Abstract
Failure to arouse from sleep is a possible mechanism leading to sudden infant death. Using a controlled pulsatile air jet applied alternately to the nostrils we have made multiple measures of arousal threshold both between and within sleep states. Infants (n = 22) born at term were studied at 2-3 wk postterm (mean age 13 d, range 9-17 d, study 1) and again at 2-3 mo postterm (mean age 78 d, range 56-98 d, study 2). Arousal threshold (stimulus driving pressure, cm H2O) was determined in both active sleep (AS) and quiet sleep (QS). At both ages arousal threshold in QS was significantly higher (251 ± 24 and 298 ± 35) than in AS (163 ± 19 and 144 ± 29) (p < 0.001). In a morning sleep period, the first and second QS epochs were compared in each baby. In both study 1 and study 2, respectively, arousal thresholds were significantly higher in the second QS epoch (270 ± 34 and 497 ± 100) than in the first QS epoch (198 ± 29 and 252 ± 69) (p < 0.05 and p < 0.02). There was a significant correlation in individual infants between arousal thresholds in the two states at both ages (p< 0.005 and p < 0.007, respectively). Regression analysis showed no correlation between the length of time the infant had been in a particular sleep state and the arousal threshold in either state in study 1 or in AS in study 2; however, arousal threshold increased significantly(p < 0.01) with time in QS in study 2. This study has expanded on previous findings that arousability is sleep state-dependent by demonstrating that arousability in QS is also altered by the length of time that the infant has been asleep.
Similar content being viewed by others
Main
It has been suggested that SIDS could arise from an inability to arouse from sleep(1). Because apnea is common in healthy babies(2, 3), arousal or some other process presumably intervenes to prevent profound asphyxia(4). Asphyxia is a potent stimulus that increases ventilation by well described reflex mechanisms(5). Moreover, cortical arousal also serves to protect infants from the asphyxic consequences of apnea by increasing neural drive to the respiratory neurons, thus enhancing ventilation(4, 5). Accordingly, it has often been hypothesized that an infant with an acute or chronic arousal deficit could become vulnerable to SIDS if a prolonged apneic event was complicated by a coincident failure in arousal. Supporting this view, one of the few markers in prospective studies that distinguished babies who subsequently died of SIDS from healthy infants was the occurrence of fewer body movements during sleep in the SIDS infants, indicating fewer episodes of arousal(6, 7).
Previous studies in QS have demonstrated that infants thought to be at increased risk for SIDS have impaired arousal responses to hypoxia(8, 9); however, this is by no means a universal finding(10). However, the use of respiratory stimuli such as hypoxia and hypercarbia for the measurement of arousability in infants has two major limitations. First, ethical constraints limit the levels of asphyxial stimuli that can be used to elicit arousal from sleep, particularly as arousability to hypoxia appears to be low in QS(11). Second, test procedures that fail to elicit arousal do not allow quantification of an arousal threshold. Other indices of arousability, such as the probability of arousal or nonarousal, may be too insensitive to detect infants with low arousability who may be at higher risk for SIDS.
In an attempt to overcome ethical limitations, several more benign forms of stimulation have been used to test arousability from sleep, such as partial nasal occlusion and vibrotactile or auditory stimulation(12–15). Although these types of stimulation usually allow multiple tests to be performed in both QS and AS, infants often fail to arouse and, as with the hypoxia and hypercapnia tests, this precludes the calculation of a reliable index of arousability.
A reliable method to measure arousability in quantitative terms with a simple, controlled, and safe stimulus in the human infant across sleep states is clearly needed. Because it has been suggested that airstream stimulation could be used to screen infants at risk for SIDS(16), we have developed and evaluated a method of using a pulsatile jet of air applied to the nostrils in varying intensities to evoke trigeminal stimulation and estimate arousal threshold. By using a modified method based on that of Cornsweet(17), we show that reliable arousal thresholds can be calculated. The methodology permits multiple measures of arousal threshold across sleep states, and has for the first time shown the dynamic changes that occur in arousability as the infant cycles through AS and QS in a sleep epoch. This has allowed us to address such important issues as distinguishing in which of the two sleep states (AS or QS) the infant is less arousable, and whether arousability is affected by the length of time the infant has been asleep and by its postnatal age.
