Abstract
Circulatory changes occur during perinatal life that increase cardiac output and left ventricular contractile reserve. To examine postnatal changes in left ventricular systolic function and ventricular-vascular coupling, piglets underwent cardiac catheterization at 1, 2, 4, and 6 wk of age. We measured end-systolic elastance (Ees), preload-recruitable stroke work, dP/dtmax, the dP/dtmax end-diastolic volume relation, cardiac index, heart rate, arterial elastance (Ea), and the ratio Ea/Ees at rest, during isoproterenol infusions (0.05-1.0 μg/kg/min), and after propranolol (1 mg/kg i.v.). Resting heart rate and cardiac index decreased between 1 and 6 wk. In 1 wk olds, resting Ees was at maximum and was unchanged during isoproterenol infusion; isoproterenol increased other contractility indices. Two, 4, and 6 wk olds demonstrated reserve using all contractility indices. Contractile efficiency was not different between ages. In 1 wk olds, Ea decreased during isoproterenol infusion; isoproterenol did not change Ea at 6 wk .Ea/Ees was higher at rest at 6 wk than at 1 wk, and fell significantly on isoproterenol; isoproterenol did not change Ea/Ees at 1 wk. Withβ-adrenergic stimulation, 1 wk olds increased cardiac index by increasing heart rate and decreasing afterload, 6 wk olds increased cardiac index by increasing heart rate and contractility; no change in contractile efficiency was found in either group. In summary, contractile reserve is limited at 1 wk when measured by Ees, but other indices demonstrated reserve. Indexed Ea falls in response to β-adrenergic stimulation in all ages but 6 wk. Ventricular-vascular coupling is optimized at 1 wk even under baseline conditions.
Similar content being viewed by others
Main
Many changes occur during the perinatal period in the mammalian cardiovascular system. Birth-related changes in the cardiovascular system include a dramatic increase in left ventricular output and an increase in left ventricular inotropic state(1). At birth, left ventricular output increases 2-fold. This increase is caused by an increase in contractility(2, 3), an increase in preload(1, 4, 5), and an improved circulatory environment that the left ventricle faces(6), particularly with improvement in left ventricular preload(7, 8). The newborn left ventricle functions at close to its maximum contractile state. After birth, the resting inotropic state falls relative to its maximum, allowing for development of contractile reserve during the first weeks of life(9). Many previous studies of left ventricular function in the newborn heart have examined indices of function that are dependent upon factors such as preload, afterload, and heart rate, which confound precise evaluation of changes in ventricular contractility(10–13). Few studies in newborn hearts have examined indices that specifically measure left ventricular systolic performance which are relatively independent of loading conditions(9).
Afterload has been shown to have direct effects on the contractility of the immature heart. Fetal and newborn myocardium has been shown to be very sensitive to increased afterload, in that increasing left ventricular afterload is known to limit left ventricular stroke volume(11, 14–16). Additionally, in young animals, it has been recently demonstrated that increasing afterload can increase indices of contractile function(17). Little is known about changes in afterload, systemic vascular resistance, and coupling of contractility and afterload during the postnatal period.
We wished to examine left ventricular systolic function, arterial elastance, and ventricular-vascular coupling in the piglet during the first few weeks of life. We hypothesized that, in the piglet during the early postnatal period, resting contractile function would be at a high level relative to that achieved during maximum stimulation using β-adrenergic agonist infusion, and that with maturation during the first 6 wk, resting contractile state would fall relative to this maximum, allowing for“contractile reserve.” In addition, we hypothesized that contractile efficiency and arterial elastance would not change during this period, and that no age-related differences should exist in ventricular-vascular coupling as measured by endsystolic elastance and arterial elastance. To determine developmental changes in indices of left ventricular systolic and ventricular-vascular coupling, piglets were studied by cardiac catheterization at 1, 2, 4, and 6 wk of age. Conductance and manometric catheters were placed in the left ventricle of these piglets to acquire simultaneous pressure and volume data to measure indices of systolic function and afterload during different preload conditions and inotropic states.
METHODS
To evaluate maturation of left ventricular function in young piglets, healthy mixed-breed piglets underwent a nonsurvival cardiac catheterization to obtain indices of cardiovascular function derived from simultaneously recorded left ventricular pressure and volume. This study conforms with the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health. The protocol was approved by the institutional Animal Care and Use Committee. The ages of the piglets were as follows: 7 piglets were 1 wk old (range 5-9 d), 7 were 2 wk old (range 12-16 d), 8 were 4 wk old (range 25-30 d), and 8 were 6 wk old (range 39-45 d).
