Abstract
The transferrin receptor in serum provides a useful measure of tissue iron deficiency and the rate of erythropoiesis, whereas serum ferritin reflects the amount of storage iron in normal subjects. We studied the serum transferrin receptor and the transferrin receptor-ferritin ratio in 57 healthy prepubertal or early pubertal boys and followed them at 3-mo intervals for 24 mo to evaluate their iron status during puberty. The mean laboratory parameters changed as follows: Hb from 13.0 to 13.3 g/dL (p = 0.01), mean corpuscular volume from 85 to 84 fL (p = 0.0001), transferrin receptor from 6900 to 7200 μg/L (p = 0.03), ferritin from 36 to 23 μg/L (p = 0.0001), and transferrin receptor-ferritin ratio from 230 to 400 (p = 0.0001). At the start of the investigation, the serum transferrin receptor was elevated (>9000 μg/L) or ferritin low(≤12 μg/L) in fewer than 2% of the boys. During the subsequent 2 y the proportion of boys with an elevated transferrin receptor or low ferritin value increased. The two parameters were simultaneously abnormal in none of the boys initially, but in about 3% of the boys 2 y later. The change in transferrin receptor-ferritin ratio was closely correlated with genital development. The proportion of elevated transferrin receptor-ferritin ratios increased 4.5-fold during the 2 y, indicating the high responsiveness of the ratio. At the end of the study, iron therapy was started to eliminate any iron deficiency. In response to the therapy, the mean transferrin receptor-ferritin ratio fell to 210 ± 19, i.e. close to the level at the beginning of the study. The marked responses of the transferrin receptor and the receptor-ferritin ratio to iron therapy reflect the dependence of these parameters on iron status rather than on physiologic differences in the rate of erythropoiesis.
Similar content being viewed by others
Main
The soluble truncated form of the transferrin receptor present in serum reflects the mass of the tissue receptor. Consequently, an increase in serum receptor concentration provides a useful measure of functional iron deficiency or the rate of erythropoiesis(1–6). The serum transferrin receptor concentration is elevated in patients with iron deficiency anemia, sickle cell disease, and thalassemia, reduced in patients with aplastic anemia(7–9), and normal in patients with anemia of chronic disease(10). Preliminary observations indicate that the serum transferrin receptor-ferritin ratio may be a more useful parameter of tissue iron status than the transferrin receptor alone(7, 11). Only few studies of transferrin receptor are available in children(6, 8–10, 12, 13), and no data are available for the transferrin receptor-ferritin ratio under physiologic conditions.
Male puberty is a period of many important changes. Rapid growth of body and muscles and achievement of adult testosterone levels by the end of puberty cause increases in iron requirements due in part to an increase in erythropoiesis(14, 15). Iron requirements have been estimated to increase by a mean of 2.5-fold(16). We undertook a longitudinal study of 60 prepubertal or early pubertal boys and followed them at 3-mo intervals for 24 mo. The purpose of the study was to evaluate changes in the serum transferrin receptor concentration and transferrin receptor-ferritin ratio in conditions in which physiologic iron needs are rapidly increasing, and to assess the effect of iron therapy on these changes.
METHODS
Subjects. An opportunity to participate in the study was offered to 84 boys of the same secondary school level in three schools near the Children's Hospital, Helsinki. The study participants came mainly from upper middle class families. The study was approved by the parents, the school authorities, those responsible for school health care, and the Ethics Committee of the Children's Hospital. Written informed consent was obtained from the parents. Of the 84 boys, 62 agreed to participate. There were two early drop-outs, leaving 60 boys for the first 12 mo, and one later drop-out, leaving 59 boys for the next 12 mo of the study. Medical history and physical examination indicated that all the boys were healthy except three, who used seasonal medication for asthma. Epilepsy was diagnosed in one boy during the study, and he was started on oxicarbamazepine medication. None of these boys was excluded from the study. Three boys were considered to be iron-deficient during the first 6 mo of the study and were started on iron therapy for 2 mo, one after the first visit and two at 6 mo; the data for them have been excluded from the final analyses. During the 24-mo study, all the boys were studied nine times at 3-mo intervals. At the 24-mo visit, 54 of the boys were started on iron therapy consisting of 3 mg iron/kg/d as ferrous sulfate divided into 2-3 doses, for 50 d (two boys were lost to follow-up at the end of the study). Venous blood samples for determination of iron status were drawn 1 wk after discontinuation of the iron therapy.
