Abstract
The purpose of the present study was to determine developmental changes in the effect of respiratory acidosis on vascular smooth muscle contraction. Vessel diameter, intracellular pH (pHi), and calcium concentration ([Ca]i) were measured in a cannulated preparation of the small mesenteric artery of newborn and adult rabbits. In the artery precontracted by high KCl, acidosis caused a vasorelaxation both in the newborn and the adult; the vasorelaxation was greater in the newborn than in the adult. The fura-2 fluorescence ratio, an indicator of[Ca]i, decreased transiently during acidosis and the decrease was similar in the two age groups. In the artery precontracted by norepinephrine, acidosis caused a transient vasoconstriction in the adult and a vasorelaxation in the newborn. In these vessels, the fura-2 fluorescence ratio increased transiently during acidosis; the increase was similar in the two groups. Upon induction of acidosis, pHi fell rapidly in the artery precontracted by norepinephrine or high KCl, and the depression of pHi was similar in the two groups. In the skinned smooth muscle preparation, a tension-[Ca] relationship curve at pH 7.1 was not significantly different from that at pH 6.8 in the adult. In the newborn, the tension-[Ca] curve at pH 6.8 was shifted to the right, compared with that at pH 7.1. These data suggest that the vasorelaxant effect of respiratory acidosis in the premature vessel is greater than in the adult. The greater vasorelaxation in the newborn cannot be explained by the age-related difference in pHi or [Ca]i during acidosis. The greater sensitivity of myofibrils to low pHi in the newborn may, at least in part, be responsible for the greater vasorelaxation in this age group.
Similar content being viewed by others
Main
Acidosis is commonly associated with cardiovascular imbalance in both pediatric and adult patients; acidosis usually decreases the ability of the heart to contract and also causes vasodilation of systemic vessels(1–10). We showed previously that the negative inotropic effect of acidosis was less in the neonatal myocardium than in the adult myocardium(1, 2). Developmental changes in the effect of acidosis on the contraction of vascular smooth muscle have not been studied. The blood flow to the tissue is mainly regulated by the resistance artery(10), and changes in its vascular tone during acidosis may cause significant alteration in blood supply. This study was designed to determine developmental changes in the effect of acidosis on vascular tone of the resistance artery.
In the present study, we chose to use the mesenteric artery for several reasons; first, the superior mesenteric artery carries more than 10% of the systemic output and its resistance is an important component of the systemic resistance(10), second, the mesenteric resistance artery is obtained relatively easily even in newborn rabbits, and third, the mesenteric artery preparation has been used by other investigators(7, 9) and their results can be compared with the present data.
Vascular smooth muscle contraction is regulated by [Ca]i and sensitivity of myofibrils to calcium(11). Changes in pHi and pHo may alter [Ca]i and/or the sensitivity of myofibrils to calcium(12, 13). In the present study, [Ca]i and pHi were determined during acidosis and changes in mechanical function were correlated with changes in pHi and [Ca]i.
Several methods have been used to alter extracellular and/or intracellular pH in in vitro experiments; by the addition of HCl to the solution(4), by changing HCO3- concentration(1, 2), or by the addition and withdrawal of NH4Cl(14). In the present study, acidosis was produced by hypercapnea because “respiratory acidosis” is common not only in respiratory failure but also under conditions in which CO2 accumulates in the tissue due to reduction in blood flow or due to increased metabolic rate. The effect of pHi on the sensitivity of myofibrils to calcium was also measured using a chemically permealized mesenteric artery preparation.
METHODS
Solutions. The HEPES solution contained (in mM): NaCl, 142; Kcl, 5; CaCl2, 1.5; glucose, 6; MgCl2, 1; HEPES, 5; pH 7.4, with 1 M NaOH. The HEPES solution was equilibrated with room air or 100% O2. The control Krebs-Henseleit solution contained (in mM): NaCl, 118; KCl, 5; CaCl2, 1.5; glucose, 6; MgCl2, 1; NaHCO3, 24; NaH2PO4, 0.436. In a K-rich Krebs-Henseleit solution (50 mM KCl), NaCl was replaced, mole by mole, by KCl. In K-rich solutions (50 mM KCl), the NaCl was replaced, mole by mole, by KCl, and 1 μM phentolamine was added to block α-adrenergic stimulation.
These solutions were equilibrated with 95% O2-5% CO2, yielding a final pH of 7.38-7.42, Pco2 of 35-42 mm Hg, and Po2 of 660-690 mm Hg. Respiratory acidosis was produced by equilibration with 80% O2-20% CO2, and the superfusate pH was 6.80 ± 0.01. The relatively severe acidosis was chosen in the present study because this degree of acidosis (pH 6.8) had a more constant effect on mechanical function,[Ca]i, and pHi than did the milder degree of acidosis (pH 7.3- 7.1).
In experiments in which arterial contraction was induced by noradrenaline, a stock solution of noradrenaline (10-3 M) (Nakarai Chemical, Tokyo) containing 10-3 M ascorbic acid was made just before use and diluted to a final concentration of 10-6 M in a Krebs-Henseleit solution.