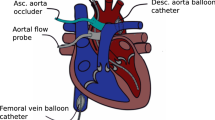
Catheterization instrumentation. The piglets were catheterized under general anesthesia, using an intramuscular injection of Telazole (6 mg/kg/dose) (Fort Dodge Laboratories, Fort Dodge, IA) and Rompun (4 mg/kg/dose) (Miles, Inc., Shawnee Mission, KS). Anesthesia was maintained using additional doses of ketamine (10 mg/kg as required) approximately once per hour. A tracheostomy was performed, and mechanical ventilation was established using room air delivered by an infant ventilator (Seachrist, Anaheim, CA). Adequacy of ventilation was determined by blood gas measurements obtained on a standard blood gas analyzer (Radiometer, Copenhagen, Denmark); mild respiratory alkalosis was achieved (pH 7.42-7.45) to abolish respiratory effort during data acquisition. The left carotid artery was isolated via the tracheostomy incision, and an introducer sheath (Cook, Bloomington, IN or Cordis, Miami, FL) was inserted into this carotid artery for placement of a 6 F pigtail 10-electrode conductance catheter (Webster Laboratories, Baldwin Park, CA) that was passed retrogradely into the left ventricle. A two-dimensional echocardiogram (Hitachi EUB-165, Tokyo, Japan) was used to guide catheter placement. Care was taken to place the pigtail into the left ventricular apex with the shaft of the catheter parallel to the ventricular septum without contacting endocardial surfaces. Intravenous sheaths were placed into a femoral artery and both femoral veins using percutaneous techniques. A 4 F micromanometer catheter (Millar Instruments, Houston, TX) was inserted into the femoral artery sheath and passed retrogradely into the left ventricle. A 5 F Berman angiographic catheter (Arrow, Reading, PA) was inserted into a femoral venous sheath and passed to the pulmonary artery. Again, a two-dimensional echocardiogram was used for guidance. A contrast echocardiogram was performed using injection of agitated 0.9% saline into the Berman catheter to confirm placement in the pulmonary artery (i.e. no saline “contrast” was introduced in the heart). A 5 F Fogarty atrial septostomy catheter (Baxter Health Care Corp., McGaw Park, IL) was placed into the contralateral femoral venous sheath and positioned in the inferior vena cava just below the junction with the right atrium.
The conductance catheter was connected to a Sigma-5 signal conditioner-processor (CardioDynamics, Leiden, Netherlands) for volume measurements. The Sigma-5 provides a small alternating current between the most proximal and distal electrodes, measures conductivity for each electrode pair between the current electrodes, and calculates a volume from the measured conductivity using the formula(18): where V(i) is the segmental volume, α is a dimensionless slope constant, L is the known interelectrode distance of the catheter, σb is the specific conductivity constant of blood, G(i) is the measured segment conductance, and Vc is the parallel conductance, a correction term which consists of electrical conductance through structures surrounding the ventricular cavity. The slope constant, α, has previously been found in the piglet to be very nearly 1(19) and was assumed to be 1 for this study. The Sigma-5 was used to measure the conductivity constant, σb, of the piglet's blood.
To measure true left ventricular volume, the raw volume signal must be corrected to by subtracting Vc, the apparent volume from electrical current flowing through structures other than left ventricular blood. Five to seven determinations of Vc were performed during each study using the saline technique(18). A small volume (0.3 mL) of saturated saline solution was injected into the pulmonary artery catheter using a minimal amount (0.5 mL) of normal saline flush. Left ventricular volume data were acquired as the saline bolus circulated through the left ventricle, causing a transient increase in blood conductivity and creating an apparent increase in left ventricular volume. Parallel conductance was then calculated by regression of end-systolic versus end-diastolic volume and extrapolating this regression to where end-systolic volume equals end-diastolic volume. At this point, blood conductivity is zero, and all measured conductance (Vc) is through surrounding structures.
Parallel conductance has been shown to vary with ventricular volume(19, 20). To try to compensate for this variation, we established a linear relationship between Vc and left ventricular volume using the five to seven separate parallel conductance determinations during the studies(21). Isoproterenol reduces ventricular volume considerably(19), allowing for measurement of Vc over a range of ventricular volumes. To define the linear relationship between Vc and end-diastolic volume, all of the Vc determinations for each piglet were used to perform linear regression between Vc values and the last uncorrected end-diastolic volume before each saline injection. The slope and intercept of this regression, and the first end-diastolic volume from each data acquisition were then used to calculate a unique Vc value for each data acquisition based upon the measured uncorrected left ventricular volume. This method allows for correction for variations of Vc with ventricular volume if a linear relationship exists. If there is no variation of Vc with volume, the slope of the regression between Vc and uncorrected diastolic volume is zero, and correction for Vc is simply subtraction of the intercept, which represents the average Vc for the separate determinations when the slope of this relation is zero.
The output from the Sigma-5 was sent to a chart recorder (Gould Electronics, Cleveland, OH) for monitoring in the laboratory and to an analog-to-digital data acquisition board (National Instruments, Austin, TX) inside a Macintosh IIx microcomputer (Apple Computers, Cupertino, CA). Data were digitized at 200 Hz and recorded on a hard disk for later analysis. Data acquisition and analyses were performed using programs written in our laboratory with the LabVIEW programming environment (National Instruments, Austin, TX)(22).
Data acquisition protocol. Simultaneous left ventricular pressures from the manometric catheter and volumes from the conductance catheter were recorded during brief pauses of mechanical ventilation at end-expiration, to minimize the effects of ventilatory changes in ventricular volume or the effects of thoracic impedance on measurement of volume or parallel conductance. Data were recorded before and during brief (5-10 s) occlusion of the inferior vena cava. Inferior vena caval occlusion was accomplished by inflating the balloon on the Fogarty catheter rapidly using air. These brief occlusions led to no change in heart rate during the time of occlusion.