METHODS
Subjects. Ethical approval for this project was granted by the Human Ethics Committee of the Monash Medical Centre. Twenty-two healthy full-term infants (7 male and 15 female) were recruited from the Moorabbin Birth Centre of the Monash Medical Centre. All infants were from a low risk obstetric group, born at term (median 40 wk; range 37-42 wk) with a birth weight (mean ± SEM) of 3578 ± 85 g (range 3040-4240 g). Median Apgar scores were 8 (range 6-10) at 1 min and 9 (range 8-10) at 5 min. Infants were recruited for polysomnographic recordings at a mean postnatal age of 13 d(range 9-17 d, study 1) and at 78 d (range 56-98 d, study 2). Studies were performed in the Paediatric Sleep Laboratory between 1000 and 1600 h. Most infants slept through the morning and afternoon, interrupted by a midday feed. Recording electrodes were attached while the baby slept or was being fed, and when drowsy the infant was placed in a bassinette under dim lighting. The experiment did not begin until the infant had entered a stable sleep state.
Recordings were made on an 8-channel Hewlett Packard thermal polygraphic recorder (HP 7558B, Hewlett Packard, San Diego CA) of EEG and ECG (HP Bioelectric Amplifiers 8811A), instantaneous HR (HP Rate Computer 8812A), abdominal breathing movements (Respiratory Inductive Plethysmograph, Respitrace Corporation, Ardsley, NY), expired CO2 (CO2/O2 Analyser, Engstrom Eliza MC, Bromma, Sweden), and transcutaneous blood SaO2 (Biox 3700e Pulse Oximeter, Ohmeda, Louisville, CO). Sleep state was assessed using EEG, behavioral, HR, and breathing pattern criteria according to the manual by Anders et al.(18).
Stimulus. A custom-built air pulse generator was designed to deliver pulsatile air jets into the nostrils of the sleeping infants. Trains of air pulses are emitted at a frequency of 3 Hz for 5 s, and travel along 160 cm of flexible polyvinyl chloride tubing (Clippard 3814-7) with a lumen diameter of 1.8 mm. The distal end of the tubing is connected to a specially designed double lumen respiratory monitoring catheter (UDLM-751-RESP-AMP1422, William A Cook, Queensland, Australia). The hand-held catheter is 43 cm in length, with the last 1 cm bent at an angle of 70° to allow the air pulses to be easily directed into the nares (Fig. 1). The driving pressure used to produce each set of air jet pulses was measured before presenting air pulses to the baby's nose using a pressure transducer(Hewlett Packard, model 1290A) connected via a T junction to the distal end of the delivery tubing. Studies using water displacement showed a linear relationship between the volume of gas delivered from the end of the catheter and the driving pressure (y = 0.49x, r2 = 0.79). At a driving pressure of 500 cm H2O, 200 mL of air were delivered per 5-s train, equivalent to 26 mL/pulse.
Diagrammatic representation of the presentation of the air jet stimulus to a sleeping infant. The hand-held catheter is held approximately 0.5 cm from the opening of the nostril. The flexible tubing of the catheter is attached to a rigid metal rod to allow accurate and steady direction of the air jet into the nostril. The inset shows the two lumens within the catheter. The larger lumen delivers the air jet stimulus, and the smaller lumen is for sampling expired CO2.
The second lumen of the catheter enabled the recording of expired nasal CO2. The dilution of the expired CO2 during stimulus presentation indicated that the stimulus was being delivered into the nares, and the prompt return of the expired CO2 record to the prestimulus levels confirmed that the catheter tip had not moved relative to the nares during the procedure. Care was taken that the catheter did not touch the baby, and it was removed between presentations for pressure calibration.