BCECF, BCECF/AM, fura-2, and fura-2/AM were purchased from Dojin (Kumamoto, Japan), and DMSO was purchased from Eastman-Kodak. BCECF, BCECF/AM, fura-2, and fura-2/AM were dissolved in DMSO to 1 mM. EGTA, TES, HEPES, saponin, l-NAME, indomethacin, phentolamine, and propranolol were purchased from Sigma Chemical Co. A23187, a calcium ionophore, was purchased from Calbiochem.
Vessel preparation. Experiments were performed using the mesenteric artery of 3-5-d-old newborn and 4-8-mo-old adult New Zealand White rabbits. The animals were heparinized (15 U/100 g of body wt, i.v.) and anesthetized with pentobarbital sodium (40 mg/kg). The abdomen was opened, and a segment (2-5 cm) of the small intestine (the proximal part of jejunum) with attached mesentery was removed and placed in ice-cold HEPES-buffered solution. Under a dissecting microscope (model SMZ, Nikon, Tokyo), a segment of the second branch resistance artery, about 2 mm in length, was isolated, as described previously(15, 16).
We showed previously that the effect of acidosis in the cannulated preparation was somewhat different from that in the wire-mounted preparation in the adult rabbit(17). Because the reasons for this difference were unclear and developmental changes in the effect of acidosis may differ depending upon the type of preparation, both the wire-mounted preparation(15) and the cannulated preparation(18) of the mesenteric artery were used in the present study. The cannulated preparation was used to determine the effect of acidosis on vessel diameter, pHi, and [Ca]i. In the wire-mounted preparation, contractile force, not pHi or[Ca]i, was measured.
In the present study, vessel contraction was induced in two ways, high KCl and noradrenaline. High KCl causes vascular contraction mainly by membrane depolarization and increasing calcium influx across the sarcolemma. Noradrenaline causes vascular contraction mainly by inducing calcium release from intracellular store sites(11).[Ca]i is regulated by both calcium influx across the sarcolemma and calcium release from intracellular store sites. It must be noted that vascular contraction caused by 50 mM KCl is only 10-15% of the maximal contraction(16), suggesting that the degree of depolarization is relatively small.
Cannulated preparation. The experimental setup of the cannulated preparation was similar to that described previously(16–20). In brief, the isolated arteriole was cannulated using two glass pipettes (100-120-μm tip diameter) and tied to the glass pipettes with strands of 10-0 nylon suture(17). The vessel was initially filled with HEPES solution equilibrated with 100% O2, using peristaltic pumps and a pressure servo system (Living Systems, Burlington, VT) at a pressure of about 2 mm Hg. The intraluminal pressure was gradually increased to 80 mm Hg in the adult and 32 mm Hg in the newborn. It was assumed that the mean pressure of the mesenteric small artery in vivo is 32 mm Hg for the newborn, and 80 mm Hg for the adult. These values were calculated based on the assumption that the pressure in the resistance artery with internal diameters of 100-400 μm is 80-90% of the arterial mean pressure and that the arterial mean pressure is 40 mm Hg in the 3-d-old newborn, and 90 mm Hg in the adult rabbit(21–23). Once set up, the perfusion of the vessel was stopped, and the vessel was superfused with oxygenated solutions at 5 mL/min.
The vessel diameter was measured as described previously(17). The image of the vessel was monitored by a low light level Newvicon television camera (model NC-70X, Dage-MTI, Michigan City, IN), and the television camera output was connected to a video motion analyzer(Percepterscope C3161, Hamamatsu Photonics, Hamamatsu, Japan). The outer diameter of the vessel was measured by the video motion detector every 32 ms and recorded on a strip-chart recorder (San-ei Sokki, Tokyo, Japan).
Dye-loading. [Ca]i was measured using fura-2, and pHi was measured using BCECF, as described previously(2, 3, 17, 24, 25). Before dye-loading, autofluorescence of the vessel was measured for 30 s using HEPES solution equilibrated with 100% O2. Equilibration with 100% O2 was performed to measure autofluorescence under oxygenated conditions. The preparation was then superfused with the solution equilibrated with 100% O2 and containing either 5 μM fura-2/AM or 5 μM BCECF/AM, for 3-5 h at room temperature (22-24°C). The final DMSO concentration was 0.2%. A vehicle control study indicated that superfusion with the solution containing this concentration of DMSO had no effect on the arteriolar responses to acidosis. After dye-loading, the superfusate was changed to a dye-free, oxygenated, control Krebs-Henseleit solution to wash out extracellular fluorescent dye. Intraluminal perfusion was resumed using the control Krebs-Henseleit solution. The temperature of the solution was gradually increased to 37°C. After 60 min of wash out, intraluminal perfusion was stopped, and the experiment to measure vessel diameter, pHi, or [Ca]i was started. Although intraluminal perfusion was halted, the intraluminal pressure was kept at 75-80 mm Hg in the adult and 30-32 mm Hg in the newborn.
Fluorescence measurements. Excitation lights of 340- and 380-nm wavelengths (for fura-2), or 439- and 490-nm wavelengths (for BCECF), were obtained alternatively using a xenon lamp (450-W), two monochrometors, and a chopper (Spex, Edison, NJ). The intensity of fura-2 fluorescence at 505 nm, or that of BCECF fluorescence at 530 nm during excitation at each wavelength, was observed for 1 s at 5-s intervals. The fura-2 fluorescence ratio was then calculated as: (fluorescence at 340-nm excitation - autofluorescence at 340-nm excitation)/(fluorescence at 380-nm excitation - autofluorescence at 380-nm excitation), as described previously(17). The magnitude of the autofluorescence was 15-20% of the total fluorescence at 340- and 380-nm excitation, respectively, in the two age groups.