Data were acquired at rest, during infusion of isoproterenol, and after administration of propranolol. To develop a doseresponse relationship to determine the dose of maximal β-adrenergic stimulation, isoproterenol infusion at rates of 0.05, 0.1, 0.2, and 0.3 μg/kg/min were used in piglets of all ages. One- and 6-wk-old piglets additionally received isoproterenol at rates of 0.5, 0.75, and 1.0 μg/kg/min to determine if any additionalβ-adrenergic effects were seen at these higher doses. At each infusion rate, the piglets' hemodynamics were allowed to achieve a steady state (5-10 min) before data acquisition. After acquiring data at the highest isoproterenol infusion rate, isoproterenol was stopped, and the piglet was allowed to “recover” for at least 20 min, at which time a second set of resting data were acquired. The piglet was then given 1 mg/kg intravenous propranolol to establish complete β-blockade, and after a brief equilibration period, pressure and volume data were again acquired. The pig was then killed using a lethal dose of pentobarbital.
Calculation of indices of function. Systolic function was analyzed by calculation of indices of function from the pressure-volume data. Load-dependent indices, including cardiac index (cardiac output/kg of body weight), stroke volume index (stroke volume/kg of body weight), stroke work, and the maximum of the first derivative of left ventricular pressure(dP/dtmax) were calculated. To define end-systole, the iterative technique described by Baan and van der Velde(23) was used. The Ees, a relatively load-independent index of systolic contractile state(24), was calculated. In addition, PRSW, another relatively load-independent index of contractility, was determined by performing linear regression of stroke work versus end-diastolic volume during reduction of preload by balloon occlusion of the inferior vena cava(25). Finally, the dP/dtmax end-diastolic volume relation was calculated by regression of dP/dtmax versus end-diastolic volume during occlusion of the inferior vena cava(26). Contractile reserve was considered to be present if there was a significant increase in an index of contractility during isoproterenol infusion.
To determine contractile efficiency at the different ages, an index was calculated relating external work performed to MVo2. The external work performed by the heart during contraction is defined as the stroke work, or the area bounded by the pressure-volume loop. Because MVo2 could not be directly measured in this model, PVA was used as an index of MVo2 as described by Suga(27) and Suga et al.(28) (Fig. 1). The PVA is defined as the area bounded by the end-diastolic and end-systolic pressure volume curves, the systolic pressure-volume trajectory, the end-systolic pressure-volume relationship from the end-systolic pressure-volume point to the volume axis intercept, and the volume axis. The PVA is linearly related to MVo2; changes in inotropic state do not affect the slope of this linear relation, but rather change its intercept(29). Contractile efficiency can be calculated as the ratio of external work (stroke work) to MVo2(30), and in this model was calculated as the ratio of stroke work to PVA × 100%. Although this produces a load-dependent index, loading conditions weigh heavily in the work performed during ejection and therefore affect efficiency. The PVA-MVo2 relation is dependent upon contractile state(29). Increases in inotropic state alter the absolute relation between MVo2 and pressure-volume area, but the change is a parallel shift, with an increase in the intercept of the MVo2-PVA regression with unchanged slope, and should not affect the calculation of the stroke work-PVA ratio(30). Although the ratio of stroke work to true MVo2 may decrease slightly during inotropic stimulation, this effect is likely to be small and not physiologically significant. In addition, the potential energy at the end of contraction was calculated, and changes within animals during β-adrenergic stimulation were examined.
Calculation of PVA. Representative data are shown for the determination of PVA and contractile efficiency from the pressure-volume plane from a 1-wk-old piglet at baseline (A) and on isoproterenol(B), and a 6 wk old at baseline (C) and on isoproterenol(D). Pressure is represented on the y axis and volume on the x axis. The solid diagonal lines represents the Ees defined in the text. The hatched areas represents the potential energy (PE) remaining at end systole. The areas within the pressure-volume loop of a contraction represent the stroke work (SW) of that contraction. The sum of PE + SW = PVA. In these particular examples, contractile efficiency values are 58, 71, 55, and 66 for A, B, C, and D, respectively.
Ea was used as an index of afterload .Ea combines compliance, resistance, and characteristic impedance of the vasculature(31–33). To facilitate comparison between age groups, indexed Ea was calculated as the ratio of end-systolic pressure to stroke volume indexed to piglet weight (ESP/SVI) for the normally loaded beats during data acquisitions. The use of Ea allows for examination of ventricular-vascular coupling, by comparison to simultaneous Ees. To evaluate ventricular-vascular coupling, the ratio of Ea to Ees was calculated for each acquisition. For calculation of the Ea/Ees ratio,Ea was calculated as the ratio of end-systolic pressure to stroke volume not indexed to body weight, as Ea/Ees should be independent of size. External stroke work performed by the ventricle should be optimal when the ratio of Ea to Ees is approximately 1(32).
Data analysis. Data are expressed as mean (SD) in the tables, or as mean with SE bars in graphs. All statistical analyses were performed on a Macintosh 7100/66 microcomputer (Apple Computers), using StatView 4.5(Abacus Concepts, Berkeley, CA) and SuperAnova (Abacus Concepts). To determine if age and isoproterenol dose had any significant effect on a given index of function, two-way ANOVA was performed with repeated measures used for experimental condition (baseline, isoproterenol dose, or propranolol). To determine if high rate infusion of isoproterenol had additional effects on indices of function over doses up to 0.3 μg/kg/min, this ANOVA was separately performed for all ages at isoproterenol infusion rates up to 0.3μg/kg/min, and for 1 and 6 wk olds only for all infusion rates. To evaluate differences in indices within each age group from baseline to the different isoproterenol dosages, multiple linear regression and Fisher's least significant difference test was used(34) to analyze magnitudes of differences between conditions quantitatively as well as qualitatively, and to eliminate variation of indices of function related to differences between animals. Independent variables used included dummy variables assigned to each pig to determine effect of variability between pigs, and dummy variables to code for isoproterenol dose. To further determine specific differences between age groups at baseline and at each isoproterenol infusion rate, multiple regression analysis with Fisher's least significant difference test was again used. A p < 0.05 was considered to be significant.
