loss of pulmonary function. Treatment of these monkeys 1-5 h after birth with BAL with dilute KL4-Surfactant produced clearing of chest radiographs and a rapid improvement in pulmonary function with ratios of partial pressure of O2 in arterial blood to the fraction of O2 in the inspired air rising into the normal range where they remained through the 20-h period of study. The studies indicate that pulmonary function in two models of severe meconium injury respond rapidly to BAL with dilute KL4-Surfactant.
Similar content being viewed by others
Main
Meconium-stained amniotic fluid is present in 5-20% of all births in the United States. Each year, approximately 26,000 newborn infants in the United States develop MAS (1,2), involving progressive respiratory distress, hypoxia, hypercapnea, and acidosis requiring long-term ventilatory treatment. Experimental studies have shown that, after inhalation of meconium, collapse of subpleural alveoli takes place (3,4), and gross and microscopic atelectasis develops (5–9). Atelectasis, may result from mechanical obstruction (10–12) caused by the particulate meconium, a so-called chemical pneumonitis and meconium-induced dysfunction of surfactant. Although mechanical obstruction may play a role in meconium-induced pulmonary injury, the use of filtered meconium, obviating mechanical obstruction, led to loss of pulmonary function and alveolar collapse (13). This indicated a direct effect in vivo of the meconium on surfactant in the lung tissue. Surfactant extracts of atelectatic lung taken after meconium aspiration revealed poor surface tension (5,7,14).
A direct action of meconium on surfactant has been shown in vitro. A dose-dependent loss of surface activity of surfactant was produced by human meconium (14,15). Both chloroform and aqueous extracts of meconium have been found active (7,15), although in a separate study (5), only the organic extract was stated to be active. Constituents of meconium that may contribute to alteration of the physical properties of surfactant include fatty acids, cholesterol, bile salts, bilirubin, and proteolytic enzymes (5,7,15–17).
Another factor in the development of pulmonary dysfunction has been stated to be a "chemical pneumonitis" (2,10,18). The chemical pneumonitis apparently results from both a direct action of meconium on the lung and from the inflammatory reaction that ensues (6,10,14).
With the evidence that surfactant function is impaired by meconium aspiration, efforts have been directed toward therapeutic intervention with exogenous surfactant. Studies in animal models of the efficacy of surfactant treatment have yielded mixed results (6,8,19–20). Partly in response to this, attention has been focused on approaches employing pulmonary lavage with saline or surfactant. Treatment of experimental animals by lavage with surfactant has shown improved lung function greater than that achieved by lavage with saline (21,22) or bolus administration of surfactant (23).
In studies in human infants, Auten et al. (24) treated neonatal infants with MAS with calf lung surfactant extract and observed improvement in lung function and minimal clearing of chest radiographs. A majority of the patients required additional surfactant treatment. Data showing a positive effect were also found by Davis et al. (25). Lotze et al. (26) and Lotze (27) compared the response in newborn infants to multiple bolus doses of surfactant or air placebo. A decreased need for extracorporial membrane oxygenation in the surfactant group was observed in those patients with the least severe disease. No difference was found in time on ventilation, oxygen requirements, time to discharge, or incidence of pneumothorax, pulmonary interstitial emphysema, and chronic lung disease. In a separate study, bolus administration of bovine surfactant resulted in improved pulmonary function over a 6-h period (28). Additionally, two human infants with severe MAS, both destined for extracorporial membrane oxygenation, were treated with repeated saline lavage, 10 mL/kg, followed by instillation of bovine surfactant. Both infants responded rapidly with an increase in a/A and clearing of chest radiographs in 4-5 h (29).
Considering the variability in efficacy using exogenous surfactant given as a bolus in the treatment of experimental and clinical MAS and the early experimental data using lavage methods, we undertook studies to determine the effect of removal of meconium from the airways by lavage with dilute surfactant or saline in improving pulmonary function.
The exogenous surfactant used is a totally synthetic, peptide-containing preparation, the 21-residue peptide being a mimic of human surfactant protein B, consisting of repeated units of four hydrophobic leucine (L) residues, bounded by basic polar lysine (K) residues (KL4). Combined with the phospholipids dipalmitoyl phosphatidylcholine and palmitoyl oleoylphosphatidyl glycerol (3:1) and palmitic acid, the phospholipid peptide aqueous dispersion has been termed KL4-Surfactant. Its efficacy in experimental and clinical studies has been reported (30–33).
METHODS
Animals. Adult New Zealand White rabbits, approximately 2.5 kg in weight, and rhesus monkeys (Macaca mulatta) delivered at full-term by cesarean section and weighing approximately 520 g were used. Rabbits were studied at The Scripps Research Institute and rhesus monkeys, at the California Primate Research Center, Davis, CA. Studies were approved by the Animal Research Committee of The Scripps Research Institute, and the Animal Use and Care Committee, University of California, Davis. All studies conformed to the requirements of the Animal Welfare Act and National Institutes of Health Guidelines.
Meconium preparation. The first stool passed by human infants was collected and stored frozen until pools were made representing meconium from 5-11 infants. Sterile water was added until a stirrable slurry was achieved. After freezing, the mixture was lyophilized and a dry weight obtained. Sterile saline was added in amounts calculated to yield a concentration of 50 mg (dry weight)/mL. The stock solutions were homogenized in a blender, filtered through gauze to remove particulate material, and stored frozen until diluted for use. For most samples, the dry weight was 25-30% of the original wet weight. Meconium quantities are expressed as dry weight.
KL4-Surfactant. KL4-Surfactant, a synthetic peptide-containing surfactant consisting of dipalmitoyl-sn-glycero-3-phosphatidylcholine, 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoglycerol, palmitic acid, and a synthetic peptide of the sequence KLLLLKLLLLKLLLLKLLLLK, was prepared as described previously (32,33). Dilutions with saline were made from the 30 mg/mL stock solution. The KL4-Surfactant was supplied by the Johnson and Johnson Company, Raritan, NJ.