Clinical and laboratory methods. At each examination, body weight was measured on the same platform scale, the boys wearing small shorts only. Height was measured on a Harpenden scale with 0.1-cm precision. Genital(G) and pubic hair (P) stages were assessed according to Tanner(17). The length and width of the testicles of all subjects were also measured with a ruler to the nearest millimeter in a lying position by the same pediatrician (R.A.). Testicular volume was calculated from the formula: 0.52 × longitudinal axis in cm × (transverse axis in cm)2(18). Means of the two testicular volumes were used.
Venous blood samples were drawn between 0830 and 1400 h. Serum transferrin receptor measurements were performed using a two-site ELISA as described by Flowers et al.(3). The assay employs a double MAb raised against transferrin-saturated receptor purified from human placenta. The sensitivity is 500 μg/L. On samples with concentrations ranging between 3 500 and 10 000, the within-assay variability for a single measurement is 3-5%. The interassay variability measured over a 12-mo period is 1.9% and 2.8% on sera containing 5200 and 9700 mg/L(3), respectively. All unknowns were measured in triplicate against a standard curve constructed with quadruplicate measurements of six standards ranging from 0.5 to 50 μg/L. Serum ferritin concentrations were determined by a two-site chemiluminometric immunoassay with an automatic analyzer (Ciba Corning ACS:180, Switzerland). The receptor-ferritin ratio was calculated by dividing the serum transferrin receptor (μg/L) by the serum ferritin value (μg/L). Serum concentrations of testosterone were determined by RIA after separation of the steroid fractions on a Lipidex-5000 microcolumn(19). Serum sex hormone-binding globulin concentrations were determined by fluoroimmunoassay(DELFIA; Wallac, Turku, Finland). To have a more sensitive measure of the androgen status, we also used the free androgen index calculated as total testosterone × 100/sex hormone-binding globulin(20).
Statistical analysis. Values are expressed as means ± SEM. We used analysis of variance and the paired and unpaired t tests when the distribution of the parameters was normal. Because of skewness, we used nonparametric tests (Spearman and Wilcoxon) for analyses of testosterone values and of testicular volumes. The transferrin receptor values were normally distributed (skewness, -0.005-0.397). The ferritin values had a near normal distribution (skewness, 0.2-1.6), except at the age of 12 y (3.0). The transferrin receptor-ferritin ratios also had near normal distributions(skewness, 0.6-2.3), except at the age of 11.7 (4.8). Nevertheless, we used nonparametric tests for all ferritin and transferrin receptor-ferritin ratio values.
RESULTS
At the start of the study the mean age of the boys was 11.7 ± 0.05 y. Of the 57 boys at the start, 34 had mean testicular volumes of less than 2.0 mL and were considered prepubertal (Tanner stage G1)(17). The other 23 boys had testicular volumes ranging from 2.0 to 6.8 mL (G2). During the study, the mean testicular volume increased from 2.4 ± 0.2 mL to 9.8 ± 0.8 mL. At 24 mo, the boys' mean age was 13.7 ± 0.05 y and, of the 56 boys remaining in the study, none was prepubertal and five had gone through puberty: the numbers of boys at stages G1, G2, G3, G4, and G5 were 0, 9, 20, 22, and 5, respectively.
We observed a small rise, from 13.0 ± 0.1 to 13.3 ± 0.1 g/dL(p = 0.01), in mean blood Hb concentration during the 24-mo follow-up. The mean serum transferrin concentration increased slightly, from 2.8 ± 0.04 to 3.0 ± 0.05 g/L (p = 0.0003). The mean serum transferrin saturations were 22 ± 0.9% at the beginning and 22± 1.2% at the end of the study. The mean corpuscular volume decreased from 85 ± 0.4 to 84 ± 0.5 fl (p = 0.0001). After iron medication, we observed responses in all of our parameters of iron status except mean corpuscular volume. The average rise in Hb was 0.7 g/dL, with a range of -0.8 to 2.1 g/dL (p = 0.0001)(21). The Hb increment was 1.0 g/dL or more in 15 boys and more than 2.0 g/dL in two boys.