RESULTS
For clarity, results will be categorized and presented as isoproterenol dose-response, global cardiovascular function, ventricular contractile function, and ventricular vascular coupling. Basic growth and baseline hemodynamic data are shown in Table 1.
Isoproterenol dose response. To determine the isoproterenol infusion rate that caused the greatest degree of β-adrenergic stimulation, we examined heart rate and indices of contractile function in the different age groups at all the isoproterenol infusion rates that were used.Table 2 shows the within-age group analysis for heart rate. Heart rate increased to maximum at doses of 0.1-0.3 μg/kg/min, depending upon piglet age. No group demonstrated further increase in heart rate at infusion rates above 0.3 μg/kg/min. In Table 3, within-group analysis of Ees is shown. No change in Ees occurred at infusion rates higher than 0.1μg/kg/min in 2, 4, and 6 wk olds. In 1 wk olds, a progressive fall in Ees occurred until an infusion rates of 0.5μg/kg/min, but no significant difference existed between Ees at 0.3 and 0.5 μg/kg/min. Similar analysis of PRSW, dP/dtmax, and the dP/dtmax end-diastolic volume index revealed that no further increases in these indices of systolic function occurred at isoproterenol infusion rates higher than 0.2μg/kg/min in any age group.
In general, within group dose-response analyses demonstrated that isoproterenol infusion rates higher than 0.3 μg/kg/min in 1- and 6-wk-old piglets did not alter the significance of age or isoproterenol dose on any index of function, except for the Ees effects demonstrated in 1 wk-olds described above. Otherwise, effects that were significant at doses lower than 0.3 μg/kg/min remained significant at higher doses; effects not significant at doses lower than 0.3 μg/kg/min remained not significant at higher doses. Because of these analyses, we have chosen an isoproterenol infusion rate of 0.3 μg/kg/min to represent maximumβ-adrenergic stimulation for the purposes of comparison of indices of function between age groups.
Global cardiovascular function. Heart rate values are shown in Figure 2. Although heart rates tended to be lower at baseline in older piglets, no significant differences existed at baseline between age groups. During isoproterenol infusion, heart rates were significantly lower in 6-wk-old piglets than other groups. After propranolol administration, heart rates were very similar in all age groups, with heart rate in 6 wk olds significantly lower than 1 wk olds (p < 0.05).
Age-related differences in heart rate. One graph represents each of the age groups, with 1WK, 2WK, 4WK, and 6WK indicating graphs for 1-, 2-, 4-, and 6-wk-old age groups, respectively. Bars indicate heart rate at baseline, during isoproterenol infusion at 0.3 μg/kg/min, and after administration of propranolol, 1 mg/kg intravenously. The asterisk (*) indicates p < 0.05 vs 6-wk-old age group, and the dagger(†) indicates p < 0.05 vs all other age groups.
The changes in cardiac output and stoke volume from baseline during isoproterenol infusion are shown in Table 4; no differences between age groups were found in the change in either cardiac output or stroke volume. The response of cardiac index to isoproterenol infusion is shown in Figure 3. Again, by ANOVA, both age and isoproterenol significantly affected cardiac index. Six-week-old piglets had a significantly lower cardiac index at baseline and after propranolol than did 1 and 4 wk olds, and lower than at all other ages on isoproterenol. Similarly, stroke volume index was affected by both age and isoproterenol infusion, but to a lesser degree. Overall stroke volume index tended to be lower in 6 wk olds than at other ages, reaching statistical significance at some isoproterenol infusion rates.
Age-related differences in cardiac index. One graph represents each of the age groups, and abbreviations are the same as in Figure 2. Bars indicate cardiac index at baseline, during isoproterenol infusion at 0.3 μg/kg/min, and after adminstration of propranolol, 1 mg/kg, intravenously. The asterisk (*) indicates p < 0.05 vs 6-wk-old age group, and the dagger (†) indicates p < 0.05 vs all other age groups.
No age-related differences were seen in contractile efficiency. Isoproterenol infusion-increased contractile efficiency occurred in all age groups. In all ages a significant and similar rise in efficiency was from 51± 11% at baseline to 69 ± 6.7% at an isoproterenol infusion rate of 0.3 μg/kg/min. Because of the differences in heart size in piglets of different ages, the absolute magnitude of the PVA indices (such as stroke work and potential energy) cannot be compared directly. However, examination of potential energy changes within age groups provided an interesting observation. As shown in Figure 4, in 1-wk-old piglets, no change occurred in potential energy with isoproterenol infusion. However, in older age groups, isoproterenol infusion decreased the potential energy at end-systole, suggesting the presence of an energy reserve that could be tapped during increased inotropic states in older piglets. This is also graphically demonstrated in Figure 1.