Biochemical assays. Protein assay was performed using a BCA reagent kit (Pierce, Rockford, IL) according to the manufacturer's instructions. MPO was measured using a modification of the o-dianisidine method described by Steinman and Cohn (34) for the measurement of horseradish peroxidase. Typically, 200 µl of 125 µg/mL o-dianisidine in 100 mM citrate buffer, pH 6.0, were added to 20 µl of sample. The change in absorbance at 405 nm over a 10-min period after the addition of 20 µl of 2 mM H2O2 was proportional to the amount of MPO present. A standard reference solution which caused an absorbance change of approximately 0.072 unit µl-1 10 min-1 was used. One unit of MPO activity was defined as the activity in 1 µl of the reference solution. Because of extensive variability in background values, it was not possible to obtain meaningful MPO data using rabbit lungs. Rabbit and monkey IL-8 were quantitated by a previously described ELISA method (35). Surfactant was isolated from rabbit or monkey lavage fluids by taking the supernatant from a 5-min, 1000 rpm spin (which removed cells and debris) and subjecting it to a 1-h spin at 40,000 × g at 4°C. The precipitate from this high speed spin was resuspended in 1/20 the original volume of normal saline and quantitated as to the amount of phospholipid present by a modification of the method of Rouser et al. (36). Meconium was quantitated spectrophotometrically. Samples were centrifuged at 40,000 × g for 1 h. The OD of the supernatant (or dilutions in saline) was read at 260 and 300 nm, and the following formula applied: OD300 - (0.13)(OD260). This formula was derived empirically from spectral analyses of multiple rabbit and monkey lavage samples with and without meconium; it was found to yield values of approximately zero for samples not containing meconium, and a linear relationship was found to exist for solutions containing from 1 to 1600 µg/mL meconium.
Lavage samples from animals with pulmonary inflammation, determined microscopically, were found to have a unique absorption peak at approximately 400-405 nm, which was not present in lavage fluids of normal animals. Using this observation and correcting for absorption of proteins with peaks <300 nm, an arbitrary "inflammatory index" was defined as OD400 - (0.45)(OD305).
Rabbit studies. Rabbits were anesthetized with i.m. xylazine (2 mg/kg) and ketamine (50 mg/kg) and maintained anesthetized throughout the study. Tracheostomy was performed, and a 3.0-mm internal diameter endotracheal tube was inserted to a position at least 1 cm above the carina. An arterial line for blood samples was placed in the auricular artery. All animals were then placed on a pressure-cycled ventilator (Bird; 3M). To induce pulmonary injury, meconium was instilled intratracheally. The appropriate volume of meconium was divided into two equal portions, one given with the rabbit held at 45° with head up and the right side of the animal down, and the second half as before, but with the left side down. Meconium was instilled through a cannula threaded through the endotracheal tube and reaching <0.5 cm beyond the tip of the endotracheal tube. Rabbits were placed on 100% O2 for 10-15 min before instillation of meconium and received 100% O2 for the duration of the study. Initial studies were performed to determine the response of adult rabbits to varying doses of a slurry of human meconium given intratracheally. Rabbits received the following doses of meconium (mg/kg in 5-7.5 mL/kg): 93.8 (n = 3), 125 (n = 4), 187.5 (n = 7), 281.3 (n = 2), and 375 (n = 1). Although doses of 93.8 and 125 mg/kg produced a fall in PaO2 and partial atelectasis in the lung, the effect was not consistent, and 71% of the rabbits showed signs of spontaneous recovery of PaO2 by 5 h after instillation of the meconium. At the dose of 187.5 mg/kg, a consistent fall in PaO2 occurred within 1 h of instillation of meconium, and spontaneous recovery of PaO2 by 5 h was not observed. Doses higher than 187.5 mg/kg also induced a fall in PaO2, but with death occurring in two of the three rabbits. The dose of 187.5 mg/kg in 7.5 mL/kg was selected for the studies. Comparison was made with rabbits receiving 7.5 mL/kg 0.9% saline as a control. After the meconium instillation mechanical ventilation was begun with PIP of 25 cm H2O, PEEP of 2 cm H2O, and ventilatory rate of 20 breaths/min. Blood gas determinations were performed approximately 10, 30, and 50 min after meconium instillation. After 1 h, the rabbits were placed into one of four treatment groups.
Group 1 rabbits had BAL with KL4-Surfactant diluted to 2 mg/mL, receiving 20 mL/kg divided into two equal portions, one lavaged into the right lung, and the second half into the left lung. After verification that anesthesia was fully effective, the rabbit was given i.v. vecuronium bromide 0.1 mL/kg to induce paralysis, found to be essential for appropriate drainage in the lavage procedure. The KL4-Surfactant was instilled through the endotracheal tube with the ventilator on PEEP alone, at a pressure of 5 cm H2O. The animal was held head up at approximately 45° with, first, the right-side down to instill surfactant into the right lung and then left-side down for instillation into the left lung. Immediately after instillation of the dilute surfactant, rabbits received IMV (five breaths). The rabbit was then disconnected from the ventilator and the lavage-surfactant and meconium were drained by tipping the rabbit head down at 30-45°. Drainage was continued with gentle massage of the chest until flow slowed considerably. The total time required for the lavage procedure was approximately 90 s. The rabbit was immediately placed back on IMV for 2-5 min, i.e. until O2 saturation reached ≥90%. The lavage procedure was then repeated. This constituted the first lavage. Two repeated lavages were performed similarly, each divided between right and left lungs. A final lavage was performed with KL4-Surfactant using a concentration of KL4-Surfactant 15 mg/mL. In a separate group of animals, a bolus of KL4-Surfactant was given at 30 mg/mL(100 mg/kg), rather than the final lavage to determine whether a large amount of retained surfactant improved pulmonary function; group 2 had BAL with sterile saline, performed as in group 1, but using equal volumes of sterile saline; group 3 had instillation of a bolus of KL4-Surfactant (i.e., without lavage) at a concentration of 30 mg/mL, using 100 mg/kg divided equally between right and left lungs; and group 4 had no treatment of the meconium injury.
Assays of surfactant function. Two additional rabbits were treated as above with meconium (187.7 mg/kg), and after 1 h, when PaO2 had fallen, the rabbits were killed, and the atelectatic lower lobes of the lungs were lavaged twice with 5 mL of saline to obtain surfactant for assay. The fluid obtained was centrifuged (4°C) at 1000 × g 5 min to remove cells and then 40,000 × g 60 min to isolate surfactant. The pellet of surfactant was washed once with 1.5 mL of 0.9% saline and resuspended in 1.5 mL of 0.9% saline. The concentration of surfactant was adjusted to 3 mg/mL based on phospholipid concentration. The capacity of the surfactant to lower surface tension at an air-liquid interface at 37° was measured using a pulsating bubble surfactometer as described (37).