In the present study, an in vivo [Ca]i calibration using a calcium ionophore, ionomycin, was not performed because the effect of ionomycin to permealize the cell membrane was not stable(2). We therefore used an in vitro calibration curve of [Ca]i to estimate absolute values of[Ca]i, as described previously(1, 2, 17). The BCECF fluorescence ratio(490/439 nm) was calculated in a similar fashion. Acidosis did not alter the autofluorescence at the BCECF excitation wavelength. Calibration of the BCECF fluorescence ratio was performed using an in vitro calibration curve, as described previously(2, 17).
Wire-mounted preparation. The experimental setup of the wire-mounted preparation was similar to that described previously(15, 17). In brief, two tungsten wires (25 μm in diameter, Thermionic Products Company, North Plainfield, NJ) were threaded into the lumen, and the preparation was placed in a myograph (Living Systems, Burlington, VT). Isometric tension was monitored using the force transducer(Kulite Semiconductor, Leonia, NJ) and a recorder (San-ei Sokki, Tokyo, Japan). Once set up, the preparation was superfused with oxygenated solutions at 5 mL/min.
Axial vessel length, distance between the wires, and force were measured while the vessels were stretched gradually. Wall tension was calculated by dividing force (mN) by axial vessel length (mm) and expressed as mN/mm. Exponential curves of vessel circumference versus tension were constructed(16). The slopes of the curves were similar in the two age groups. Transmural pressure was calculated from the vessel circumference and wall tension using LaPlace's equation(15, 16, 20). The artery was stretched until the resting wall tension reached the level corresponding to the transmural mean pressure in vivo (80 mm Hg in the adult and 32 mm Hg in the newborn). The vessel circumference at this point was expressed asLop. The vessel circumference was then adjusted so that the operating circumference was 80% of Lop(L80%). The resting tension at this point was 0.24 ± 0.05 mN/mm in the newborn, and 0.36 ± 0.04 mN/mm in the adult. We previously studied the length-tension curve at various KCl concentrations in the two age groups and showed that at the muscle length atL80%, although absolute values of contractile force induced by high KCl increased with age, sensitivity to high KCl was similar in the two age groups(16).
Experimental protocol. All preparations were stabilized for at least 30 min in an oxygenated Krebs-Henseleit solution at 37°C. The vascular contraction was induced by either 50 mM KCl or 10-6 M noradrenaline. After the mechanical function reached a new steady state, the vessel was superfused with an acidosis solution containing 50 mM KCl or 10-6 M noradrenaline for 10 min.
In additional experiments, the role of endothelium in the age-related difference in the effect of acidosis was studied. The endothelium was removed by gently rubbing the internal wall of the vessel with a polyethylene tube(PE-50), which had been tapered to about 100 μm in diameter. The absence of a relaxation response to acetylcholine (1 μM) in vessels precontracted with 50 mM KCl or norepinephrine was taken as evidence that the endothelium was removed. In some experiments, 30 μM l-NAME, a blocker of nitric oxide synthesis, was added to the solutions throughout the experiment, and the effect of acidosis was examined. In the presence of 30 μM l-NAME, 1 μM acetylcholine did not cause a vasorelaxation in vessels precontracted with 50 mM KCl. In additional experiments, the role of prostaglandins andβ-adrenergic receptors in the age-related difference in the effect of acidosis was studied. Indomethacin (10 μM) and 0.1 μM propranolol (0.1μM) were added to the solutions throughout the experiment, and the effect of acidosis was examined. These concentrations of drugs have been used to block prostaglandin synthesis and β-adrenergic receptors, respectively(26–31).
Skinned smooth muscle fibers. Sensitivity of myofibrils to calcium was measured at pH 7.1 and 6.8 in the two age groups. Skinned smooth muscle fibers of the mesenteric artery were prepared using the method of Saidaet al.(32, 33). The vessel was mounted in a wire-myograph with a volume capacity of 0.5 mL, and contractile force was monitored using a force transducer. The tissue bath was initially filled with a control HEPES solution. The solution was then changed to the relaxing solution (30 mM TES, 10 mM EGTA, 3.3 mM Na2-ATP, 10 mM creatine phosphate, 0.3 mg/mL creatine phosphokinase, 5 mM MgCl2, 130 mM KCl, pH 7.1, with 1 M KOH), containing 100 μg/mL saponin and 10 μM calcium ionophore A23187. Saponin was used for chemical skinning of the smooth muscle cells and A23187 was used to inactivate the function of the sarcoplasmic reticulum. The incubation was carried out for 25 min in the adult and for 15 min in the newborn at 25°C. In a preliminary experiment, the incubation time was varied from 5 to 60 min, and membrane permeabilization was obtained at 25 min in the adult and at 15 min in the newborn. After the incubation, the skinned smooth muscle was washed once with the relaxing solution which did not contain saponin and A23187. Contraction of the skinned smooth muscle was induced by changing the solution to the relaxing solution containing various amounts of CaCl2. The pCa (- log [Ca]) of the solution was changed from 8 to 4.5. The amounts of CaCl2 to be added were calculated as described previously(1). The pH was adjusted with 1 M KOH to 7.1 or 6.8 and in each muscle preparation developed tension was measured at two different pH values. Developed tension at variousp Ca was normalized by the maximal developed tension obtained in each preparation and the pCa value for the half-maximal activation(Kd) of developed tension was calculated.