Graphic representation of within-group potential energy at baseline, changes in potential energy during graded infusions of isoproterenol, and after administration of 1 mg/kg propranolol. Abbreviations are as in Figure 2. Note that only within-group comparisons are made in this figure. The asterisk (*) indicates p < 0.05 vs all other conditions in that age group, the double asterisk (**) indicates p < 0.05 vs all other conditions except baseline, the dagger (†) indicates p< 0.05 vs all other conditions except propranolol, and the double dagger (‡) indicates p < 0.05 vs the condition immediately to the left.
Indices of contractile function. The different indices of contractile function that were examined yielded somewhat different results. To compare Ees changes between age groups (because of different heart sizes), percent change in Ees during the different isoproterenol infusions and other experimental conditions were compared. ANOVA showed that age, experimental condition, and the interaction between the two significantly affected the percent change in Ees from baseline (Fig. 5). In 1 wk olds, isoproterenol infusion decreased Ees; this decrease became significant at infusion rates greater than 0.5μg/kg/min. In all other age groups, isoproterenol infusion increased Ees, even at low infusion rates of 0.05μg/kg/min in 2 and 4 wk olds and 0.1 μg/kg/min in 6 wk olds. One-week-old piglets were significantly different from the other age groups at all isoproterenol infusion rates. The 2-, 4-, and 6-wk-old piglet groups behaved similarly to one another.
Between-age group comparisons in the percentage change in Ees (%ΔEes) during isoproterenol infusion and after administration of propranolol 1 mg/kg. Other abbreviations are as in Figure 2. The asterisk(*) indicates p < 0.05 vs 6 wk olds, the double asterisk (**) indicates p < 0.05 vs 4 and 6 wk olds, and the dagger (†) indicates p < 0.05 vs 4 wk olds. Within-group comparisons of Ees values are presented in Table 2.
Other indices of contractile function showed significant increases caused by isoproterenol in all age groups. No significant differences were found between age groups in dP/dtmax by ANOVA .dP/dtmax increased to a similar degree in all age groups. Average baseline dP/dtmax was 308 ± 131 kPa/s, and maximum dP/dtmax was 797 ± 147 kPa/s in all piglets. When corrected for preload by examining the regression of dP/dtmax end-diastolic volume, results were similar. No significant differences between age groups were found in this index by ANOVA. In all piglets, the dP/dtmax end-diastolic relation increased from 37.1 ± 37.6 kPa/s/mL at baseline to a maximum of 103 ± 64 kPa/s/mL on isoproterenol. No significant differences were found between age groups in PRSW by ANOVA. In all piglets, PRSW increased from 6.91 ± 3.2 kPa·mL/mL at baseline to a maximum of 10.7 ± 2.5 kPa·mL/mL on isoproterenol.
Ventricular-vascular coupling. Indexed Ea was found by ANOVA to vary with both piglet age and isoproterenol dose. These findings are summarized in Figure 6. Indexed Ea was not significantly different between age groups at baseline. Differences between age groups in indexed Ea during isoproterenol infusion are demonstrated in Figure 6. At infusion rates of 0.75 μg/kg/min and higher, indexed Ea was higher in 6-wk olds than in 1 wk olds. In 1-, 2-, and 4-wk-old groups, indexed Ea decreased significantly from baseline at isoproterenol infusion rates of 0.2 to 0.3 μg/kg/min and higher. No such decrease was seen in 6-wk-old piglets, where no significant change in indexed Ea from baseline was found at any isoproterenol infusion rate.
Between-age group comparisons in indexed arterial elastance (ESP/SVI) at baseline, during isoproterenol infusion, and after administration of propranolol 1 mg/kg intravenously. Abbreviations are as in Figure 2. The asterisk (*) indicates p< 0.05 vs 6 wk olds. For within-group comparisons, a dagger(†) indicates p < 0.05 vs baseline, and a double dagger (‡) indicates p < 0.05 vs propranolol.
The ratio of Ea/Ees was found to vary with isoproterenol dose by ANOVA; age-related differences approached significance. Some interesting patterns of this ratio were found. In 1 wk olds, isoproterenol infusion had no significant effect on Ea/Ees, which remained close to 1. In older piglet groups, the resting Ea/Ees ratio was higher at rest, falling to values close to 1 at isoproterenol infusion rates of 0.05 or 0.1 μg/kg/min. These findings are shown in Figure 7.
Between-age group comparisons in the Ea/Ees ratio at baseline, during isoproterenol infusion, and after administration of propranolol 1 mg/kg intravenously. Abbreviations are as in Figure 2. The asterisk (*) indicates p< 0.05 vs 6 wk olds. For within-group comparisons, a dagger(†) indicates p < 0.05 vs baseline, a double dagger (‡) indicates p < 0.05 vs all conditions except baseline and isoproterenol infusion at 0.05 μg/kg/min, and a section sign (§) indicates p < 0.05 vs all other conditions.
DISCUSSION
This study demonstrates that contractile reserve exists in piglets as young as 1 wk of age. It may be some what limited, as reserve is not demonstrated by all indices of contractility. Significant differences exist in changes in indices of contractile function in response to β-adrenergic stimulation between younger and older piglets. In addition, important changes in afterload and ventricular-vascular coupling occur with maturation. These changes potentially allow for increased cardiac output reserve in older piglets. Other indices of cardiac function including heart rate and cardiac index changed with age in a similar manner to what has been found in previous studies(10, 12).
