Abstract
Spleens from 1-20-wk-old guinea pigs infected in utero withTreponema pallidum and age-matched controls, born to normal and heat-killed (56°C, 2 h.) T. pallidum-injected mothers, were examined for their in vitro lymphoproliferative response to phytohemagglutinin, concanavalin A, and lipopolysaccharide. Additionally. T cell surface markers (μ-chain, pan T, CD4, and CD8) were determined in spleen, lymph node, and peripheral blood from 10-wk infected and normal pups by single and dual parameter fluorescence-activated cell sorter analysis. Compared with control animals, congenitally infected animals showed a remarkable prolonged naive-type of immune response as reflected by the higher(p < 0.01) proliferative responses to both T cell mitogens (up to 20 wk of age), and the weaker response to the B cell mitogen, significantly different (p < 0.01) at 10 wk of age. As opposed to controls, in all organs examined the level of CD8+ (cytotoxic/suppressor) T cells was significantly diminished (p < 0.01); consequently, the CD4/CD8 ratio was significantly elevated (p < 0.05). The role of C4 complement component and the nature and potential role of the immature T and B lymphocyte responses in asymptomatic congenital syphilis is discussed.
Similar content being viewed by others
Main
We have elaborated the gp model of experimental neonatal(1) and congenital(2) syphilis and showed that congenital and neonatal infection display a different pattern of humoral immune responses(3). Like human infants,in utero infection of two genetically different strains of gps (C4D and Albany) elicits an early and extended (up to 2-4 mo of age) production of IgM anti-treponemal antibodies, IgM-rheumatoid factor, and circulating immune complex-containing IgM antitreponemal antibodies, and a delayed switch to IgG antibodies(2). On the other hand, neonatally infected C4D and Albany gps respond similarly to their infected mothers but with relatively lower levels of IgM and IgG antibodies(1, 3). As opposed to in utero infection, neonatally infected animals did not produce IgM-RF or IgM-circulating immune complex(3). To investigate the cellular basis of the immune response in congenital infection, in this report we examine the mitogenic response of T and B cells from congenitally infected C4D gps for a 20-wk period, and the relative levels of B cells and subpopulations of T cells in various organs at 10 wk of age.
METHODS
The C4D strain of gp was used in this study. These animals have genetically controlled total deficiency of the fourth component of complement(4), although their immunologic competence is similar to that of a complement-sufficient strain(5–8). Of five strains studied, C4D gp is the most susceptible to cutaneous infection with T. pallidum(ID50 = 102) as adult(9). But, as in the rabbit(10), it shows temporary resistance when infected intradermally as a neonate(1), and as in >50% of congenitally infected human infants, does not present obvious clinical symptoms when infected in utero(2).
The present experiment includes 1-, 10-, and 20-wk-old congenitally infected C4D pups that showed in a previous study(11) to be serologically (IgM treponemal antibodies) and T. pallidum-DNA(polymerase chain reaction) positive. Controls included serologically negative, age-matched pups born to normal sows or injected i.v. at midpregnancy (30-40 d of gestation), with a dose of 1 × 108 HKTP similar to that used for infection of pregnant dams with virulent T. pallidum(11). A total of 63 animals, 28 congenitally infected and 35 age-matched controls, were included in the study. Lymphoproliferative response to T (Con A and PHA) and B cell (LPS) mitogens was done as described previously(12) using Ficoll-Hypaque-purified SP lymphocytes obtained from infected and noninfected pups at 1. 