Statistical analysis. Results were expressed as mean ± SEM. For comparison between two groups, statistical significance was analyzed using the modified t test with the Bonferroni method. Statistical significance in changes in mechanical function, [Ca]i, and pHi during acidosis was tested using repeated measures ANOVA. Statistical significance in changes in Kd of developed tension in skinned fibers at two different pH values was tested using pairedt test. The probability was considered to be significant if thep value was less than 0.05(34, 35).
RESULTS
Cannulated preparation. The outer diameter of the mesenteric artery at an intraluminal pressure of 0 mm Hg was 167 ± 12 μm in the newborn (n = 25) and 270 ± 14 μm in the adult (n= 24). The outer diameter increased to 265 ± 13 μm at an intraluminal pressure of 32 mm Hg in the newborn, and to 432 ± 13 μm at an intraluminal pressure of 80 mm Hg in the adult. The outer diameter of the vessel contracted by noradrenaline was 171 ± 30 μm in the newborn and 265 ± 23 μm in the adult. The outer diameter of the vessel contracted by high KCl was 186 ± 18 μm in the newborn and 317± 28 μm in the adult.
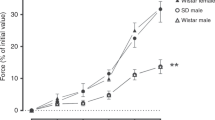
Respiratory acidosis in the vessel precontracted by high Kcl. The effect of acidosis in the adult was similar to the previously reported data from our laboratory(17). In the cannulated preparation, respiratory acidosis caused a vasorelaxation both in the newborn and adult (Fig. 1A). When the vessel contraction was expressed as a percentage of the change induced by high KCl, the vessel contraction decreased transiently to 72 ± 3% and it recovered to 90± 2% in the adult (n = 12). In contrast, the vessel contraction decreased to 64 ± 4% and it did not recover significantly during acidosis in the newborn (n = 12). The vasorelaxation in the newborn was significantly (p < 0.05) greater than in the adult(Fig. 2).
Recordings showing the effect of respiratory acidosis on vessel diameter, pHi, and [Ca]i in the cannulated preparation of the mesenteric artery precontracted by 50 mM KCl in the newborn. Respiratory acidosis caused a vasorelaxation (A). In the newborn, the decrease in the vessel contraction did not recover during acidosis. pHi fell rapidly during acidosis (B). Upon induction of acidosis, the fura-2 fluorescence ratio decreased transiently and then increased to a level greater than control(C).
Graph showing the effect of respiratory acidosis on vessel diameter in the cannulated preparation precontracted by 50 mM KCl. The vessel contraction decreased transiently and recovered in the adult. In the newborn, the vessel contraction decreased and it did not recover during acidosis. The values are mean ± SEM. *Significantly different(p < 0.05) from the adult value.
Upon induction of acidosis, pHi fell rapidly, from 7.05± 0.05 to 6.83 ± 0.06 in the adult (n = 6) and from 7.05 ± 0.05 to 6.84 ± 0.06 in the newborn (Fig. 1B). The net decrease in pHi was similar in the two age groups (0.22 ± 0.03 in the adult and 0.21 ± 0.03 in the newborn).
Upon superfusion with the K-rich solution, the fura-2 fluorescence ratio increased in the two age groups (n = 6). The fluorescence ratio decreased transiently upon induction of acidosis, and it then returned to a level slightly greater than the control both in the newborn and adult(Fig. 1C). Absolute values of [Ca]i were estimated using in vitro calibration curves, as described previously(17). Respiratory acidosis caused a small but significant decrease in [Ca]i, from 115 ± 16 to 93 ± 12 nM(n = 6) in the adult and from 239 ± 46 to 221 ± 42 nM in the newborn (n = 6). There was no significant difference in the net decrease in [Ca]i between the two age groups.[Ca]i returned to a level greater than the control in the two age groups (123 ± 15 nM in the adult and 256 ± 46 nM in the newborn).
Respiratory acidosis in the vessel precontracted by noradrenaline. In the cannulated preparation, respiratory acidosis caused a transient vasoconstriction in the adult, as described previously(17). In contrast, respiratory acidosis caused a small vasorelaxation in the newborn (Fig. 3A). When the vessel contraction was expressed as a percentage of the contraction induced by noradrenaline, it significantly increased to 134 ± 3% of control during acidosis in the adult and significantly decreased to 83 ± 5% of control in the newborn (Fig. 4).
Recording showing the effect of respiratory acidosis on vessel diameter, pHi, and [Ca]i in the cannulated preparation of the mesenteric artery precontracted by noradrenaline in the newborn. Respiratory acidosis caused a small vasorelaxation in the newborn (A). pHi fell rapidly during acidosis(B). Upon induction of acidosis, the fura-2 fluorescence ratio increased transiently (C).