In this study, the different indices used to examine left ventricular contractility yielded different results. These indices of contractility examined different intervals of systole and therefore have different strengths and weaknesses. The preejection index dP/dtmax occurs before aortic valve opening during physiologic conditions, and unless afterload is low, dP/dtmax is insensitive to alterations of afterload. It is very sensitive to acute changes in contractility(35). Despite its known preload sensitivity and the reduction in left ventricular volume (preload) that accompanies isoproterenol infusion(9, 19),dP/dtmax increased significantly during isoproterenol infusion, because it is more sensitive to changes in contractility than to preload(35). Given its preload sensitivity, it is likely that the increase in dP/dtmax caused by isoproterenol infusion demonstrated a true increase in contractility in this study. PRSW, an ejection phase index, is relatively preload-insensitive by nature of its inclusion of end-diastolic volume(25, 36). However, as with other ejection phase indices, it is afterload-sensitive, although it has been shown to demonstrate little afterload dependence in the physiologic range(36). Changes in afterload during each isoproterenol experiment and differences in afterload between age groups may have influenced the findings using this index. The decrease in afterload caused by isoproterenol infusion in 1-wk-old piglets may have led to an artifactual rise in PRSW that mimicked a real change in contractility. Conversely, in circumstances with relatively higher afterload, a larger increase in contractility may be masked and not as apparent using PRSW.
The lack of change in Ees in 1-wk-old piglets in response to isoproterenol infusion in the presence of the increases in the other indices of contractility is of great interest. Although Ees is relatively insensitive to alterations in preload(36) and sensitive to only extreme alterations in afterload(37), it is also known to be somewhat less sensitive to changes in contractility than are other indices(36). It is possible that the lack of change in Ees in 1-wk-old piglets is related to insensitivity to changes in inotropic state, but this is unlikely because the same phenomenon was not seen in the older age groups that had similar changes to 1-wk-olds in the other indices of contractility. More likely,Ees, an end-systolic index, measures an aspect of contractility not measured by preejection and ejection phase indices. It is conceivable that lack of change in Ees in 1-wk-olds represents a deficiency in the extent of sarcomere shortening or of force generation at end-systole, possibly due to a deficiency in actin-myosin cross-bridge formation in response to β-stimulation. The exact mechanism causing this finding is not clear.
The incongruity of Ees and other indices of function in immature piglets is not without precedent. This finding parallels the finding of Klautz and Teitel(38) in piglets that blockade of sarcoplasmic reticulum function by ryanodine had no effect on the end-systolic pressure-volume relationship, but significantly diminished the dP/dtmax end-diastolic volume relationship. This could be because the maximum rate of pressure development, dP/dtmax, is dependent upon the rate of actin-myosin cross-bridge cycling, but end-systolic stiffness, Ees, is dependent upon the extent of cross-bridge cycling. The rate of cross-bridge cycling and the extent of cycling may depend differently on the source of intracellular calcium, such as the sarcolemma and sarcoplasmic reticulum. Because of immaturity of their sarcoplasmic reticulum, the younger piglets may be more dependent upon sarcolemmal calcium and may not be as responsive to adrenergic stimulation as the older piglets. The precise biochemical reason for the inability of the 1-wk-old hearts to increase Ees in response to β-adrenergic stimulation merits further study. One must consider that any single index of contractile function may not be sufficient to adequately describe global changes in contractility in all maturational states or heart diseases.
It is possible that some of the differences found in contractile function between this study and previous works are due to interspecies differences. Most prior examination of postnatal changes in function have been performed in lambs or in humans. It is quite possible that cardiac function in piglets matures at earlier ages than other species, accounting for the presence of contractile reserve using some of the indices of contractility in this study. Examination of piglets younger than 1 wk of age was not possible because of the very small size of the newborn piglet, and the relatively larger sizes of the catheters that were available for this study.
Another important finding of the present study is the response at different ages of indexed Ea to isoproterenol administration .Ea is an afterload term that combines arterial compliance, characteristic impedance, and resistance(33). Ea falls substantially during isoproterenol infusion in 1 wk olds and to a lesser degree in 2 and 4 wk olds. However, Ea does not change significantly in 6 wk olds in response to β-adrenergic stimulation from isoproterenol. This decrease allows for an increase in cardiac output in the youngest age groups, despite what may be a somewhat limited ability to increase contractility. If one considers only within-age group values of indexed Ea, it is possible that Ea is at its highest levels at rest in younger piglets and can be reduced by β-adrenergic stimulation. In 6 wk olds, resting Ea is closer to that found duringβ-adrenergic stimulation. Because contractility in immature hearts is particularly sensitive to the effects of increased afterload, this may be in part responsible for limiting Ees in the youngest piglets. To compensate for the high resting Ea, 1-wk-old piglets maintain an Ea/Ees ratio that is very close to 1, so that ventricular-vascular coupling is optimized even during baseline conditions(32), maximizing the amount of external work performed by the heart under given afterload conditions. In contrast, at rest, 6-wk-old piglets had an Ea/Ees ratio that was greater than 2, but decreased to approximately 1 after infusion of isoproterenol, the decrease occurring as a result of an increase in Ees. Younger piglets increase their cardiac output in response to β-adrenergic stimulation primarily by an increase in heart rate and a decrease in afterload; 6-wk-old piglets increase cardiac output by an increase in heart rate and an increase in contractility. This suggests that, at rest, ventricular-vascular coupling does not need to be optimized in the older piglets and that reserve exists for increasing cardiac output by a number of mechanisms.