10, and 20 wk of age. Also SP, LN, and PB from 10-wk-old congenitally infected and age-matched controls were submitted to single and dual FACS analysis using MAb against gp: μ-chain (B cells), CD5 (pan T, MAb 8BE6), CD4 (MAb H155), and CD8 (MAb Msgp6) surface markers, as described previously(12). Irrelevant mouse MAb IgG2b and rat MAb IgG2 (Dako Corporation, Carpinteria, CA), were used as primary antibodies in control experiments. Affinity-purified fluorescein-conjugated goat F(ab) anti-mouse IgG (G/M FITC) and goat F(ab) anti-rat (G/R FITC) (Jackson Immuno Research Laboratories, Westgrove, PA) were used as second antibodies. Protein A-purified phycoerythrin-conjugated mouse IgG (MAb 8BE6) anti-pan T(M/gpT-PE). was prepared by Chromoprobe Inc. Immunobiologist, Mountain View, CA. In a single-parameter analysis, the cells were stained by the indirect procedure using first the ascites containing the MAb followed by G/M FICT or G/R FICT. T cells were stained directly by M/gpT-PE. For two-color analysis, cells were stained first by the indirect method, washed and incubated for 15 min with 5% mouse serum in PBS to block any free antibody binding site, before the reaction with M/gpT-PE. Background staining was obtained by reacting cells with mouse or rat MAb IgG2 directed to irrelevant antigen. The stained cells were analyzed on a B-D Vantage Flow cytometer (Becton Dickinson). Forward and 90° light scatter were used to gate on the lymphocyte population, and the FICT and PE emission signals from 10 000 events were collected using a log integral amplification system. Differences in mitogenic responses and cell surface markers were calculated by the parametric, unpaired t test(Fisher's exact test) and two-tailed p values.
RESULTS
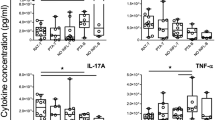
Regardless of age, the blastogenic response of SP cells to both T cell mitogens. Con A, and PHA, expressed either as increment counts/min or as stimulation index, was significantly elevated (p < 0.05 to 0.01) throughout the first 20 wk of life in congenitally infected gps compared with offspring born to HKTP-injected or normal dams. On the other hand, the response to LPS was mostly lower in infected offspring, reaching statistical significance (p < 0.01) at 10 wk of age. Although less pronounced, a similar trend was still observed at 20 wk. No major differences in mitogenic responses were noticed between progeny born to normal or HKTP-injected sows (Fig. 1).
SP lymphocytes from 51 congenitally infected and control animals (22 1-wk-old, 17 10-wk-old, and 12 20-wk-old), were examined for their in vitro lymphoproliferative response to Con A (1 μg), PHA (1 μg), and LPS (10 μg/2 × 105 cells/well). There is a significant difference between infected and noninfected controls in their lymphoproliferative response to T and B cell mitogens expressed either as increment (Δ) counts/min (mean counts/min of stimulated - mean counts/min unstimulated cultures) ± SEM. or as stimulation index (SI)(mean counts/min of stimulated/mean counts/min unstimulated cultures) ± SEM for a period of 10-20 wk of age.
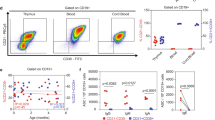
Single- and double-parameter analysis of PB, SP, and LN lymphocytes from 10-wk-old progeny born to syphilitic and normal dams provided additional pieces of information. As opposed to LN and PB, in both, normal and infected SP, not all CD4 and CD8 T cells coexpress the pan T cell marker possibly accounting for the remarkable lower percentage of pan T+ cells in SP. Although in all organs of infected animals the relative level of CD4+ T cells did not differ from that of uninfected controls, in the former, the CD4/CD8 ratio was significantly elevated (p < 0.05-0.01) as a results of a sharp reduction in the levels of CD8+ T cells(p< 0.01-0.001). This in turn seems to account for the tendency to lower levels of pan T cells in infected animals, reaching significant values in PB(p < 0.01) (Table 1 and Fig. 2). No major differences were seen between infected and control animals in the levels of B cells, except for the slight increase in PB in the former.
Single and dual FACS analysis of SP, LN, and PB from a normal and a congenitally infected guinea pig. While B cells constitute the largest population in SP, T cells are predominant in LN and PB. Dual color analysis shows that in guinea pig not all CD4 and CD8 T cells coexpress the pan T cell marker.