Arousal criteria. Three variables were used to define arousal from sleep: 1) a change in breathing pattern, 2) a HR acceleration, and 3) an observed behavioral response, occurring within 7 s of the stimulus presentation, to allow for the time delay to reach peak HR acceleration. EEG criteria were not used as an α rhythm has not developed at this age. The 10 s of recording immediately preceding the presentation of the stimulus was the baseline level used to assess the change in each variable. A significant change in breathing pattern was defined as at least two breaths in which there was an increased amplitude followed by either movement artefact, periodic breathing, or a return to the basal pattern. HR responses were considered significant when there was an increase of greater than 10%. A behavioral response occurred when one or more of the following events was observed: brief opening of the eyes, grimacing, frowning, or turning of the head away from the stimulus(18). Care was taken that any tests in which a movement occurred within the 10 s immediately before the stimulus administration were excluded from the study and repeated. After the stimulus presentation, a score of 1 (response) or 0(no response) was assigned for each of the three variables and summed to obtain a total response score for each stimulus presentation. A cut-off total response score of 2 or 3 was chosen as the designation of an arousal response.
Arousal threshold. The method used to determine arousal threshold was based on that of the double staircase method of Cornsweet(17). The stimulus was presented alternately to the left and right nostrils; if the infant failed to arouse, the air jet pressure was increased and the stimulus again presented to that nostril. Whenever an arousal response occurred the pressure was then decreased (Fig. 2). The changes of pressure were in the range of±25 to ±200 cm H2O, but were usually ±100 cm H2O. Stimuli were presented at intervals of ≥40 s, allowing up to 33 stimulus presentations within a single epoch of QS or AS. Arousal threshold was calculated as the mean pressure between each arousal and nonarousal response.
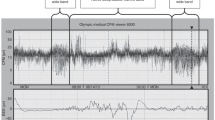
An example of a typical sleep study (baby 11, study 2). Stimuli are presented successively across an epoch of QS and AS. Data for left and right nostrils are presented separately. Calculated arousal thresholds are shown by arrows on the lines between points where the infants response changed from either arousal or nonarousal. Responses which were incorrectly designated at the time of the study have been deleted.
Data analysis. For each infant AS and QS epochs were numbered in sequence, and the time of presentation of each stimulation from the onset of each sleep epoch was recorded. The site of presentation (left or right nostril), stimulus driving pressure (cm H2O), and the response score were noted on the chart recording at the time each stimulation was given. Each recording was subsequently reviewed to confirm that arousal and nonarousal responses had been correctly identified. The records were then corrected by removing the next successive stimulus presentation at that site from the analysis, providing it occurred in the same sleep epoch. Overall the observer correctly identified arousal (sensitivity) in 85 and 80% of the trials and identified nonarousal (specificity) in 99 and 98% in study 1 and study 2, respectively.
The probability of a spontaneous arousal, using the same arousal criteria, was determined by identifying the number of arousals that coincided with calibration of the stimulus before each stimulus presentation. This probability was expressed as a percentage of the total number of calibrations.
Arousal thresholds for left and right nostrils were compared using a one-way nested ANOVA, and arousal thresholds for AS and QS were compared within individual studies and between studies using a two-way nested ANOVA for repeated measures. Comparisons of the spontaneous and test arousal probabilities were made using χ2 analysis. Relationships between arousal threshold in a given sleep state, and the total time asleep for each sleep state, for each infant, were determined by linear regression analysis. Levene's test was used to examine whether the variance in arousal threshold differed between states and studies. All values are presented as mean ± SEM, and a p value of <0.05 was considered significant.
RESULTS
Arousal thresholds for the left and right nostrils were not different in AS or QS in either study. Accordingly, the data for each nostril have been pooled for all subsequent analyses of threshold.
The probability of spontaneous arousal was significantly lower (p< 0.001) than the probability of arousal in response to the stimulus in both sleep states and in both studies (Table 1). The probability of spontaneous arousal was greater in AS than QS (p < 0.001) in both studies and was significantly greater in study 2 than in study 1 in both QS (p < 0.05) and AS (p < 0.001)(Table 1).
Arousal threshold for QS was significantly higher than for AS (p< 0.001) in both study 1 and study 2 (Fig. 3). Arousal thresholds for QS and AS did not change between study 1 and study 2.
Figure 2 shows the data from one infant across an epoch of QS and AS during study 2. This figure illustrates that arousal threshold alters abruptly with change in sleep state and that arousal threshold increases with time in QS while remaining consistent across AS.