Afterload plays an important role in the neonatal circulation. Studies in fetal and neonatal sheep have suggested that high afterload limits increases in stroke volume with volume expansion(15, 16), which may account for the previous belief that newborns did not exhibit the Frank-Starling response to volume loading. Afterload may, in fact, be in part responsible for the high level of resting contractility in the newborn. A recent report by Klautz et al.(17) demonstrated homeometric auto-regulation in newborn hearts. They demonstrated that increasing afterload in newborn lambs caused a significant increase in contractility to maintain cardiac output. This finding would account for the“optimized” resting Ea/Ees ratio found in 1-wk-old piglets in the present study. In the newborn, to provide adequate cardiac output to meet the high metabolic demands, contractility would have to be high in the face of high afterload.
The age-related differences in potential energy represent a potential for cardiac output reserve in older piglets. Because no significant change in potential energy at end-systole in 1 wk olds occurs in response to isoproterenol, it is unlikely that a further increase in stroke volume can occur from either increased contractility or decreased afterload. In contract, isoproterenol causes a significant fall in potential energy in older piglets. This decrease is in part to a steeper end-systolic pressure volume relationship. It would seem that pharmacologic agents which decrease the afterload that the ventricle faces at end-systole could potentially tap this energy reserve in older piglets, independent of any changes in contractility.
There is lack of agreement between the Ea and potential energy findings in this study. The proportion of potential energy to external work should be proportional to afterload, so that with extremely high afterload, no external work is performed, and all contractile energy would be“potential.” Because a fall in Ea was found at increasing doses in isoproterenol in 1 wk olds, one would expect a parallel fall in potential energy, but this was not found. One possible explanation for this discrepancy is that peripheral resistance may change to a greater degree in younger piglets and is underrepresented by Ea as an index of afterload. Because arterial pressures were not recorded during these studies, arterial resistance cannot be directly calculated from our data to address this question.
In summary, we have demonstrated that, although 1-wk-old piglets demonstrated contractile reserve to β-adrenergic stimulation by some indices of contractility, Ees did not demonstrate any increase in response to isoproterenol infusion in 1 wk olds, suggesting that resting contractility is higher in younger newborn piglets .Ees did demonstrate an increase in older groups of piglets in response to isoproterenol infusion. Ventricular-vascular coupling is optimized in 1-wk-old piglets, even in the resting anesthetized state. This may in part maintain the high cardiac output necessary to meet metabolic demands in the newborn and young piglet.

Abbreviations
- E es :
-
end-systolic elastance
- E a :
-
arterial elastance
- ESP/SVI:
-
arterial elastance indexed to body weight
- PRSW:
-
preload-recruitable stroke work
- dP/dt max :
-
maximum first derivative of left ventricular pressure
- V c :
-
parallel conductance
- MVo2:
-
myocardial oxygen consumption
- PVA:
-
pressure-volume area
References
Rudolph AM 1985 Distribution and regulation of blood flow in the fetal and neonatal lamb. Circ Res 57: 811–821.
Friedman WF 1972 The intrinsic physiologic properties of the developing heart. Prog Cardiovasc Dis 15: 87–111.
Anderson PA, Manring A, Glick KL, Crenshaw CC Jr 1982 Biophysics of the developing heart. III. A comparison of the left ventricular dynamics of the fetal and neonatal lamb heart. Am J Obstet Gynecol 143: 195–203.
Teitel DF 1988 Circulatory adjustments to postnatal life[review]. Semin Perinatol 12: 96–103.
Friedman AH, Fahey JT 1993 The transition from fetal to neonatal circulation: normal responses and implications for infants with heart disease. Semin Perinatol 17: 106–121.
Teitel DF, Dalinghaus M, Cassidy SC, Payne BD, Rudolph AM 1991 In utero ventilation augments the left ventricular response to isoproterenol and volume loading in fetal sheep. Pediatr Res 29: 466–472.
Grant DA, Maloney JE, Tyberg JV, Walker AM 1992 Effects of external constraint on the fetal left ventricular function curve. Am Heart J 123: 1601–1609.
Grant DA, Kondo CS, Maloney JE, Walker AM, Tyberg JV 1992 Changes in pericardial pressure during the perinatal period. Circulation 86: 1615–1621.
Teitel DF, Sidi D, Chin T, Brett C, Heymenn MA, Rudolph AM 1985 Development changes in myocardial contractile reserve in the lamb. Pediatr Res 19: 948–955.
Klopfenstein HS, Rudolph AM 1978 Postnatal changes in the circulation and responses to volume loading in sheep. Circ Res 42: 839–845.
Romero TE, Friedman WF 1979 Limited left ventricular response to volume overload in the neonatal period: a comparative study with the adult animal. Pediatr Res 13: 910–915.
Riemenschneider TA, Brunner RA, Mason DT 1981 Maturational changes in myocardial contractile state of newborn lambs. Pediatr Res 15: 349–356.
Riemenschneider TA, Allen HD, Mason DT 1986 Maturational changes in myocardial pump performance in newborn lambs. Am Heart J 111: 731–736.
Shaddy RE, Tyndall MR, Teitel DF, Li C, Rudolph AM 1988 Regulation of cardiac output with controlled heart rate in newborn lambs. Pediatr Res 24: 577–582.
Van Hare GF, Hawkins JA, Schmidt KG, Rudolph AM 1990 The effects of increasing mean arterial pressure on left ventricular output in newborn lambs. Circ Res 67: 78–83.