Monkey studies. Studies were limited to 10 newborn rhesus monkeys. Rhesus monkeys were delivered by cesarean section at the time of full-term gestation (157-160 d), performed by the veterinary staff of the Primate Center of the University of California, Davis. After delivery of the head and neck under continuous anesthesia, vecuronium bromide was injected i.m., and tracheotomy was performed with placement of a 2.0-mm internal diameter endotracheal tube with the distal tip being 0.5-1 cm above the carina before delivery of the body and clamping of the umbilical cord. The endotracheal tube was clamped to prevent gasping, and delivery was completed. The newborn monkey was weighed and placed under a radiant warmer, and meconium was instilled through the endotracheal tube into the fluid-filled lungs before the first breath. The monkey was placed on a mechanical ventilator set on IMV: FIO2 of 0.8-1.0, PIP of 30-35 cm H2O, PEEP of 4 cm H2O, and 40 breaths/min with inspiratory time of 0.4 s. A 3.5 French umbilical artery catheter was inserted into the aorta (to L4) to obtain arterial blood samples and for administration of fluids. The monkey received 5-8.3 mL kg-1 h-1 of 5% dextrose in water with 0.5 U/mL heparin throughout the study. Continuous measurements of heart rate, arterial blood pressure, arterial blood oxygen saturation, and rectal temperature were maintained through the study. Arterial blood samples were obtained every 20-60 min for blood gas and pH analyses. Mechanical ventilation and FIO2 were adjusted to maintain PaO2 of 50-70 mm Hg, PaCO2 of 40-50 mm Hg, and pH > 7.25. Chest radiographs were obtained within 1 h of birth and meconium administration, within 1 h of surfactant treatment, and at various intervals thereafter as noted. Anesthesia and paralysis were maintained throughout the study.
Lavage with dilute KL4-Surfactant was performed through a 3.5 French umbilical catheter inserted through the endotracheal tube and cut so the tip was just distal to the tip of the tube. The monkeys received 100% O2 throughout the procedure. Lavage was performed with KL4-Surfactant diluted to 2 mg/mL, using 10-20 mL/kg, divided equally between the right and left sides and administered as described for rabbits (above). Monkeys received one to three subsequent lavages using KL4-Surfactant at 2 mg/mL and either a final lavage at 15 mg/mL or a bolus of 133 mg/kg at 30 mg/mL.
Pressure-volume assays in the lung. Static pressure-volume assays were performed on rabbits before instillation of meconium, at 0.9 h after meconium, i.e. just before treatment, and at about 5 h after meconium instillation. Anesthetized rabbits were treated with vecuronium bromide (0.1 mL/kg i.v.), briefly removed from the ventilator, and connected to a pressure/volume monitoring circuit, with recording of volumes of air corresponding to increments of 1 cm H2O pressure up to and down from 7 cm H2O. Further increase in pressure in the normal or atelectatic lung does not alter the relationships observed with a maximum pressure of 7 cm H2O. Pressures above 8 cm H2O resulted in a diminished increase in volume and, in the injured rabbit lung, led frequently to pneumothorax.
Postmortem examination. Rabbits and rhesus monkeys were killed with i.v. barbiturate. The thorax was opened, and the heart and lungs were removed en bloc with the trachea clamped under 10 cm H2O pressure. Gross examination was performed with the tracheal pressure at both 10 cm H2O and atmospheric pressure. One lung was placed in 10% zinc-formalin after ipsilateral ligation of the bronchus at 10 cm H2O pressure. After fixation, a 2-mm section was cut through the widest portion of each lobe, i.e. vertical to the bronchus, for embedding for microscopic examination. For counts of PMNs in histologic sections of the lung, a straight line was drawn on the coverslip within 2 mm of the mainstem bronchus of the lower lobe, traversing the entire section as a cross-section of the lung. Microscopic counts were then performed along the line from one pleural edge to the other. This method was chosen in view of the consistent injury produced by meconium to this area of the lower lobe and the consistency of treatment with KL4-Surfactant or saline to this region of the lung. The other lung received two lavages of the lower lobe of approximately 2 mL/kg sterile saline each, placed in the same segment. After removal of 0.1 mL for cell counts, lavage fluids were centrifuged at 1000 × g for 5 min to remove cells, and then 40,000 × g for 60 min, both at 4°C, to remove large aggregate surfactant and provide lavage fluid for biochemical studies.
Statistical analyses. ANOVA was performed to show differences between groups using both Fisher protected least significant difference and Scheffe F test significance at 95%. Repeated measures ANOVA was used where appropriate with significance taken at p < 0.05.
RESULTS
Injury of rabbit lungs by intratracheal instillation of human meconium. Seven rabbits were instilled intratracheally with 187.5 mg/meconium. A rapid and persistent decrease in PaO2 resulted as shown in Figure 1. Static pulmonary pressure-volume recordings (assays) were performed before, and 1 and 4-5 h after, instillation of meconium. As shown in Table 1, the normal pressure-volume curve, observed before instillation of meconium, was converted to a flat curve 1 h after, and remained flat through 5 h. Autopsies at 5-6 h after instillation of meconium revealed marked atelectasis of the lungs (Fig. 2A), with dark red, nonexpanded areas in at least 80% of the lung. Small rims or caps of expanded lung existed in the apical region of the upper lobes and in some instances, the middle lobes, presumably due to a failure of the meconium to reach these areas. Histologically, at 1-2 h after administration of meconium, the alveoli were collapsed, but little evidence of inflammation was observed. With periodic acid-Schiff stain, the meconium could be detected by its bright violet color, forming fine, amorphous clumps in the alveolar spaces, generally abutting the septae. Histologically, at 5-6 h after meconium was administered, there were no obstructive plugs of meconium observed in the bronchi, presumably as a result of prior filtration of the meconium preparation. The lungs revealed widespread atelectasis, with dense infiltration of edema and inflammatory cells, primarily, PMNs, together with red blood cells (Fig. 2A).