To examine whether there was a difference in arousal threshold between successive cycles of QS in a sleep period, arousal thresholds were compared in the first and second QS epochs, occurring in the morning sleep period. Arousal thresholds were significantly higher in the second QS epoch in both studies 1(p < 0.05) and in study 2 (p < 0.02)(Fig. 4).
Figure 5 shows the relationship between arousal thresholds in QS and AS of each infant at each age. There was a significant correlation of the arousal thresholds in the two states at both ages(p < 0.005 and p < 0.007, respectively). One infant at each age (circled) was removed as it was designated a statistical outlier. There was no correlation between mean arousal thresholds in study 1 and study 2 for either sleep state.
Arousal threshold is plotted against time within each sleep state in Figure 6,A-D. There was no correlation between the length of time the infant had been in a particular sleep state and the arousal threshold in either state in study 1. In study 2, arousal threshold increased significantly (p < 0.01) with time in QS. In contrast, the arousal threshold during AS showed no correlation with time in sleep state. Arousal threshold was consistently low in AS in study 2 (<250 cm H2O) and the variance was significantly less than that in study 1(p < 0.03), and also significantly less than in QS in both study 1 and study 2 (p < 0.001).
DISCUSSION
Arousal from sleep is an important protective response to life-threatening stimuli during sleep. It has been known since the 1940s that arousal is governed by the ascending reticular activating system(19). Recent studies have confirmed these findings(20) and demonstrated that the reticular activating system is activated irrespective of the sensory modality (visual or somatosensory) that initiates the arousal(21). This study reports for the first time a method that allows the “arousal threshold” to be determined continuously both within and between sleep cycles in the human infant. The multiple determinations made using a pulsatile jet of air applied to the nares overcomes the limitations of other methods used previously that rely upon determinations of the probability of arousal or the response latency to determine arousability(12–15). Our results are in broad agreement with previous studies in showing that infants arouse from sleep to an external stimulus more easily during AS compared with QS(12–15). Additionally, we have demonstrated that over a sleep cycle the arousal threshold oscillates from high to low arousability as the infant's sleep state cycles from AS to QS.
Air stream stimulation to the nares has been used to study the“diving reflex” in premature infants as a means of assessing the maturation of cardiac and respiratory responses mediated by the trigeminal nerves during REM sleep in human infants(16, 22). However, its use as a graded stimulus to obtain arousal thresholds has not previously been described. The use of a modified form of the double staircase method of Cornsweet(17) for applying the air jet alternately to both nares to determine arousal thresholds has many advantages. First, it reduces any tendency for the infant to habituate to the stimulus, which may occur after repeated stimuli presentations(23). It does this by alternating the site of stimulus presentation and thus increasing and varying the time between successive presentations at the same site. Additionally, the stimulus intensity is varied between successive presentations at the same site. Habituation to the stimulus seems unlikely as provided by the findings that although arousal thresholds varied across the sleep study, infants continued to arouse to the stimulus throughout, and arousal threshold did not change with time in either sleep state in study 1, or in AS in study 2. Second, the methodology maximizes the number of threshold determinations as successive stimuli oscillate around the arousal threshold. Finally, the reliability of threshold calculations is increased by providing two independent and completely separate measures of threshold. The correlation between left and right nostrils was extremely high, and allowed the data to be pooled, thus increasing the sample size substantially for each infant.
The measurement of arousal threshold, in terms of the air pressure used to generate the pulse puffs of air, is arbitrary in that it is a function of 1) the particular catheters used to make up the device and 2) where the pressure is measured within the actual system. Measurement of the peak air flow at the tip of the catheter might be preferable, but this is more difficult. Also, the sensory stimulation to which the baby actually arouses can only be speculated. Possible mechanisms are the mechanical deformation of the skin, changes in temperature, movement of dry and moist air within the nose, or a combination of these. However, the present system is a very reliable way to determine arousal threshold between sleep states within the same infant, and is reliable in estimating thresholds between infants so long as no changes are made to the system during the study.