Graph showing the effect of respiratory acidosis on noradrenaline-induced contraction in the cannulated preparation of the mesenteric artery. The vessel contraction increased during acidosis in the adult and decreased in the newborn. The values are mean ± SEM.* Significantly different (p < 0.05) from the adult value.
Upon induction of respiratory acidosis, pHi fell rapidly, from 7.04 ± 0.08 to 6.83 ± 0.09 (n = 6) in the adult, and 7.03 ± 0.10 to 6.81 ± 0.10 (n = 6) in the newborn(Fig. 3B). The depression of pHi was similar in the two age groups.
Superfusion with a solution containing noradrenaline caused increases in the fura-2 fluorescence ratio and the ratio reached a new steady state in the two age groups. Upon induction of acidosis, the ratio increased transiently both in the newborn and adult (Fig. 3C). Respiratory acidosis caused a small but significant increase in [Ca]i from 125 ± 12 to 149 ± 14 nM in the adult (n = 7) and from 159 ± 10 to 184 ± 10 nM in the newborn (n = 6). The net increase in [Ca]i was similar in the two age groups.
The role of endothelium, prostaglandins, and adrenergicreceptors. The vessel from which the endothelium was removed was precontracted by either high KCl (n = 4 in each age group) or norepinephrine (n = 4 in each age group). In vessels precontracted by high KCl or norepinephrine, changes in vessel diameter during acidosis without endothelium were identical to those in the vessels with endothelium in the two age groups. In the presence of l-NAME, changes in vessel diameter were also identical to those in the absence of l-NAME in the two age groups(n = 4). Thus, the removal of the endothelium did not significantly alter the effect of acidosis.
The vessels were precontracted by either high KCl (n = 4 in each age group) or norepinephrine (n = 4 in each age group) in the presence of 10 μM indomethacin and 0.1 μM propranolol. Changes in vessel diameter were identical to those in the absence of the blockers in the two age groups in the vessels precontracted by high KCl and norepinephrine.
Wire-mounted preparation. Developed tension induced by 50 mM KCl was 0.31 ± 0.03 mN/mm in the newborn, and 0.42 ± 0.03 mN/mm in the adult. Developed tension induced by norepinephrine was 0.54 ± 0.04 mN/mm in the newborn, and 0.96 ± 0.08 mN/mm in the adult. These values in the newborn were significantly less than in the adult. In the wire-mounted preparation precontracted by high KCl, respiratory acidosis caused a small transient increase followed by a gradual decrease in tension in the adult, as described previously(17). In the newborn, however, respiratory acidosis caused only a vasorelaxation(Fig. 5). If the new steady state before induction of respiratory acidosis was considered to be a 100% contraction, respiratory acidosis increased the contractile tension significantly (p < 0.01) to 118 ± 7% (n = 6) in the adult. In the newborn, however, respiratory acidosis significantly (p < 0.01) decreased the contractile tension to 70 ± 7% (n = 6)(Fig. 6).
Graph showing the effect of respiratory acidosis on KCl-induced contraction in the wire-mounted preparation of the mesenteric artery. Respiratory acidosis increased the contractile tension transiently in the adult and decreased it in the newborn. The values are mean ±SEM.* Significantly different (p < 0.05) from the adult value.
In the wire-mounted preparation precontracted by noradrenaline, respiratory acidosis caused a transient increase in contraction in both the adult and newborn (Fig. 7). When the net increase in vessel contraction was expressed as percent of the maximal contraction induced by noradrenaline, the net increase during acidosis in the newborn was 26 ± 13% (n = 6) and the value was significantly (p = 0.01) less than in the adult (85 ± 14%, n = 6).
Recordings showing the effect of respiratory acidosis in the wire-mounted preparation of the mesenteric artery precontracted by noradrenaline in the newborn. Noradrenaline caused an increase in contractile tension followed by a gradual decrease. Respiratory acidosis caused a transient increase in contraction. Upon returning to the control solution containing noradrenaline, contractile tension decreased transiently and then recovered gradually.
Skinned fibers. The tension-[Ca]i relationship was determined in the skinned smooth muscle fibers to estimate sensitivity of myofibrils to calcium in the two age groups. In the adult, the tension-[Ca] relationship at pH 7.1 was not significantly different from that at pH 6.8(Fig. 8A). In contrast, the tension-[Ca] relationship curve at pH 6.8 was shifted to the right compared with that at pH 7.1 in the newborn (Fig. 8B). In the adult, the half-maximal activation (Kd) at pH 7.1 was observed at [Ca] of 0.71± 0.20 μM (n = 6), and it did not change significantly when the pH value of the solution decreased to 6.8 (Kd = 0.75± 0.09 μM, n = 6). In the newborn, the Kd value at pH 7.1 was 1.48 ± 0.16 μM (n = 6), and it increased significantly (p = 0.04) to 1.90 ± 0.15 μM(n = 6) when the pH value decreased to 6.8. At pH 7.1, theKd value in the newborn was greater than in the adult. It is possible that during the skinning procedure, some contractile elements, including calmodulin, might have been lost(33), and the myofibrils became less sensitive to calcium in the newborn. Therefore, calmodulin (1 μM) was added to the solution in additional experiments. Although the tension-[Ca] relationship shifted to the left in the presence of calmodulin in the two age groups, the age related difference in the sensitivity to calcium still existed and the pH sensitivity was observed only in the newborn.