Hawkins J, Van Hare GF, Schmidt KG, Rudolph AM 1989 Effects of increasing afterload on left ventricular output in fetal lambs. Circ Res 65: 127–134.
Klautz RJ, Teitel DF, Steendijk P, van Bel F, Baan J 1995 Interaction between afterload and contractility in the newborn heart: evidence of homeometric autoregulation in the intact circulation. J Am Coll Cardiol 25: 1428–1435.
Baan J, van der Velde ET, de Bruin HG, Smeenk GJ, Koops J, van Dijk AD, Temmerman D, Senden J, Buis B 1984 Continuous measurement of left ventricular volume in animals and humans by conductance catheter. Circulation 70: 812–823.
Cassidy SC, Teitel DF 1992 The conductance volume catheter technique for measurement of left ventricular volume in young piglets. Pediatr Res 31: 85–90.
Boltwood CM, Appleyard RF, Glantz SA 1989 Left ventricular volume measurement by conductance catheter in intact dogs: parallel conductance volume depends on left ventricular size. Circulation 80: 1360–1377.
Cassidy SC, McGovern JJ, Chan DP, Allen HD 1996 Effects of commonly used adrenergic agonists on left ventricular function and systemic vascular resistance in young piglets. Am Heart J 133: 174–183.
Cassidy SC, Teitel DF 1997 Left ventricular pressure and volume data acquisition and analysis using LabVIEW. Comput Biol Med 27: 141–149.
Baan J, van der Velde ET 1988 Sensitivity of left ventricular end-systolic pressure-volume relation to type of loading intervention in dogs. Circ Res 62: 1247–1258.
Sagawa K, Maughan L, Suga H, Sunagawa K 1988 Chamber pressure-volume relation versus muscle tension-length relation. In: Cardiac Contraction and the Pressure-Volume Relationship. Oxford University Press, New York, pp 42–109.
Glower DD, Spratt JA, Snow ND, Kabas JS, Davis JW, Olsen CO, Tyson GS, Sabiston DCJ, Rankin JS 1985 Linearity of the Frank-Starling relationship in the intact heart: the concept of preload recruitable stroke work. Circulation 71: 994–1009.
Little WC 1985 The left ventricular dP/dtmax-end-diastolic volume relation in closed-chest dogs. Circ Res 56: 808–815.
Suga H 1979 Total mechanical energy of a ventricle model and cardiac oxygen consumption. Am J Physiol 236:H498–H505.
Suga H, Hayashi T, Shirahata M, Ninomiya I 1980 Critical evaluation of left ventricular systolic pressure volume areas as predictor of oxygen consumption rate. Jpn J Physiol 30: 907–919.
Suga H, Hisano R, Goto Y, Yamada O, Igarashi Y 1983 Effect of positive inotropic agents on the relation between oxygen consumption and systolic pressure volume area in canine left ventricle. Circ Res 53: 306–318.
Suga H, Goto Y, Kawaguchi O, Hata K, Takasago T, Saeki A, Taylor TW 1993 Ventricular perspective on efficiency. Basic Res Cardiol 88: 43–65.
Sunagawa K, Maughan WL, Burkoff D, Sagawa K 1983 Left ventricular interaction with arterial load studied in isolated canine ventricle. Am J Physiol 245:H773–H780.
Sagawa K, Maughan L, Suga H, K. S 1988 Cardiovasular interaction. In: Cardiac Contraction and the Pressure-Volume Relationship. Oxford University Press, New York, pp 232–298.
Kass DA, Grayson R, Marino P 1990 Pressure-volume analysis as a method for quantifying simultaneous drug (amrinone) effects on arterial load and contractile state in vivo. J Am Coll Cardiol 16: 726–732.
Glantz SA, Slinker BK 1990 Primer of Applied Regression and Analysis of Variance. McGraw-Hill, New York, pp 50–109.
Braunwald E 1988 Assessment of cardiac function. In: Braunwald E (ed) Heart Disease: A Textbook of Cardiovascular Medicine. WB Saunders, Philadelphia, pp 449–470.
Kass DA, Maughan WL, Guo ZM, Kono A, Sunagawa K, Sagawa K 1987 Comparative influence of load versus inotropic states on indexes of ventricular contractility: experimental and theoretical analysis based on pressure-volume relationships. Circulation 76: 1422–1436.
Teitel DF, Klautz R, Steendijk P, van der Velde ET, van Bel F, Baan J 1991 The end-systolic pressure-volume relationship in the newborn lamb: effects of loading and inotropic interventions. Pediatr Res 29: 473–482.
Klautz RJM, Teitel DF 1993 Sarcoplasmic reticulum (SR) blockade has limited effects on LV contractility in the newborn pig heart. J Am Coll Cardiol 21( Suppl A): 191A( abstr).
Acknowledgements
The authors thank Amy Crews for her tireless efforts in the laboratory, and John Hayes for his advice on the statistical analysis of the data.
Author information
Authors and Affiliations
Additional information
Supported in part by the Bremer Foundation.
Rights and permissions
About this article
Cite this article
Cassidy, S., Chan, D. & Allen, H. Left Ventricular Systolic Function, Arterial Elastance, and Ventricular-Vascular Coupling: A Developmental Study in Piglets. Pediatr Res 42, 273–281 (1997). https://doi.org/10.1203/00006450-199709000-00005
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199709000-00005