The PaO2 response of rabbits receiving intratracheal meconium. Adult rabbits were given 7.5 mL/kg saline (open circles, n = 3) or a 25 mg/mL slurry of human meconium (closed squares, n = 7) at t = 0 h. PaO2 was followed for 5 h. Data are expressed as mean ± SEM. The time points of -0.5 and 0 h are the mean values obtained predosing at times varying from -0.77 to -0.03 h; remaining time points are those nearest to the stated times. ANOVA, p = 0.0001.
Gross and microscopic appearance of rabbit lungs injured with meconium approximately 5.5 h before euthanasia and treated as follows: (A) no treatment, (B) KL4-Surfactant lavage, (C) sterile saline lavage, and (D) KL4-Surfactant bolus. Gross photographs on the left show dark red atelectatic collapse in A, C, and D, but with generalized expansion in B. The light regions of the upper lobes in A, C, and D were devoid of meconium and were not injured. Microscopic photographs are of the same lungs shown in the gross photographs; magnification of center photographs, 60×, of right-side photographs 190×. All microscopic sections were taken midway up the lower lobe. Although most of the alveoli in the KL4-Surfactant-lavaged lung show gas expansion with a minimum of edema and PMNs, alveoli in A, C, and D contain abundant edema, PMNs, and red blood cells. Meconium appears as bright reddish amorphous material. Some alveoli in D contain gas bubbles, presumed to be retained from the bolus treatment with KL4-Surfactant. Periodic acid-Schiff-stained.
Surfactant function was assessed in lavage fluids of rabbits 1 h after receiving 187.5 mg of meconium/kg (see "Methods"). Although normal rabbit surfactant showed minimal and maximal surface tension values (3 mg/mL phospholipid phosphorous) of 2.9 ± 0.9 and 33.5 ± 0.6 mN/m at 1 min (mean ± SEM of six determinations), surfactant from meconium-treated rabbits showed values of 22.4 ± 1.3 and 56.5 ± 2.5 mN/m.
As controls, three rabbits received intratracheally equal volumes of sterile saline instead of meconium. The effect on PaO2 levels is shown in Figure 1. A statistical difference in PaO2 values (p = 0.0001) was seen between the saline control and meconium-treated rabbits. Static pressure-volume measurements of these rabbits showed a decrease in volumes from 14.2 to 8.0 at 0.9 h and then recovery by 5 h.
The effect of BAL with dilute KL4-Surfactant on lung expansion, gas exchange, and pulmonary inflammatory response in meconium-injured adult rabbits. Ten rabbits were given meconium at 187.5 mg/kg to induce 1) loss of endogenous surfactant activity in the lungs with associated collapse of the alveoli and 2) development of inflammation at 3-5 h after meconium instillation. Five of the rabbits were lavaged with KL4-Surfactant as noted in "Methods." Five rabbits were similarly lavaged with equal volumes of sterile saline. The FIO2 was maintained at 1.0, and the ventilators were adjusted to PIP of 25-28 and PEEP of 2-10 cm H2O, with a ventilatory rate of 20-32/min as required to maintain adequate ventilation.
The removal of meconium by three sequential lavages with dilute KL4-Surfactant was determined. About 29% of the instilled meconium was removed by the first lavage, 7.5% more in the second lavage, and less than 5% in the third lavage. As will be noted below, this correlated with diminished meconium observed microscopically in lung sections taken at necropsy than was observed in rabbits not lavaged. After the third lavage with dilute KL4-Surfactant, a single divided lavage using KL4-Surfactant at 15 mg/mL was performed to assure that sufficient functional surfactant was present in the lungs.
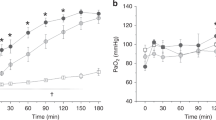
The response in PaO2 of the rabbits to the BAL with KL4-Surfactant occurred rapidly, most often within 2 min after the first lavage (Fig. 3). The PaO2 rose from <100 mm Hg to approximately 300 mm Hg with the rabbit ventilated at FIO2 of 1.0. The PaO2 remained elevated through 5 h, although a drop to a mean of about 200 mm Hg was seen 3-5 h after instillation of the meconium.
Changes in PaO2 in meconium-injured rabbits after lavage with dilute KL4-Surfactant. Ten rabbits were injured with 187.5 mg/kg meconium instilled intratracheally at t = 0. Approximately 1 h later, five rabbits were lavaged three times with dilute KL4-Surfactant (2 mg/mL) using 20 mL/kg followed by one lavage with a higher concentration (15 mg/mL) of the surfactant (solid squares). Five control animals were lavaged four times with 20 mL/kg saline (open circles). Data are expressed as mean ± SEM. The time points of -0.5 and 0 h are the mean values obtained predosing at times varying from -1.0 to -0.03 h; remaining time points are those nearest to the stated time. ANOVA of values after 1 h, p = 0.0007.
Rabbits receiving lavage with saline instead of dilute KL4-Surfactant failed to show improvement in gas exchange (Fig. 3) although comparable amounts of meconium were removed. Increases in PEEP did not increase appreciably the PaO2 in saline-lavaged animals. The difference in PaO2 values between rabbits treated with KL4-Surfactant lavage and those lavaged with saline was statistically significant (p = 0.0007) after treatment.
Static pressure-volume assays, performed at time 0, 0.9 h after instillation of meconium (immediately before lavage treatment), and at approximately 5 h after injury with meconium (i.e. approximately 4 h after lavage treatment), are shown in Table 1. As noted previously, the pressure-volume values fell after instillation of meconium. Lavage treatment with KL4-Surfactant resulted in a 3.4-fold increase in pressure-volume response at the 5-h time point as shown.
Autopsies of KL4-Surfactant-lavaged rabbits, performed at approximately 5.5 h after instillation of meconium, showed generalized expansion of the lungs, with patchy zones of partial atelectasis generally in the lower lobes (Fig. 2B). These atelectatic zones nevertheless contained regions of fine air expansion. Microscopically, the lungs exhibited clear, expanded alveoli, although the partially atelectatic areas revealed the presence of pink-stained alveolar fluid, containing moderate numbers of PMNs, but few red cells. The expanded alveoli contained less protein-rich fluid and strikingly fewer PMNs than did the atelectatic alveoli (Table 2, Fig. 2B). Although not quantitated at the microscopic level, there appeared to be distinctly less meconium in the alveoli in the lavaged than in nonlavaged rabbits.