Graphs showing a tension-[Ca]i relationship, determined in the chemically skinned smooth muscle fibers. In the adult (A), the tension-[Ca] curve at pH 7.1 was not significantly different from that at pH 6.8. In the newborn (B), the tension-[Ca] curve at pH 6.8 was shifted to the right, compared with that at pH 7.1.
DISCUSSION
The present study determined, for the first time, developmental changes in the effect of acidosis on vascular contraction in the mesenteric small artery. Several investigators(9, 36–38) have used a wire-mounted preparation of the mesenteric arteriole of the adult rat, precontracted by high KCl or noradrenaline, and showed that acidosis caused a transient vasoconstriction, followed by a gradual vasorelaxation. The effect of acidosis in the adult in the present study was similar to previously reported data(9, 17, 36–38). In the present study, acidosis caused a vasorelaxation only in the newborn in the wire-mounted vessels precontracted by high KCl (Fig. 5). In the wire-mounted vessels precontracted by noradrenaline, the transient vasoconstriction was observed, but the vasoconstriction in the newborn was significantly less than in the adult. These data indicate that acidosis can cause both vasoconstriction and vasorelaxation and that the former is less and the latter is greater in the newborn than in the adult in wire-mounted preparations.
In the cannulated preparation precontracted by high KCl, only a vasorelaxation was observed during acidosis, and the vasorelaxant effect in the newborn was greater than in the adult (Fig. 2). In the vessel precontracted by noradrenaline, acidosis caused a vasoconstriction in the adult and only a vasorelaxation in the newborn (Fig. 4). These data suggest that in the cannulated preparation, as in the wire-mounted preparation, the vasorelaxant effect of acidosis in the newborn is greater than in the adult. The experimental conditions in a cannulated preparation have been considered closer to in vivo conditions than those in the wire-mounted preparation(20). It must be noted, however, that both preparations are only in vitro models and the vessel in vivo may respond to acidosis differently. Furthermore, responses of the mesenteric arteriole to acidosis may be different from those of other resistance arteries such as the skeletal muscle arteriole.
In the present study, the decrease in pHi in the newborn vessel was similar to that in the adult and, therefore, it is unlikely that the greater vasorelaxant effect of acidosis in the newborn vessel can be explained by the age-related difference in changes in pHi.
Although the precise mechanisms for the regulation of vascular tone during acidosis are not yet clear, previous studies have shown that vascular contraction during acidosis is mainly regulated by [Ca]i and the sensitivity of contractile apparatus to calcium(11, 13, 38, 39). In the wire-mounted preparation of the adult rat mesenteric artery, the transient increase in contractile tension during acidosis was associated with an increase in[Ca]i(36, 37). In the cannulated preparation, a transient vasoconstriction was associated with a transient increase in [Ca]i in the adult vessel precontracted by noradrenaline. Furthermore, a transient vasorelaxation was associated with a transient decrease in [Ca]i in the adult vessel precontracted by high KCl. These data suggested that [Ca]i was, at least partly, regulating vascular contraction and relaxation during acidosis in the adult.
In the present study, an increase in [Ca]i was also observed during acidosis in the newborn vessel precontracted by noradrenaline(Fig. 3). However, the increase in [Ca]i was not associated with a vasoconstriction. In the vessel precontracted by high KCl, a similar decrease in [Ca]i in the two age groups was associated with the greater vasorelaxation in the newborn. Because the change in [Ca]i during acidosis was similar in the two age groups, the age-related difference in the effect of acidosis on vascular tone cannot be explained by [Ca]i.
In the present study, fura-2 was used to estimate [Ca]i. One may argue that fura-2 fluorescence is sensitive to pH and alteration of pHi may have changed fura-2 signals(40). However, changes in pHi were similar in the two age groups. Furthermore, calibration of[Ca]i using the in vitro calibration curve resulted in the similar findings to the 340/380 ratio measurement regarding[Ca]i changes during acidosis in the two age groups.
The decrease in pHi during acidosis may reduce the sensitivity of myofibrils to calcium and induce vasorelaxation(13, 39). If the sensitivity of myofibrils to low pH is different in the two age groups, this could result in different degrees of vasorelaxation for the same degree of changes in pHi and[Ca]i during acidosis. To test this hypothesis, the sensitivity of myofibrils to low pH was examined in the skinned smooth muscle fibers. In the present study, pHi changed in the range between 7.1 and 6.8 during respiratory acidosis. Within this range of pHi changes, the sensitivity of myofibrils to calcium was not altered in the adult (Fig. 8). This may partly explain the good correlation between changes in vascular tone and [Ca]i observed during acidosis in the adult. Endo et al.(41) also showed that a tension-calcium relationship was not affected by changes in pH in the skinned muscle fibers of guinea pig teniae ceci. In the newborn, however, the sensitivity of myofibrils to calcium was indeed altered when the pH value decreased from 7.1 to 6.8(Fig. 8). In the present study, absolute values of[Ca]i were about 100-150 nM in the adult and 150-250 nM in the newborn. The contractile force was especially sensitive to changes in pH in the [Ca]i range between 100 nM (pCa 7) and 1000 nM (pCa 6) (Fig. 8). Therefore, it is likely that the vasorelaxant effect of acidosis was greater in the newborn because the pH sensitivity of myofibrils was greater.