Previous studies have failed to clearly define arousal. Recent studies in adult patients with obstructive sleep apnea have demonstrated that not all arousals at apnea termination result in cortical EEG arousal. The authors concluded that the arousal response is continuous and not discrete, and is variable in the sense that a number of different cardiorespiratory and EEG changes can occur at arousal(24). Furthermore, full arousal in infants has been shown to be preceded by a highly stereotyped fixed reflex sequence of a sigh followed by a startle, regardless of whether tactile or auditory stimuli were used to elicit the arousal(25). Our choice of arousal criteria (HR acceleration, respiratory pattern change, and behavioral response) and the definition of an arousal as being a response in two or all of the three criteria allowed us to identify arousal responses without producing arousal to full wakefulness.
Our studies did not demonstrate any difference between arousal threshold at 2-3 wk and 2-3 mo of age in either sleep state. These findings are in keeping with those of Newman et al.(13) who assessed arousal in response to vibrotactile stimulation in infants from 1 wk to 6 mo of age. In that study no difference in the percentage of failure to arouse was observed from QS between 1 wk and 4 mo of age, whereas in AS percentages of failure to arouse fell significantly from 1 wk to 2 mo of age, showed a transient increase at 3 mo to levels similar to 2 wk, before declining again at 4 mo. As our study was carried out only at 2-3 wk and 2-3 mo, it is conceivable that we did not show this trend.
The principal finding of this study, that arousal threshold is consistently lower in AS compared with QS, appears to be at variance with many findings in newborn lambs where arousal latency is shorter in QS compared with AS(26–29). It is difficult to know whether this difference is entirely one of species, whether the human infant is at a stage of development in which AS is more easily disrupted, or whether the depth of QS is greater in the human infant. Alternately, it may be that the stimuli used in the animal studies, hypertension, hypotension, and hypoxia, are all “internal” stimuli and act differently to the“external” stimulus used in the human infant, recognizing that responses may differ according to the specific stimulus. Nevertheless, the greater responsiveness to trigeminal stimulation that we have found in the human infant during AS contrasts with the depression in somatic reflex responsiveness that is seen in adult animals during AS, a depression that is considered to be a characteristic motor pattern accompanying this state(30).
In this study the arousal thresholds in AS and QS were positively correlated in both study 1 and 2. This indicates that arousal threshold levels were related between the two sleep states in the same infant; i.e. those infants who exhibited low thresholds in AS also exhibited low thresholds in QS. However, arousal threshold was not correlated for individual states between studies, indicating that arousal threshold alters with age in individual infants and cannot be used as a long-term predictor of arousability.
In previous studies of arousal the possibility that observed responses were actually spontaneous arousals from sleep has not been considered. In our study the probability of spontaneous arousal from sleep was calculated for each sleep epoch by using the point in time when the air pressure signal was calibrated as a sham stimulus presentation. The probability of spontaneous arousal in both AS and QS was significantly less than that elicited by the actual presentation of the air jet stimulus. The probability of occurrence of spontaneous arousals was significantly higher in AS than QS implying that infants are more arousable in AS. This is consistent with the finding that arousal threshold to the air jet stimulus was lower in AS, and suggests that arousal pathways involved in naturally occurring arousals are similar to those produced by the pulsatile air jet.
The continuous measurement of arousal threshold has allowed arousal threshold to be determined both across and within individual sleep epochs and at different ages in the same infant for the first time. The results show that the arousal threshold increases with time within QS epochs at 2-3 mo, but not AS epochs, and increases with successive epochs of QS in both studies. Similar findings have been reported using cold stimulus in 2-5-d-old infants(31). This finding is important as many previous studies of arousability have not fully taken these temporal effects into account and these may have important implications on the interpretation of results.
The finding that arousal threshold increased with time within QS in study 2 may be related to the maturation of sleep in the human infant. There is a rapid maturation of the EEG over the period between the two studies, with the development of sleep spindles and delta waves in QS at 2-3 mo of age(32). These reflect the development of thalamocortical connections which mediate forebrain inhibitory influences on the reticular formation, resulting in the ability to suppress arousal and thus maintain sleep(33). It was notable that the arousal threshold did not change within and between AS epochs, and that variability was significantly less in AS than in QS in study 2, and also less than the AS epochs in study 1. This result indicates that the infant remains readily arousable to this sensory stimulus irrespective of length of the AS cycle. In the light of these findings we postulate an infant will be more vulnerable to an external stress in QS than in AS, particularly at 2-3 mo of age. Whether this concept applies to an asphyxial episode is yet to be defined. What it does highlight, however, is the importance of understanding the mechanism of the asphyxial event, for if it involves an external stimulus such as covering of the face of an infant, then the low arousal threshold in AS may act as a protective mechanism that would not be available in QS when the arousal threshold is high.