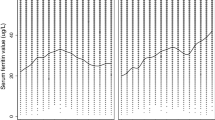
The serum transferrin receptor values increased and the serum ferritin values decreased in the healthy pubertal boys during the 24-mo follow-up. The mean transferrin receptor concentration rose from 6900 ± 100 to 7200± 200 μg/L (p = 0.03) and the mean ferritin concentration fell from 36 ± 2.2 to 23 ± 1.5 μg/L (p = 0.0001). Serum transferrin receptor concentrations were elevated (>9000 μg/L) or ferritin concentrations were reduced (≤12 μg/L) in less than 2% of the boys at the beginning of the study (Fig. 1). The proportion of boys with elevated transferrin receptor or low ferritin values increased during the follow-up. The two parameters were simultaneously abnormal in none of the boys at the start and in about 3% 2 y later (Fig. 1).
Proportion of elevated serum transferrin receptor(>9000 μg/L; closed circles) and low ferritin (≤12 μg/L;open circles) concentrations in 56 healthy boys during a 24-mo follow-up. The dashed area represents the proportion of subjects in whom the two measurements were simultaneously abnormal. The last values are those after 50 d of iron therapy.
The mean serum transferrin receptor-ferritin ratio increased from 230± 15 to 400 ± 34 during the follow-up (p = 0.0001). After iron therapy, the 90th percentile for this ratio was 350. If this proportion is taken as the criterion, 10% of the boys had elevated ratios at the beginning of the study. The proportion increased to 45% (4.5-fold) during the 24-mo follow-up (Fig. 2). The individual ratios ranged from 30 to 1250 during the study period. For comparison, the proportion of moderately low (<25 μg/L) serum ferritin values increased from 30 to 57% (1.9-fold) in 2 y and decreased again to 20% after iron medication.
When the transferrin receptor-ferritin ratios were grouped according to Tanner's genital stages (G1-G5), they varied with the follow-up time and with genital development (Table 1). Only at a mean age of 13.4 y were all genital stages present. The ratio was 130 ± 20 in boys at G1 and increased to 580 ± 220 in those at G5. The transferrin receptor-ferritin ratio was positively correlated with testicular volume at the mean ages of 12.6, 12.9, and 13.4 (r = 0.30, p = 0.02;r = 0.36, p = 0.007 and r = 0.28, p = 0.04, respectively) and was positively correlated with the free androgen index at the mean ages of 12.9 and 13.4 y (r = 0.34, p = 0.01 and r = 0.28, p = 0.04). Among the boys who remained prepubertal (G1) the transferrin receptor-ferritin ratio decreased throughout the study period (Table 1).
The 50 d of iron medication at the mean age of 13.7 y resulted in a decrease in the mean transferrin receptor concentration and the transferrin receptor-ferritin ratio and an increase in serum ferritin(Table 2). When the boys were divided into two subgroups with low (<1 g/dL) and high (≥1 g/dL) Hb responses to iron medication, the preceding transferrin receptor-ferritin ratio separated the two subgroups better than the traditional iron parameters (p = 0.002;Table 3).
DISCUSSION
This study was an evaluation of iron status, not an attempt to define iron sufficiency or deficiency in pubertal boys. The transferrin receptor-ferritin ratio increased remarkably in relation to age and pubertal development evaluated by the Tanner stages (Table 1), testicular volumes, or serum testosterone concentrations. The proportion of elevated ratios increased 4.5-fold during the 2 y, reflecting the high responsiveness of the transferrin receptor ferritin ratio in this physiologic setting (Fig. 2). The preceding transferrin receptor-ferritin ratio predicted the response in Hb concentration to iron medication better than the traditional iron parameters (Table 3).
When a cut-off point of the serum transferrin receptor concentration of>9000 μg/L, or of ferritin concentration of ≤12 μg/L was used as the indicator of iron deficiency, fewer than 2% of the boys met these criteria at the beginning of the follow-up. When the two parameters were used at the same time, none of the boys was iron-deficient at the start. Measurements of the receptor-ferritin ratio in several hundred normal adult subjects over 18 y of age have shown median values of 46 in men, 180 in menstruating women, and 1200 in pregnant women in the 3rd trimester who were not receiving iron supplements during pregnancy (J. D. Cook, unpublished observations). In our study, the overall range of transferrin receptor-ferritin ratios varied from 30 to 1250, and the mean ratio rose during the study from 230 to 400, indicating a high responsiveness of the ratio to the increase in iron needs during puberty in boys with adequate iron reserves.