The lungs of rabbits receiving lavage with saline, rather than KL4-Surfactant, were totally atelectatic (Fig. 2C), except for small caps of expanded lung on the apical portions of the upper lobe. Microscopically, the alveoli were densely packed, and no air expansion was present. The alveolar spaces contained protein-rich fluid and an abundance of PMNs and red cells (Fig. 2C, Table 2). Small amounts of meconium, similar to those seen in surfactant-lavaged rabbits, were found.
As recorded in Table 2, analysis of BAL fluids, taken at the time of autopsy from rabbits lavaged with KL4-Surfactant, revealed lower levels of protein, MPO, IL-8, PMNs, and red cells and a lower inflammatory index than in fluids from rabbits not receiving lavage. These values in KL4-Surfactant-lavaged rabbits were also lower, except for IL-8 levels, than those in saline-lavaged rabbits.
Five additional meconium-injured rabbits received two lavages with KL4-Surfactant at 2 mg/mL as above, but this was followed by bolus administration of KL4-Surfactant of 100 mg/kg at 30 mg/mL concentration. The improvement in PaO2, compliance, and autopsy findings were similar to those in the KL4-Surfactant lavage group (data not shown).
The efficacy of treatment of meconium-injured rabbit lungs with bolus instillation of KL4-Surfactant (without lavage). Five rabbits were given 187.5 mg of meconium intratracheally as before, and after 1 h, each received a 100 mg/kg bolus of KL4-Surfactant at 30 mg/mL. The results are shown in Figure 4. There was a moderate rise in PaO2 from 56 mm Hg to approximately 200 mm Hg between 1 and 2 h after instillation of the KL4-Surfactant. After this time period, however, the PaO2 gradually subsided, falling below 100 mm Hg by 3 h after treatment. Values for lung volume at insufflation pressure of 6 cm H2O at 5.1 h after meconium injury were nearly the same as those 0.9 h after meconium injury, i.e. before treatment with KL4-Surfactant bolus (Table 1). At autopsy, the lungs were almost completely collapsed, except for the small caps of uninvolved, expanded lung on the apices of the upper lobes that were apparently not exposed to meconium (Fig. 2D). Microscopically, approximately 80% of alveoli were filled with proteinaceous fluid and leukocytes (Fig. 2D, Table 2), and abundant meconium, but with scattered groups of alveoli showing expansion. Analysis of terminal BAL fluid revealed a marked inflammatory response (Table 2).
Changes in PaO2 in meconium-injured rabbits after a bolus instillation of KL4-Surfactant. Five rabbits were injured with 187.5 mg/kg meconium instilled intratracheally at t = 0. Approximately 1.1 h later, each animal was given 100 mg/kg KL4-Surfactant in a volume of 3.33 mL/kg. Data are expressed as the mean ± SEM. The time points of -0.5 and 0 h are the mean values obtained predosing at times varying from -0.15 to -0.03 h; remaining time points are those nearest to the stated time.
Response of meconium-injured newborn rhesus monkeys to lavage treatment with KL4-Surfactant. Ten rhesus monkeys were delivered by cesarean section at term. Human meconium was instilled into the tracheal fluid before the first breath. In five animals, a second instillation of meconium was given. Increasing doses of meconium were given: two monkeys receiving 187.5 mg/kg, five receiving 563 mg/kg, two receiving 750 mg/kg, and one receiving 843.8 mg/kg (mean = 553.1 mg/kg). The a/A ratio in all meconium-treated monkeys within 1 h after instillation of the full dose of meconium fell to approximately 0.20 or less. Chest radiographs showed diffuse opacity, characteristic of MAS. Noteworthy was a marked sensitivity of the monkeys to handling, with sharp decrements in oxygen saturation occurring that lasted 2-10 min. Seven of the monkeys were treated by lavage with dilute KL4-Surfactant at times ranging from 1.6 to 5.4 h after birth and instillation of meconium. The other three monkeys served as controls and were maintained for 20-24 h with ventilatory support. The control animal treated with the lowest dose of meconium (187.5 mg/kg), given in two doses, recovered with a rise in a/A ratio into the normal range (>0.4) noted about 2 h after the second instillation of meconium. The dose of meconium used in this animal and in a lavage-treated animal receiving the same dose was, therefore, considered insufficient to elicit and maintain severe pulmonary deficiency over a 20-h period; data from these two monkeys were not included in the results discussed below.
Figure 5 shows the mean a/A ratio over time for the six monkeys receiving ≥563 mg/kg (mean = 656 mg/kg) of meconium and treated with lavage with dilute KL4-Surfactant. Of the two control animals treated with a comparable amount of meconium, a/A ratios were not obtainable for one due to an inability to establish an arterial line. The a/A ratio in the other control animal remained at approximately 0.1 over an 18-h period (Fig. 5). In addition, three monkeys, receiving 563, 750, and 843.8 mg meconium/kg were monitored for 2.6, 3.2, and 5.4 h, respectively, before treatment with surfactant lavage. a/A ratios remained at approximately 0.1 to 0.25 until initiation of surfactant-lavage treatment.
Effect of treating meconium-injured newborn rhesus monkeys with KL4-Surfactant lavage. Seven newborn rhesus monkeys were given meconium (656 mg/kg mean quantity) into the tracheal fluid at birth. Six were treated at a mean time of 2.8 h with three or four lavages of KL4-Surfactant diluted to 2 mg/mL, followed by either lavage using KL4-Surfactant at 15 mg/mL or a bolus of 133 mg/kg at 30 mg/mL; one remained untreated and served as a control animal. Data shown are the mean ± SEM values for the a/A ratio at the indicated time points. Time 0 is defined as the start of the lavage procedure.
In the treatment group receiving lavage with dilute KL4-Surfactant, an abrupt rise in a/A ratio was observed immediately after the lavage (Fig. 5). The FIO2 was maintained at 1.0 during the period of lavages with PaO2 values after treatment typically being >300 mm Hg. This resulted in aberrantly high a/A ratios, and when the FIO2 was lowered to the point that PaO2 was in the range of 50-70 mm Hg, the a/A ratios decreased as shown 1 h after surfactant lavage in Figure 5. In a few monkeys the a/A ratio fell transiently to <0.2 even in the presence of clearing chest radiographs (see below). By approximately 4-8 h after lavage treatment with KL4-Surfactant, the a/A ratios rose to the normal range. All treated animals were breathing room air by the termination of the study period (mean time to FIO2 = 0.21 was 11.2 h). As shown in Figure 6, an appreciable clearing of the chest radiographs could be seen within 30 min after the start of surfactant lavage. Nearly complete clearing was observed within 18-20 h. Generalized opacity of the chest radiographs remained in the two control animals over the 18-h period with some evidence of peripheral clearing after approximately 10 h.