Abbreviations
- SIDS:
-
sudden infant death syndrome
- QS:
-
quiet sleep
- AS:
-
active sleep
- HR:
-
heart rate
- SaO2:
-
oxygen saturation
References
Hunt CE 1992 The cardiorespiratory control hypothesis for sudden infant death syndrome. Clin Perinatol 19: 757–771.
Adamson T, Cranage S, Maloney J, Wilkinson M, Wilson F, Yu Y 1981 The maturation of respiratory patterns in normal full-term infants during the first six post-natal months. II. Sleep states and apnea. Aust Paediatr J 17: 257–261.
Glotzbach SF, Ariagno RL, Harper RM 1995 Sleep and Sudden Infant Death Syndrome. In: Ferber R, Kryger M (eds) Principles and Practice of Sleep Medicine in the Child. WB Saunders, Philadelphia, PP 231–244.
McGinty DJ, Hoppenbrouwers T 1983 The reticular formation, breathing disorders during sleep, and Sudden Infant Death Syndrome. In: Tildon JT, Roeder LM, Steinschneider A. (eds) Sudden Infant Death Syndrome. Academic Press, New York, PP 375–400.
Phillipson EA . 1978 Control of breathing during sleep. Am Rev Respir Dis 118: 909–939.
Kahn A, Groswasser J, Rebuffat E, Sottiaux M, Blum D, Foerster M, Franco P, Bochner A, Alexander M, Bachy A, Richard P, Verghote M, Le Polain D, Wayenberg JL 1992 Sleep and cardiorespiratory characteristics of infant victims of sudden infant death: a prospective case-control study. Sleep 89: 287–292.
Schechtman VL, Harper RM, Wilson AJ, Southall DP 1992 Sleep state organization in normal infants and victims of sudden infant death syndrome. Pediatrics 89: 865–870.
Hunt CE, McCulloch K, Brouillette RT 1981 Diminished hypoxic ventilatory responses in near-miss sudden infant death syndrome. J Appl Physiol Respir Environ Excercise Physiol 50: 1313–1317.
McCulloch K, Brouillette R, Guzzetta A, Hunt C 1982 Arousal responses in near-miss sudden infant death syndrome and in normal infants. J Pediatr 101: 911–917.
Ariagno R, Nagel L, Guilleminault C 1980 Waking and ventilatory responses during sleep in infants near-miss for sudden infant death syndrome. Sleep 3: 351–359.
Davidson Ward SL, Bautista DB, Keens TG 1992 Hypoxic arousal responses in normal infants. Pediatrics 89: 860–864.
Newman NM, Frost JK, Bury L, Jordan K, Phillips K 1986 Responses to partial nasal obstruction in sleeping infants. Aust Paediatr J 22: 111–116.
Newman N, Trinder J, Phillips K, Jordan K, Cruikshank J 1989 Arousal deficit: mechanism of the sudden infant death syndrome?. Aust Paediatr J 25: 196–201.
Trinder J, Newman N, Kay A, Whitworth F, LeGrande M, Jordan K 1989 Arousal from sleep and the Sudden Infant Death Syndrome. In: Bond N Siddle D (eds) Psychobiology: Issues and Applications. Elsevier Science Publishers B.V, North Holland, PP 325–335.
Trinder J, Newman NM, Le Grande M, Whitworth F, Kay A, Pirkis J, Jordan K 1990 Behavioural and EEG responses to auditory stimuli during sleep in newborn infants and in infants aged 3 months. Biol Psychol 31: 213–227.
Allen GL, Howard G, Smith JB, McCubbin JA, Weaver RL 1979 Infant heart rate response to trigeminal airstream stimulation: determination of normal and deviant values. Pediatr Res 13: 184–187.