DISCUSSION
In a previous report it was observed age-associated functional and phenotypic changes in the susceptible C4D and resistant Albany strain of gps(12). Examination of SP cells from normal C4D and Albany gps at various ages (1 wk to 16 mo) showed that, although the lymphoproliferative response to T cell mitogens (Con A and PHA) was substantially high in 1-7-d-old neonates, lower adult values were reached at approximately 1 mo of age. The opposite occurred with the proliferative response to B cell mitogens (LPS and dextran sulfate). The high T and low B blastogenic response of neonates versus the lower T and higher B blastogenic response of young-adult animals were taken as a normal expression of immature versus mature T and B cell responses taking place during ontogenic development. In this report using C4D gps we corroborated those results with the control groups. However, the immature type of T and B cell lymphoproliferative response was remarkably higher (p < 0.05 to 0.01) and of longer duration (up to 20 wk) in transplacentally infected animals compared with controls (Fig. 1). In agreement with our findings, Fitzgerald and Froberg(13) reported that, in newborn rabbits with asymptomatic congenital syphilis, the Con A proliferation of SP lymphocytes was dramatically enhanced at 2 and 5 wk of age, when compared with age-matched controls. Unfortunately, there are very few reports regarding the lymphoproliferative response in human congenital syphilis. Friedman(14) reported that PB lymphocytes from six 2-4-mo-old with active and one 2-y-old infant with asymptomatic infection (VDRL positive), did not differ from age-matched controls in their proliferative response to T. pallidum Nichols or purified protein derivative antigen when expressed as a stimulation index. Samson et al.(15) reported that PB of 17 1-7-d-old neonates with active congenital syphilis showed no differences compared with age- and sex-matched controls in their response to PHA or levels of CD3, CD4, and CD8 phenotypes. Differences in age, source of cells (PB versus SP), and disease activity (symptomatic versus asymptomatic) prevent us from making any comparison between our results and those found in human infants.
We have not examined phenotypic markers or lymphoproliferative response in normal or congenitally infected complement-sufficient inbred strain 13(genetically associated to C4D). Several reports have indicated that the humoral and cellular response of C4D do not differ from those of complement-sufficient gps(6–8). Moreover, exploration of the immune response in normal and T. pallidum-infected C4D and three additional complement-sufficient strains of gps(5), provided sufficient information to rule out the possibility that the genetic deficiency of C4 plays a mayor role in the dynamics of the immune response to T. pallidum infection, either locally or systemically. Like rabbits, C4D gps are resistant to cutaneous infection with T. pallidum when infected as neonates but are highly susceptible when infected as adults, whereas the opposite occurs in Albany guinea pigs(5). The pattern of humoral response to neonatal or congenital infection in C4D does not differ from that of the Albany line(1–3). Moreover, the kinetics of elimination of virulent T. pallidum and HKTP from skin and testes of C4D animals closely resembles that of rabbits(32).
The fact is that the role of complement and antibodies in syphilitic infections seems to depend upon factors inherent to both, the pathogen and the host. Depletion of C3 by cobra venom factor increased the susceptibility of normal hamsters to infection with Treponema pertenue, the causative agent of frambesia (yaws), and abrogated the ability of immune serum to protect normal hamsters against frambesial infections(16). However, total depletion of C3 by cobra venom factor, with consequent abrogation of hemolytic activity for a period of 14 d, did not alter the cutaneous resistance to T. pallidum infection in Albany guinea pigs(17). Antibody and complement do not prevent natural or experimental transplacental infection, and passive immunization with purified specific or cross-reacting antibodies to T. pallidum did not afford protection to cutaneous infection in inbred strain 2(18). Additionally, Baughn et al.(19) reported that complement levels were unaffected in human primary syphilis. Moreover, in 12 of 15 patients with secondary syphilis and showing substantial amounts of circulating immune complex, eight had low levels of C3, four had low levels of CH50, and nine had increased levels of C4. Collectively these and the present data strongly suggest that the effect of congenital infection on the maturation of cellular effector mechanisms appears to be the major contributor to the protracted nature of the immune response and systemic dissemination of the microorganism.