In the vascular smooth muscle, sensitivity of myofibrils to calcium is, at least in part, regulated by the degree of myosin light chain phosphorylation(42), which in turn is dependent on myosin light chain kinase and phosphatase activities. Whether an age-related difference in the sensitivity of these enzymes to pH exists or not is unknown. Vascular smooth muscle contraction is activated by calcium which is bound to calmodulin(11). Saida et al.(33) showed that addition of calmodulin to the solution increased the calcium sensitivity in the saponinskinned muscle. It is unlikely, however, that the greater sensitivity of myofibrils to pH in the newborn is an artifact of the skinning procedure, because the pH sensitivity was still observed in the skinned fibers of the newborn muscle when calmodulin (1 μM) was added to the solution.
One may argue that acidosis may cause release of endothelium-derived relaxant factors and cause a vasorelaxation, especially in the newborn. Changes in vascular tone in vessels without endothelium were, however, identical to those in the vessels with endothelium. Thus, it is unlikely that the endothelium was involved in the age-related difference in the acidosis-induced vasorelaxation. It is also unlikely that prostaglandins and adrenergic-receptors are involved in the greater vasorelaxation in the newborn, because indomethacin and β-blocker did not alter the acidosis-induced vasorelaxation.
In the present study, only the effect of “respiratory” acidosis was examined. Austin and Wray(43), however, have shown that a passive entry of H+ across the membrane during“metabolic” acidosis in the resistance artery is much greater than in cardiac cells, suggesting that the difference between“respiratory” and “metabolic” acidosis on pHi might be small in the vascular smooth muscle. Developmental changes in intracellular pHi regulation, including a passive entry of H+ into the cell, must be studied further in vascular smooth muscle cells.
In conclusion, the present study has shown that the vasorelaxant effect of acidosis in the newborn was greater than in the adult. The greater vasorelaxation in the newborn cannot be explained by differences in pHi or [Ca]i during acidosis. The greater sensitivity of myofibrils to low pHi in the newborn may be responsible, at least in part, for the greater vasorelaxation in this age group.
Abbreviations
- [Ca]i:
-
intracellular calcium concentration
- pHi:
-
intracellular pH
- pHo:
-
extracellular pH
- BCECF:
-
2,7-biscarboxyethyl-5(6)-carboxyfluorescein
- AM:
-
acetoxymethyl ester
- L-NAME:
-
nitro-L-arginine methyl ester
- TES:
-
N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid
References
Nakanishi T, Okuda H, Nakazawa M, Takao A 1985; Effect of acidosis on contractile function in the newborn rabbit heart. Pediatr Res 19: 482–488.
Nakanishi T, Seguchi M, Tsuchiya T, Yasukouchi S, Takao A 1990; Effect of acidosis on intracellular pH and Ca concentration in the newborn and adult rabbit myocardium. Circ Res 67: 111–123.
Nakanishi T, Gu H. Seguchi M, Cragoe EJ, Momma, K 1992; HCO3- -dependent intracellular pH regulation in the premature myocardium. Circ Res 71: 1314–1323
Loutzenhiser R, Matsumoto Y, Okawa W, Epstein M 1990; H+ -induced vasodilation of rat aorta is mediated by alterations in intracellular calcium sequestration. Circ Res 67: 426–439.
Rinaldi GJ, Gattaneo A, Gingolani HE 1987; Interaction between calcium and hydrogen ions in canine coronary arteries. J Mol Cell Cardiol 19: 773–784.
Kontos HA 1981; Regulation of the cerebral circulation. Annu Rev Physiol 43: 397–407.
Aalkjaer C, Cragoe Jr EJ 1988; Intracellular pH regulation in resting and contracting segments of rat mesenteric resistance vessels. J Physiol 402: 391–410.
Krampetz IK, Rhoades RA 1991; Intracellular pH: effect on pulmonary arterial smooth muscle. Am J Physiol 260:L516–L521.
Nielsen H, Aalkjaer C, Mulvany MJ 1991; Differential contractile effects of changes in carbon dioxide tension on rat mesenteric resistance arteries precontracted with noradrenaline. Pflugers Arch 419: 51–56.
Berne RM, Levy MN 1993; Physiology. Mosby Year Book, St. Louis, MO 510–531.
Karaki H, Weiss GB 1988; Calcium release in smooth muscle. Life Sci 42: 111–122.
Aalkjaer C 1990; Regulation of intracellular pH and its role in vascular smooth muscle function. J Hyperten 8: 197–206.
Wray S 1988; Smooth muscle intracellular pH: measurement, regulation, and function. hysiol 254:C213–C225.
Roos A, Boron WF 1981; Intracellular pH. Physiol Rev 61: 296–434.
Mulvany MJ, Halpern W 1977; Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res 41: 19–26.
Nakanishi T, Gu H, Momma K 1997; Developmental changes in contractile system of the mesenteric small artery of rabbit. Pediatr Res 41: 1–5.