At 18-24 h of age, the animals were killed. Gross inspection of the meconium-injured lungs of monkeys that were lavaged with KL4-Surfactant revealed generalized expansion, with scattered small zones of dark red atelectasis, mostly in the dorsal (dependent) regions of the lower lobes. Cut sections of these atelectatic zones showed that they involved 1-2 mm of the surface, with light-pink, expanded lung beneath. In contrast, lungs of the two monkeys receiving meconium, but without KL4-Surfactant treatment, were >80% atelectatic. Microscopically, the lungs of monkeys treated with KL4-Surfactant lavage were expanded with clear alveoli and a small amount of meconium, edema, and leukocytes. In the zones of atelectasis, meconium was present in large quantities along with PMNs and modest amounts of edema fluid. The lungs of monkeys not treated with surfactant were collapsed and filled with meconium, neutrophils, and some edema fluid. There was little to no expansion. The lobes of the lung not taken for microscopic analysis were lavaged twice with saline for analysis of the inflammatory reaction. The results of these analyses are shown in Table 3. The data show a diminution in the markers of inflammation in KL4-Surfactant-lavaged animals.
DISCUSSION
The effect of lavage treatment of meconium-injured lungs using KL4-Surfactant. The data in this study indicate that in models of MAS in rabbits and newborn monkeys, pulmonary function can be greatly improved within minutes by lavage of the lungs with dilute KL4-Surfactant followed by instillation of a sustaining dose. PaO2, a/A ratio, pulmonary pressure-volume relationships, and chest radiographs all revealed rapid improvement.
Two species were used, adult rabbits that exhibit a brisk and marked inflammatory response to instillation of human meconium, and newborn primates (M. mulatta) that were given human meconium intratracheally at the time of cesarean delivery and before the first breath. The latter model mimics closely the situation in human infants who aspirate meconium in utero shortly before birth. Of particular interest, the improvement in pulmonary function in rhesus monkeys persisted and the clearing of chest radiographs continued over the course of the study, approximately 20 h. The monkeys were breathing room air at approximately 11 h after receiving KL4-Surfactant lavage.
In both rabbits and rhesus monkeys, the instilled meconium induced a rapid fall in gas exchange associated with the development of atelectasis. Three mechanisms have been proposed to explain the meconium-induced decrement in pulmonary function: 1) particles of meconium may obstruct small bronchioles in the lung, 2) meconium may inhibit surfactant directly, and 3) meconium-induced inflammation may serve to inhibit surfactant. The current studies support the theory that meconium-induced dysfunction of surfactant is a major factor in the loss of pulmonary function in MAS. The decrement in lung function in rabbits was associated with a loss of surfactant activity as evidenced by atelectasis associated with dysfunction of surfactant removed from the lung. These data are consistent with previous in vitro data showing that mixing meconium, or organic and aqueous extracts of meconium, with surfactant leads to surfactant dysfunction (5,7,15). The mechanism of the inactivation and the constituents of the meconium responsible remain unknown. In contrast, loss of pulmonary function owing to particulate meconium appears unlikely in the present study, given that the meconium was filtered, plugs were not observed microscopically in bronchi, and lavage with saline alone failed to expand the alveoli and improve lung function.
The present data suggest that lavage with dilute KL4-Surfactant removed sufficient amounts of meconium in both rabbits and monkeys to allow the KL4-Surfactant, possibly coupled with residual native surfactant, to expand the alveoli, improve pulmonary function and pressure-volume relationships, and diminish the development of inflammation (see below). In contrast, lavage of the lungs with saline, rather than surfactant, failed to improve pulmonary function and, in fact, resulted in a greater inflammatory reaction in the lung. The detrimental effect of saline lavage (38) or bolus instillation of saline have been noted (6,14).
Treatment with KL4-Surfactant by lavage versus bolus. Studies of the efficacy of treatment with KL4-Surfactant by lavage as opposed to bolus instillation revealed that the method of lavage was superior both from the standpoint of improved gas exchange, and also the lessened inflammatory response. The data showed that although bolus instillation, without lavage, resulted in a modest increase in PaO2 over the first 2 h, the effect was ephemeral, with PaO2 values returning to levels at or below 100 mm Hg within about 2 h. This transient effect may be explained by the continued presence of meconium in the lungs, which may have directly inactivated the instilled KL4-Surfactant (and native surfactant) in the lung. Additionally, bolus treatment with KL4-Surfactant failed to reduce the inflammatory response to meconium at 3-5 h, allowing the inflammation to inactivate further the surfactant and also impede pulmonary function independently of its action on surfactant.
The effect of KL4-Surfactant lavage on the development of inflammation in the lungs. The amount of pulmonary inflammation in meconium-injured adult rabbits was compared in four treatment groups: KL4-Surfactant-lavaged rabbits, saline-lavaged rabbits, rabbits treated with KL4-Surfactant by bolus instillation, and rabbits receiving meconium alone (Table 2). The data indicate that meconium-injured rabbits receiving lavage treatment with KL4-Surfactant exhibited less pulmonary inflammation than did untreated controls, saline-lavaged rabbits, or bolus surfactant-treated rabbits. This was observed both in the terminal lavage fluids and microscopic sections of the lungs. The diminution of inflammation was striking in microscopic sections of lung of KL4-Surfactant-lavaged rabbits in which small zones of atelectasis contained abundant edema and PMNs and red blood cells, whereas the neighboring expanded lung was nearly devoid of each. Comparison of the amount of inflammation in the surfactant-lavaged and saline-lavaged rabbits suggests that the KL4-Surfactant lavage reduced the inflammatory reaction, even though nearly equal amounts of residual meconium were present in lungs in the two groups. The mechanism of the inhibitory effect on inflammation by surfactant is unclear. One possible explanation is that surfactants, including KL4-Surfactant, have been found to inhibit the function of inflammatory cells (39–44), which may diminish their contribution to the inflammatory reaction. Similarly, expansion of the alveoli and restoration of lung function by the KL4-Surfactant lavage may diminish the formation of edema, thus reducing the amount of potential substrate for mediators of inflammation. The effect of sustained ventilatory pressure on the diminution of inflammation in surfactant-depleted animals has been the subject of a number of reports (e.g. see Refs. 45–48). The means by which surfactant lavage inhibits recurrence of inflammation will require additional studies.