Several studies(20–22) have indicated that human T lymphocytes defined by MAbs 2H4 and 4B4 identify two different subsets of CD4+ T lymphocytes, virgin and memory T cells, respectively. The characterization and properties of naive and memory T cells have been explored in the murine model(23) and in humans(20–22). Virgin (CD4+, 2H4+) T cells have increased proliferative response to T cell mitogens but react poorly to antigens. They are associated with induction of suppression to pokeweed mitogen and antigen-driven IgG production. Memory (CD4+, 4B4+) T cells, on the other hand, have a relatively lower proliferative response to T cell mitogens and react strongly to antigens. They represent the helper-inducer subpopulation for antigenic and mitogenic response of B cells(22). Both subpopulations have different activation requirements and secretion of cytokines. IL-2 is mainly secreted by naive T cells and IL-4 and interferon γ by memory T cells(22). Although there are not readily available specific reagents to differentiate between naive and memory T cells in gp, several findings would suggest that asymptomatic congenital infection in this host is accompanied by an unusually prolonged virgin-like immune response at the T and B cell levels. This could account for the nature of the T and B cell mitogenic response, the consistent increased level and sustained production of specific primary IgM antibodies, the delayed switch to mature IgG response (> 12 wk) to the pathogen, and lack of obvious clinical symptoms in the experimental model(2, 3). Indeed, the phenotypic studies done with PB, SP, and LN, consistently show a lack of an absolute increase in CD4 with a concomitant decrease in CD8 T cells and consequently an increase in the CD4/CD8 T cell ratio. We do not know for certain whether those two populations represent the type 1 or type 2 subpopulations of T cells(24). As the experimental animals are asymptomatic although producing a prolonged naive type of humoral response, one is tempted to speculate that the effector immune mechanisms operative in asymptomatic congenital infection are most likely of type 2, nonaggressive but rather suppressive(24). Although less harmful to the host, it facilitates the dissemination and persistence of the pathogen in various organs(11). Several findings provide support for this assumption. CD8+ T suppressor cells are known to bind and be activated by IgM-rheumatoid factor and IgM-circulating immune complexes(25, 26), both of which are actively produced in early experimental(2, 3) and natural congenital infection [for review of the literature, see Wicher et al.(2)]. Moreover, in adult acquired syphilis(27) it has been reported that the level of CD4+ T cells CD4/CD8 ratio and production of IL-2 were increased in PB of asymptomatic (latent) syphilis, whereas decreased percentage of CD4+, lower production of IL-2, and increased percentage of CD8 were associated with a severe course of the disease (neurosyphilis, malignant syphilis). Van Voorhis et al.(28) found activated cytolytic CD8+ T cells in lesions of acquired primary and secondary human syphilis. Although the immunologic competence encountered by the pathogen in the fetus and adults may differ in some aspects, it is of interest that asymptomatic congenital infection and latency in acquired syphilis seem to be associated with down-regulation (suppressor-effector function), whereas the aggressive course of the disease relates to up-regulation (helper/inducer, cytotoxic activity) of the host response(27, 28).
In this as in a previous report(12), we show that in gp SP, not all CD8+ or CD4+ T cells coexpressed the pan T cell marker. This is puzzling, as the same MAb (8BE6) react with mature T cells binding to the majority of PB and LN lymphocytes but only to 10% of thymocytes(29). One of the possible explanation for this finding is that natural killer cells, also known as Kurloff cells in gp(30, 31), react with MAb to CD4 antigen (V. Wicher, unpublished information). This is quite interesting as these cells are active participants in both natural and adaptive immune responses. We are currently involved in the preparation of gp cytokine mRNA probes tht will be used for evaluation of cytokine production by the various subsets of gp pig T cells.