Nakanishi T, Gu H, Momma K 1996; Effect of acidosis on contraction, intracellular pH and calcium in the rabbit mesenteric small artery. J Mol Cell Cardiol 28: 1715–1726.
Halpern W, Kelley M 1991; In vitro methodology for resistance arteries. Blood Vessels 28: 245–251.
Bukoski RD, Bergmann C, Gairard A, Stoclet J 1989; Intracellular Ca and force determined simultaneously in isolated resistance arteries. Am J Physiol 257:H1728–H1735.
Meninger GA, Zawieja DC, Falcone JC, Hill MA, Davey JP 1991; Calcium measurement in isolated arterioles during myogenic and agonist stimulation. Am J Physiol 261:H950–H959.
Dawes GS, Handler JJ, Mott JC 1957; Some cardiovascular responses in foetal, newborn, and adult rabbits. J Physiol 139: 123–136.
Downing SE 1960; Baroreceptor reflexes in newborn rabbits. J Physiol 150: 201–213.
Mulvany MJ, Aalkjaer C 1990; Structure and function of small arteries. Physiol Rev 70: 921–961.
Nakanishi T, Seguchi M, Tsuchiya T, Cragoe EJ, Takao A, Momma K 1991; Effect of partial Na pump and Na-H exchange inhibition on[Ca]i during acidosis in cardiac cells. Am J Physiol 261:C758–C766.
Nakanishi T, Gu H, Hagiwara N, Momma K 1993; Mechanisms of oxygen-induced contraction of ductus arteriosus isolated from the fetal rabbit. Circ Res 72: 1218–1228.
Nakanishi T, Kamata K, Nojima K, Seguchi M, Takao A 1989; Inotropic effect of phenylephrine and myocardial alpha-adrenergic receptor in newborn and adult animals. J Mol Cell Cardiol 21: 975–985.
Wang Y, Coceani F 1992; Isolated pulmonary resistance vessels from fetal lambs. Circ Res 71: 320–330.
Krampetz IK, Rhoades RA 1991; Intracellular pH: effect of pulmonary arterial smooth muscle. Am J Physiol 260:L515–L521.
Li J, Bukoski RD 1993; Endothelium-dependent relaxation of hypertensive resistance arteries is not impaired under all conditions. Circ Res 72: 290–296.
Vane JR 1971; Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drug. Nature 231: 232–235.
Rubanyi GM, Vanhoutte PM 1985; Hypoxia releases a vasoconstrictor substance from the canine vascular endothelium. J Physiol 364: 45–56.
Saida K, Nonomura Y 1978; Characteristics of Ca and Mg-induced tension development in chemically skinned smooth muscle fibers. J Gen Physiol 72: 1–14.
Saida K, Manabe Y, Seki H 1991; Ca sensitization of vascular smooth muscle. J Vasc Med Biol 3: 181–186.
Wallenstein S, Zucker CL, Fleiss JL 1980; Some statistical methods useful in circulation research. Circ Res 27: 1–9.
Snedecor GW, Cochran WG 1970; Statistical Methods. Iowa State University Press, Ames, IA
Aalkjaer C, Mulvany MJ 1988; Effect of changes in intracellular pH on the contractility of rat resistance vessels. Prog Biochem Pharmacol 23: 150–158.
Jensen PE, Hughes A, Boonen HCM, C. Aalkjaer C 1993; Force, membrane potential, and [Ca]i during activation of rat mesenteric small arteries with norepinephrine, potassium, aluminum, fluoride, and phorbol ester. Circ Res 73: 314–324.
Matthews JG, Graves JE, Poston L 1992; Relationships between pHi and tension in isolated rat mesenteric resistance arteries. J Vasc Res 29: 330–340.
Nagesetty R, Paul RJ 1994; Effects of pHi on isometric force and [Ca]i in porcine coronary artery smooth muscle. Circ Res 75: 990–998.
Batlle DC, Peces R, LaPointe MS, Ye M, Daugirdas JT 1993; Cytosolic free calcium regulation in response to acute changes in intracellular pH in vascular smooth muscle. Am J Physiol 264:C932–C943.
Endo M, Yagi S, IIno M 1982; Tension-pCa relation and sarcoplasmic reticulum responses in chemically skinned smooth muscle fibers. Fed Proc 41: 2245–2250.
Kitazawa T, Gaylinn BD, Denney GH, Somlyo AP 1991; G-protein mediated Ca sensitization of smooth muscle contraction through myosin light chain phosphorylation. J Biol Chem 266: 1708–1715.
Austin C, Wray S 1993; Extracellular pH signals affect rat vascular tone by rapid transduction into intracellular pH changes. J Physiol 466: 1–8.
Author information
Authors and Affiliations
Additional information
Supported by a Research Grant 5670702 from the Japanese Ministry of Education, Science, and Culture and by a grant-in-aid from the Japan Research Promotion Society for Cardiovascular Diseases.
Rights and permissions
About this article
Cite this article
Nakanishi, T., Gu, H. & Momma, K. Developmental Changes in the Effect of Acidosis on Contraction, Intracellular pH, and Calcium in the Rabbit Mesenteric Small Artery. Pediatr Res 42, 750–757 (1997). https://doi.org/10.1203/00006450-199712000-00006
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199712000-00006