Abbreviations
- a/A:
-
ratio of PO2 in arterial blood (a) to the fraction of O2 in the inspired air (A)
- BAL:
-
bronchoalveolar lavage
- IMV:
-
intermittent mandatory ventilation
- KL4:
-
peptide KLLLLKLLLLKLLLLKLLLLK
- MAS:
-
meconium aspiration syndrome
- PaO2:
-
PO2 in the arterial blood
- PaCO2:
-
PCO2 in the arterial blood
- PEEP:
-
positive end expiratory pressure
- PIP:
-
peak inspiratory pressure
- MPO:
-
myeloperoxidase
- FIO2:
-
fraction of inspired O2
- PMN:
-
polymorphonuclear leukocytes
References
Wiswell TE, Bent RC 1993 The meconium aspiration syndrome: unasnwered questions. Pediatr Clin North Am 40: 955–981
Gregory GA, Gooding CA, Phibbs RH, Tooley WH 1974 Meconium aspiration in infants: a prospective study. J Pediatr 85: 848–852
Nieman GF, Bredenberg CE 1985 High surface tension pulmonary edema induced by detergent aerosol. J Appl Physiol 58: 129–136
Clark DA, Graeber J, Nieman GF 1979 Alveolar cinemicroscopy in experimental meconium aspiration. Pediatr Res 13: 532
Clark DA, Nieman GF, Thompson JE, Paskanik AM, Rokhar JE, Bredenberg CE 1987 Surfactant displacement by meconium free fatty acids: an alternative explanation, for atelectasis in meconium aspiration syndrome. J Pediatr 110: 765–770
Sun B, Herting E, Curstedt T, Robertson B 1994 Exogenous surfactant improves lung compliance and oxygenation in adult rats with meconium aspiration. J Appl Physiol 77: 1961–1971
Sun B, Curstedt T, Robertson B 1993 Surfactant inhibition in experimental meconium aspiration syndrome. Acta Paeditr 82: 182–189
Sun B, Curstedt T, Song GW, Robertson B 1993 Surfactant improves lung function and morphology in newborn rabbits with meconium aspiration. Biol Neonate 63: 96–104
Seo IS, Gillim SE, Mirkin CD 1990 Hyaline membrane in postmature infants. Pediatr Pathol 10: 539–548
Tyler DC, Murphy J, Cheney FW 1978 Mechanical and chemical damage to lung tissue caused by meconium aspiration. Pediatrics 62: 454–459
Gooding CA, Gregory GA 1971 Roentgen analysis of meconium aspiration of the newborn. Radiology 100: 131–135
Tran N, Lowe C, Sivierl EM, Shaffer TH 1980 Sequential effects of acute meconium obstruction on pulmonary function. Pediatr Res 14: 34–38
Chen TC, Tuong TJK, Rogers MC 1985 Effect of intraalveolar meconium on pulmonary surface tension properties. Crit Care Med 13: 233–236
Davey AM, Becker JD, Davis JM 1993 Meconium Aspiration syndrome: physiologic and inflammatory changes in a newborn piglet model. Pediatr Res 16: 101–108
Moses D, Holm BA, Spitale P, Liu M, Enhorning G 1991 Inhibition of pulmonary surfactant function by meconium. Am J Obstet Gynecol 164: 477–481
Henderson RD, Fung K, Cullen JB 1975 Bile aspiration: an experimental study in rabbits. Can J Surg 18: 64–69
Lieberman J 1966 Proteolytic enzyme activity in fetal pancreas and meconium. Gastroenterology 50: 183–190
Bascik RD 1977 Meconium aspiration syndrome. Pediatr Clin North Am 24: 463–479
Sun B, Curstedt T, Robertson B 1996 Exogenous surfactant improves ventilation efficiency and alveolar in rats with meconium aspiration. Am J Crit Care Med 154: 764–770
Wiswell TE, Peabody SS, Davis JH, Slayter MV, Bent RC, Merritt TA 1994 Surfactant therapy and high-frequency jet ventilation in the management of a piglet model of the meconium aspiration syndrome. Pediatr Res 36: 494–500
Paranka MS, Walsh WF, Stancombe BB 1992 Surfactant lavage in a piglet model of meconium aspiration syndrome. Pediatr Res 31: 625–628
Ohama Y, Itakuru Y, Koyama N, Eguchi H, Ogawa Y 1994 Effect of surfactant lavage in a rabbit model of meconium aspiration syndrome. Acta Paediatr Jpn 33: 236–238
Balaraman V, Sood SL, Finn KC, Hashiro G, Uyehara CFT, Easa D 1996 Physiologic response and lung distribution of lavage vs. bolus Exosurf in piglets with acute lung injury. Am J Respir Crit Care Med 153: 1838–1843
Auten RL, Notter RH, Kendig JW, Davis JM, Shapiro DL 1991 Surfactant treatment in full-term newborns with respiratory failure. Pediatrics 87: 101–107
Davis JM, Richter SE, Kendig JW, Notter RH 1992 High-frequency jet ventilation and surfactant treatment of newborns with severe respiratory failure. Pediatr Pulmonol 13: 108–112
Lotze A, Knight GR, Martin GR, Bulas DL, Hull WM, O'Donnell RM, Whitsett JA, Short BL 1993 Improved-pulmonary outcome after exogenous surfactant therapy for respiratory failure in term infants requiring extracorporeal membrane oxygenation. J Pediatr 122: 261–268
Lotze A 1996 Survanta® pre-ECMO trial: multi-center evaluation of Survanta® in treatment of term infants with severe respiratory failure. Pediatr Res 39: 226A( Abstr)
Khammash H, Perlman M, Wojtulewicz J, Dunn M 1993 Surfactant therapy in full-term neonates with severe respiratory failure. Pediatrics 92: 135–139
Ibara S, Ikenoue T, Murata Y 1995 Management of meconium aspiration syndrome by tracheobronchial lavage and replacement of surfactant-TA. Acta Paediatr Jpn 37: 64–67
Cochrane CG, Revak SD 1991 Pulmonary surfactant protein B (SP-B): structure-function relationships. Science 254: 566–568
Vincent JS, Revak SD, Cochrane CG, Levin IW 1991 Raman spectroscopic studies of model human pulmonary surfactant systems: phospholipid interactions with peptide paradigms for the surfactant protein SP-B. Biochemistry 30: 8395–8401