Abbreviations
- gp:
-
guinea pig
- HKTP:
-
heat-killed Treponema pallidum
- Con A:
-
concanavalin A
- PHA:
-
phytohemmaglutinin
- LPS:
-
lipopolysaccharide;
- SP:
-
spleen
- LN:
-
lymph node
- PB:
-
peripheral blood
- G/M FITC:
-
fluorescein-conjugated goat F(ab) anti-mouse Ig
- G/R FITC:
-
fluorescein-conjugated goat anti-rat Ig
- M/gpT-PE:
-
phycoerythrin-conjugated mouse IgG anti-gp pan T
- FACS:
-
fluorescence-activated cell sorter
References
Wicher V, Wicher K, Rudofsky U, Zabek J, Jakubowski A, Nakeeb S 1990; Experimental neonatal syphilis in a susceptible (C4D) and resistant (Albany) strain of guinea pig. Clin Immunol Immunopathol 55: 23–40.
Wicher K, Baughn RE, Wicher V, Nakeeb S 1992; Experimental congenital syphilis: guinea pig model. Infect Immun 60: 271–277.
Wicher V, Baughn RE, Wicher K 1994; Congenital and neonatal syphilis in guinea-pigs show a different pattern of immune response. Immunology 82: 404–409.
Ellman L, Green I, Frank MM 1970; Genetically controlled deficiency of the fourth component of complement in the guinea pig. Science 170: 74–75.
Wicher K, Wicher V 1989; Experimental syphilis: guinea pig model. Crit Rev Microbiol 16: 181–234.
Burger R, Shevach EM 1979; Evaluation of the role of C4 in the cellular immune response in vitro. J Immunol 122: 2388–2394.
Frank MM, May J, Gaither T, Ellman L 1971; In vitro studies of complement function in sera of C4-deficient guinea pigs. J Exp Med 134: 176–187.
Ellman L, Green I, Judge F, Frank MM 1971; In vivo studies in C4-deficient guinea pigs. J Exp Med 134: 162:17175
Wicher K, Miller JN, Urquhart AW, Wicher V 1991; Median Infective dose of Treponema pallidum determined in a highly susceptible guinea pig. Infect Immun 59: 453–456.
Gamboa D, Miller JN 1984; Experimental neonatal syphilis. I. Evidence of resistance to symptomatic infection in neonatal rabbits following intradermal inoculation with Treponema pallidum(Nichols strain). Pediatr Res 18: 965–971.
Wicher K, Abbruscato F, Wicher V, Baughn RE, Noordhoek GT 1996; Target organs of infection in guinea pigs with acquired or congenital syphilis. Infect Immun 64: 3174–3179.
Zhao J, Wicher V, Burger R, Schafer H, Wicher K 1992; Strain- and age-associated differences in lymphocyte phenotype and immune responsiveness in C4-deficient and Albany strains of guinea pigs. Immunology 77: 165–170.
Fitzgerald TJ, Froberg MK 1991; Congenital syphilis in newborn rabbits: immune functions and susceptibility to challenge infection at 2 and 5 wk of age. Infect Immun 59: 1869–1871.
Friedmann PS 1977; Cell-mediated immunological reactivity in neonates and infants with congenital syphilis. Clin Exp Immunol 30: 271–276.
Samson GR, Beatty DW, Malan AF 1990; Immune studies in infants with congenital syphilis. Clin Exp Immunol 81: 315–318.
Steiner BM, Schell RF, Liu H. Harris ON 1986; The effect of C3 depletion on resistance of hamsters to infection with yaws spirochete. Sex Transm Dis 13: 245–250.
Wicher K, Wicher V, Jakubowski A, Bartholomew W, Nakeeb S 1988; Effect of irradiation and depletion of C3 complement component on the course of T. pallidum infection in a resistant guinea pig strain. Int Arch Allergy Appl Immunol 86: 76–78.
Wicher K, Zabek J, Wicher V 1992; Effect of passive immunization with purified specific or cross-reacting immunoglobulin G antibodies against Treponema pallidum on the course of infection in guinea pigs. Infect Immun 60: 3217–3223.