Cornsweet, T . 1962 The staircase method in psychophysics. Am J Psychol 75: 485–491.
Anders T, Emde R, Parmelee A 1971 A Manual of Standardised Terminology, Technique, and Criteria for Scoring States of Sleep and Wakefulness in Newborn Infants. BRI Publications, Los Angeles, CA, PP 1–11.
Moruzzi G, Magoun H 1949 Brainstem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol 1: 455–473.
Steriade M 1996 Arousal: revisiting the reticular activating system. Science 272: 225–226.
Kinomura S, Larsson J, Gulyas B, Roland PE 1996 Activation by attention of the human reticular formation and thalamic intralaminar nuclei. Science 271: 512–515.
Ramet J, Praud JP, D'Allest A-M, Dehan M, Gaultier C 1990 Trigeminal airstream stimulation. Maturation-related cardiac and respiratory responses during REM sleep in human infants. Chest 98: 92–96.
Graham FK 1973 Habituation and dishabituation of responses innervated by the autonomic nervous system. In: Peeke HVS, Herz MJ(eds) Habituation 1. Academic Press, New York, PP 163–218.
Rees K, Spence DPS, Earis JE, Calverley PMA 1995 Arousal responses from apneic events during non-rapid-eye-movement sleep. Am J Respir Crit Care Med 152: 1016–1021.
Lijowska A, Thach BT 1996 Sequential brain stem reflexes preceding arousal from sleep in infants: Potential relevance for sudden infant death syndrome. Proc Fourth SIDS International Conference, Washington DC, Abstract 2-03-03
Horne RSC, Berger PJ, Bowes G, Walker AM 1989 Effect of sinoaortic denervation on arousal responses to hypotension in newborn lambs. Am J Physiol 256:H434–H440.
Horne RSC, dePreu ND, Berger PJ, Walker AM 1991 Arousal responses to hypertension in lambs: effect of sinoaortic denervation. Am J Physiol 260:H1283–H1289.
Fewell JE, Johnson P 1984 Acute increases in blood pressure cause arousal from sleep in lambs. Brain Res 311: 259–265.
Fewell JE, Baker SB 1987 Arousal from sleep during rapidly developing hypoxemia in lambs. Pediatr Res 22: 471–447.
Nakamura Y, Goldberg LJ, Chandler SH, Chase MH 1978 Intracellular analysis of trigeminal motor neuron activity during sleep in the cat. Science 199: 204–207.
Schmidt K, Birns B 1971 The behavioural arousal threshold in infant sleep as a function of time and sleep state. Child Dev 42: 269–277.
Anders TF, Sadeh A, Appareddy V 1995 Normal sleep in neonates and children. In: Ferber R, Kryger M (eds) Principles and Practice of Sleep Medicine in the Child. WB Saunders, Philadelphia, PP 7–18.
Sterman MB, McGinty DJ, Harper RM, Hoppenbrouwers T, Hodgman JE 1982 Developmental comparisons of sleep and EEG power spectral patterns in infants at low and high risk for sudden death. Electroencephalogr Clin Neurophysiol 53: 848–853.
Acknowledgements
The authors thank the staff at the Moorabbin Campus Birth Centre and the parents and infants who participated in the study. We also acknowledge the statistical advice of Dr. Phillip McCloud of the Monash University Statistical Consulting Service.
Author information
Authors and Affiliations
Additional information
Supported by the Victorian Health Promotions Fund and the National SIDS Council of Australia Ltd.
Dr. Rosemary Horne, Department of Paediatrics, Monash Medical Centre, 246 Clayton Road, Clayton VIC, Australia 3168.
Rights and permissions
About this article
Cite this article
Read, P., Horne, R., Cranage, S. et al. Dynamic Changes in Arousal Threshold during Sleep in the Human Infant. Pediatr Res 43, 697–703 (1998). https://doi.org/10.1203/00006450-199805000-00020
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199805000-00020
This article is cited by
-
The prone sleeping position and SIDS. Historical aspects and possible pathomechanisms
International Journal of Legal Medicine (2018)
-
Decreased orexin (hypocretin) immunoreactivity in the hypothalamus and pontine nuclei in sudden infant death syndrome
Acta Neuropathologica (2015)