The relative merits of using serum ferritin or transferrin receptor measurements to detect iron deficiency depends on the overall iron status of the sampled population. In adult men, iron status is best characterized by the serum ferritin measurement, because the vast majority of men have some iron reserves. In pregnant women, on the other hand, serum ferritin is abnormally low in the vast majority even when iron supplements are taken regularly. In this situation, the transferrin receptor level is a more reliable index of tissue iron need(22). It is well known that, during the growth spurt, the iron requirements increase because of blood volume expansion, the increase of red cell mass in males, and the growth of other tissues. In such a situation, mobilization of iron stores, as reflected by reduction in serum ferritin, is to be expected, just as during pregnancy. In fact, in this study, there was an appreciable decline of serum ferritin from 36 to 23 μg/L during the 2-y follow-up. To a large extent, this normal reduction accounts for much of the observed increase in the receptor-ferritin ratio because serum ferritin is the denominator of this ratio. However, there is a modest and statistically significant increase in the mean transferrin receptor level, and the changes in the receptor-ferritin ratio seem to react more sensitively to iron needs than transferrin receptor or ferritin alone.
The use of a single laboratory parameter to cover the entire spectrum of iron status offers some advantages, particularly when the iron status of a population is evaluated. As the serum ferritin reflects changes in storage iron and the serum transferrin receptor reflects changes in tissue iron, the ratio of these two key indicators of iron status provides an additional parameter, which encompasses iron status over a broad range. There are cogent theoretical reasons for using the ratio of the transferrin receptor to ferritin, because the synthesis of these two key iron proteins is strictly regulated in all body cells in a reciprocal fashion by the iron regulatory factor(23). Studies in which normal adults underwent serial phlebotomies to produce a spectrum of iron status indicated a highly consistent and quantitative relationship between the transferrin receptor-ferritin ratio and body iron status(7). These findings are supported by the results of the present study, in which the most dramatic change after iron therapy was seen in the receptor-ferritin ratio(Table 2). The mean ratio of 210 ± 20 observed after iron therapy in these pubertal boys is roughly comparable with the median value observed in healthy menstruating women. Little or no information has yet been published on mean ratios in infants, children or adolescents.
One potential limitation to employing the serum transferrin receptor as a measure of iron status is that it is also affected by the rate of erythropoiesis. Indeed, the mean elevation in patients with overt iron deficiency is modest compared with the striking increase in patients with hemolytic anemia or with thalassemia(3, 5). In the present study, relative iron deficiency or enhanced erythropoiesis could both influence the receptor-ferritin ratio. The marked fall in the transferrin receptor-ferritin ratio that occurred after administration of iron to these adolescent boys indicated that the main determinant of the value of the ratio was the iron requirement, not the rate of erythropoiesis. In other situations, the distinction between these two determinants of the serum transferrin receptor or receptor-ferritin ratio is less clear. One complex setting is found in very low birth weight infants given recombinant human erythropoietin(13). A similarly complex clinical setting is found in patients with chronic renal failure on hemodialysis, many of whom receive recombinant human erythropoietin and also develop iron deficiency because of blood loss associated with dialysis(24). Further clinical studies will be needed in these situations to clarify the relative sensitivity of the transferrin receptor-ferritin ratio to changes in iron status or in the rate of erythropoiesis.