Baughn RE, McNeely MC, Jorizzo JL, Musher DM 1986; Characterization of the antigenic determinants and host components in immune complexes from patients with secondary syphilis. J Immunol 136: 1406–1414.
Morimoto C, Letvin NL, Distaso JA, Aldrich WR, Schlossman SF 1985; The isolation and characterization of the human suppressor inducer T cell subset. J Immunol 134: 1508–1515.
Morimoto C, Letvin NL, Boyd AW, Hagan M, Brown HM, Schlossman SF 1985; The isolation and characterization of the human helper inducer T cell subset. J Immunol 134: 3762–3769.
Sanders ME, Makgoba MW, Shaw S 1988; Human naive and memory T cells: reinterpretation of helper-inducer and susppressor inducer subsets. Immunol Today 9: 195–198.
Lee WT, Yin XM, Vitetta ES 1990; Functional and ontogenic analysis of murine CD45hi and CD45Rlo CD4 T cells. J Immunol 144: 3288–3295.
Mossmann TR, Cherwinski H, Bond MW, Giedlin MA. Coffman RL 1986; Two types of murine helper T cell clones. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol 136: 2348–2357.
Moretta L, Webb SR, Grossi CI, Lydyard PM, Cooper MD 1977; Functional analysis of two human T-cell subpopulations: help and suppression of B-cell responses by T cells bearing receptors for IgM or IgG. J Exp Med 146: 184–200.
Baughn RE, Tung KSK, Musher DM 1980; Detection of circulating immune complexes in the sera of rabbits with experimental syphilis: possible role in immuno-regulation. Infect Immun 29: 575–582.
Podwinska J, Zaba R, Chomik M, Bowszyc J 1995; The ability of peripheral blood mononuclear cells (PBMC) of syphilitic patients to produce IL-2. FEMS Immunol Med Microbiol 12: 17–27.
Van Voorhis WC, Barret LK, Nasio JM, Plummer FA, Lukehart SA 1996; Lesions of primary and secondary syphilis contain activated cytolytic T cells. Infect Immun 64: 1048–1050.
Chiba J, Chused TM, Leiserson WM, Zweig SE, Shevach EM 1983; Production and characterization of monoclonal antibodies to guinea pig lymphoid differentiation antigens. J Immunol Methods 63: 247–261.
Debout C, Quillec M, Izard J 1984; Natural killer activity of Kurloff cells: a direct demonstration of purified Kurloff cell suspension. Cell Immunol 8: 674–677.
Debout C, Griveau A, Izard J 1991; The Kurloff cell in estrogenized guinea pigs as a CT7+ 8BE6- CT6- MK- CT10- IgM- lymphocyte with natural killer activity. Nat Immun Cell Growth Regul 10: 327–335.
Wicher V, Abbruscato F, Wicher K 1996; Persistence of virulent and heat-killed Treponema pallidum in rabbit and guinea pig as determined by PCR. American Society for Microbiology 96th Annual Meeting, New Orleans, LA, Abstract D51
Acknowledgements
The authors thank Dr. M. King for editorial comments. We are grateful to the immunology Core of the Wadsworth Center for Laboratories and Research for allowing us to perform the FACS analysis in their premises.
Author information
Authors and Affiliations
Additional information
Supported by National Institutes of Health Grant A121833 from the National Institute of Allergy and Infectious Diseases, U.S. Public Health Service(V.W.) and the Georg and Agnes-Blumenthal Foundation (R.B.).1Present address: Bronx Lebanon Hospital, Department of Pathology, Bronx, NY 10457.
Rights and permissions
About this article
Cite this article
Wicher, V., Zhao, J., Dilwith, R. et al. Immune Abnormalities in Guinea Pigs with Asymptomatic Congenital Syphilis. Pediatr Res 42, 794–798 (1997). https://doi.org/10.1203/00006450-199712000-00013
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199712000-00013