Cochrane CG, Revak SD, Merritt TA, Heldt GP, Hallman M, Cunningham MD, Easa D, Pramanik A, Edwards DK, Alberts MS 1996 The efficacy and safety of KL4-Surfactant in preterm infants with RDS. Am J Respir Crit Care Med 152: 404–410
Revak SD, Merritt TA, Cochrane CG, Heldt GP, Alberts MS, Anderson DW, Kheiter A 1996 Efficacy of synthetic peptide-containing surfactant in the treatment of respiratory distress syndrome in preterm infant rhesus monkeys. Pediatr Res 39: 715–724
Steinman RM, Cohn ZA 1972 The interaction of soluble horseradish peroxidase with mouse peritoneal macrophages in vitro. J Cell Biol 55: 186–204
Schraufstatter IU, Barritt DS, Ma M, Oades ZG, Cochrane CG 1993 Multiple sites on IL-8 responsible for binding to α and β IL-8 receptors. J Immunol 151: 6418–6428
Rouser G, Fleischer S, Yamamoto A 1970 Two dimensional thin layer chromatographic separation of polar lipids and determination of phospholipids by phosphorous analysis of spots. Lipids 5: 494–496
Revak SD, Merritt TA, Hallman M, Cochrane CG 1986 Reconstitution of surfactant activity using purified human apoprotein phospholipids measured in vitro and in vivo. Am Rev Respir Dis 134: 1258–1265
Carson BS, Losey RW, Bowes WA, Simmons AS 1976 Combined obstetric and pediatric approach to prevent meconium aspiration syndrome. Am J Obstet Gynecol 126: 712–715
Hayakawa H, Myrvik QN, St Clair RW 1989 Pulmonary surfactant inhibits priming of rabbit alveolar macrophage: evidence that surfactant suppresses the oxidative burst of alveolar macrophage in infant rabbits. Am Rev Respir Dis 140: 1390–1397
Geertsma MF, Broos HR, van den Barselaar MT, Nibbering PH, van Furth R 1993 Lung surfactant suppresses oxygen-dependent bactericidal functions of human blood monocytes by inhibiting the assembly of the NADPH oxidase. J Immunol 150: 2391–2400
Suzik H, Otake K, Yakuwa N, Suwabe A, Sato S, Tomoike H, Takahashi K 1992 Effects of artificial surfactant on human peripheral blood polymorphonuclear neutrophil function. Am Rev Respir Dis 145: A876( abstr)
Yoshida K, Mohsenin V 1991 Unsaturated phosphatidylcholines inhibit superoxide production in human neutrophils. Life Sci 49: 1359–1365
Chao W, Spragg RG, Smith RM 1995 Inhibitory effects of porcine surfactant on the respiratory burst oxidase in human neutrophils: attenuation of p47phox and p67phox membrane translocation as the mechanism. J Clin Invest 96: 2654–2660
Ahuja A, Oh N, Chao W, Spragg RG, Smith RM 1996 Inhibition of the human neutrophil respiratory burst by native and synthetic surfactant. Am J Respir Cell Mol Biol 14: 496–503
Jackson JC, Truog WE, Standaert TA, Murphy JH, Juul SE, Chi EY, Hildebrandt J, Hodson WA 1994 Reduction in lung injury after combined surfactant and high-frequency ventilation. Am J Respir Crit Care Med 150: 534–539
McCulloch PR, Forkert PG, Froese AB 1988 Lung volume maintenance prevents injury during high frequency oscillatory ventilation in surfactant-deficient rabbits. Am Rev Respir Dis 137: 1185–1192
Imai Y, Kawano T, Miyasaka K, Takata M, Imai T, Okuyama K 1994 Inflammatory chemical mediators during conventional ventilation and during high frequency oscillatory ventilation. Am J Respir Crit Care Med 150: 1550–1554
Sugiura M, McCulloch PR, Wren S, Dawson RH, Froese AB 1994 Ventilator pattern influences neutrophil influx and activation in atelactasis-prone rabbit lung. J Appl Physiol 77: 1335–1365
Acknowledgements
The authors acknowledge the contributions of Drs. Thomas Haverty and Sandra Cottrell for discussions at the initiation of these studies; Monica Cochrane for critical reading and preparation of the manuscript; veterinarians Beth Ford, Kent Osborn, and Susan Spray for superb assistance and advice on the rabbit studies; and Ahmed Kheiter, Sarah Davis, and the excellent staff of the University of California Primate Center, Davis, CA.
Author information
Authors and Affiliations
Additional information
Supported by a Parker B. Francis Fellowship in Pulmonary Research (R.C.H.).
This is publication No. 10361-IMM from the Department of Immunology, The Scripps Research Institute.
This study could not have been performed without the contributions of Pete Glavinos, Jr., Ph.D., who died on July 11, 1996, at the age of 34, and this publication is dedicated to his memory.
Rights and permissions
About this article
Cite this article
Cochrane, C., Revak, S., Merritt, T. et al. Bronchoalveolar Lavage with KL4-Surfactant in Models of Meconium Aspiration Syndrome. Pediatr Res 44, 705–715 (1998). https://doi.org/10.1203/00006450-199811000-00013
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199811000-00013
This article is cited by
-
Pulmonary surfactant kinetics of the newborn infant: novel insights from studies with stable isotopes
Journal of Perinatology (2009)
-
Bronchoalveolar lavage with pulmonary surfactant/dextran mixture improves meconium clearance and lung functions in experimental meconium aspiration syndrome
European Journal of Pediatrics (2008)
-
(K)eine Surfactant-Therapie bei Patienten mit „acute respiratory distress syndrome“?
Der Anaesthesist (2006)