Abbreviations
- G:
-
genital stage (according to Tanner)
- P:
-
pubic hair stage (according to Tanner)
References
Kohgo Y, Nishisato T, Kondo H, Tsushima N, Niitsu Y, Urushizaki I 1986 Circulating transferrin receptor in human serum. Br J Haematol 64: 277–281
Kohgo Y, Niitsu Y, Kondo H, Kato J, Tsushima N, Sasaki K, Hirayama M, Numata T, Nishisato T, Urushizaki I 1987 Serum transferrin receptor as a new index of erythropoiesis. Blood 70: 1955–1958
Flowers CH, Skikne BS, Covell AM, Cook JD 1989 The clinical measurement of serum transferrin receptor. J Lab Clin Med 114: 368–377
Shih YJ, Baynes RD, Hudson BG, Flowers CH, Skikne BS, Cook JD 1990 Serum transferrin receptor is a truncated form of tissue receptor. J Biol Chem 265: 19077–19081
Huebers HA, Beguin Y, Pootrakul P, Einspahr D, Finch CA 1990 Intact transferrin receptors in human plasma and their relation to erythropoiesis. Blood 75: 102–107
Locatelli F, Zecca M, Beguin Y, Giorgiani G, Ponchio L, de Stefano P, Cazzola M 1993 Accelerated erythroid repopulation with no stem-cell competition effect in children treated with recombinant human erythropoietin after allogeneic bone marrow transplantation. Br J Haematol 84: 752–754
Skikne BS, Flowers CH, Cook JD 1990 Serum transferrin receptor: a quantitative measure of tissue iron deficiency. Blood 75: 1870–1876
Shinghal A, Cook JD, Skikne BS, Thomas P, Serjeant B, Serjeant G 1993 The clinical significance of serum transferrin receptor levels in sickle cell disease. Br J Haematol 84: 301–304
Musto P, Lombardi G, Centra M, Modoni S, Carotenuto M, Di Giorgio G 1993 Soluble transferrin receptor in β-thalassemia. Lancet 342: 1058
Martini A, Ravelli A, Di Fuccia G, Rosti V, Cazzola M, Barosi G 1994 Intravenous iron therapy for severe anaemia in systemic-onset juvenile chronic arthritis. Lancet 344: 1052–1054
Thorstensen K, Egeberg K, Romslo I, Dalhoj J, Wiggers P 1991 Variations in serum erythropoietin and transferrin receptor during phlebotomy therapy of hereditary hemochromatosis: a case report. Eur J Haematol 47: 219–222
Kivivuori SM, Anttila R, Viinikka L, Pesonen K, Siimes MA 1993 Serum transferrin receptor for assessment of iron status in healthy prepubertal and early pubertal boys. Pediatr Res 34: 297–299
Kivivuori SM, Heikinheimo M, Teppo AM, Siimes MA 1994 Early rise in serum concentration of transferrin receptor induced by recombinant human erythropoietin in very-low-birth-weight infants. Pediatr Res 36: 85–89
Thomsen K, Riis B, Krabbe S, Christiansen C 1986 Testosterone regulates the haemoglobin concentration in male puberty. Acta Paediatr Scand 75: 793–796
Daniel WA 1973 Hematocrit: maturity relationship in adolescence. Pediatrics 52: 388–394
Dallman PR 1992 Changing iron needs from birth through adolescence. In: Fomon SJ, Zlotkin S (eds) Nutritional Anemias. Raven Press, New York, pp 29–38
Tanner JM 1962 Growth at Adolescence, Ed. 2. Blackwell Scientific Publications, Oxford
Hansen P, With TK 1952 Clinical measurements of the testes in boys and men. Acta Med Scand Suppl 266: 457–465
Apter D, Jänne O, Karvonen P, Vihko R 1976 Simultaneous determination of five sex hormones in human serum by radioimmunoassay after chromatography on Lipidex-5000. Clin Chem 22: 32–38
Selby C 1990 Sex hormone binding globulin: origin, function and clinical significance. Ann Clin Biochem 27: 532–541
Anttila R, Siimes MA 1996 Development of iron status and response to iron medication in pubertal boys. J Pediatr Gastroenterol Nutr 22: 312–317
Carriaga MT, Skikne BS, Finley B, Cutler B, Cook JD 1991 Serum transferrin receptor for the detection of iron deficiency in pregnancy. Am J Clin Nutr 54: 1077–1081
Melefors Ö, Hentze MW 1993 Iron regulatory factor-the conductor of cellular iron regulation. Blood Rev 7: 251–258
Lindsay RM, Burton JA, Edward N, Dargie HJ, Prentice CRM, Kennedy AC 1973 Dialyzer blood loss. Clin Nephrol 1: 29–34
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Anttila, R., Cook, J. & Siimes, M. Body Iron Stores Decrease in Boys during Pubertal Development: The Transferrin Receptor-Ferritin Ratio as an Indicator of Iron Status. Pediatr Res 41, 224–228 (1997). https://doi.org/10.1203/00006450-199702000-00012
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199702000-00012
This article is cited by
-
Iron Status of Preadolescents Without Clinical Pallor Attending a Tertiary Care Hospital in South India- A Cross-Sectional Analytical Study
Indian Journal of Pediatrics (2023)
-
Lower Plasma Soluble Transferrin Receptor Range in Healthy Indian Pediatric Cohort as Compared to Asian and Western Data
Indian Journal of Hematology and Blood Transfusion (2017)
-
Sexual dimorphism in immune response genes as a function of puberty
BMC Immunology (2006)